 Found 88 hits with Last Name = 'sheppard' and Initial = 'a'
Found 88 hits with Last Name = 'sheppard' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-1
(Homo sapiens (Human)) | BDBM50289397
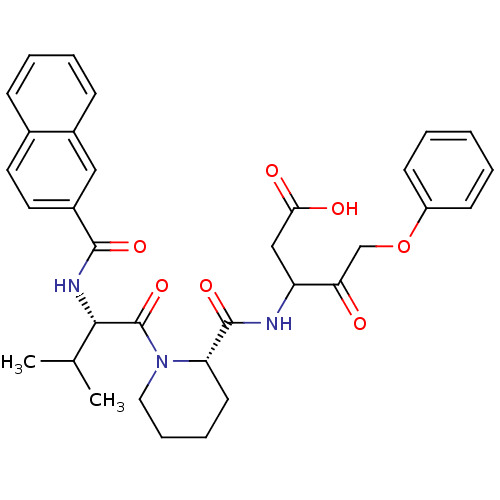
(3-[(1-{3-Methyl-2-[(naphthalene-2-carbonyl)-amino]...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N1CCCC[C@H]1C(=O)NC(CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C33H37N3O7/c1-21(2)30(35-31(40)24-16-15-22-10-6-7-11-23(22)18-24)33(42)36-17-9-8-14-27(36)32(41)34-26(19-29(38)39)28(37)20-43-25-12-4-3-5-13-25/h3-7,10-13,15-16,18,21,26-27,30H,8-9,14,17,19-20H2,1-2H3,(H,34,41)(H,35,40)(H,38,39)/t26?,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289410
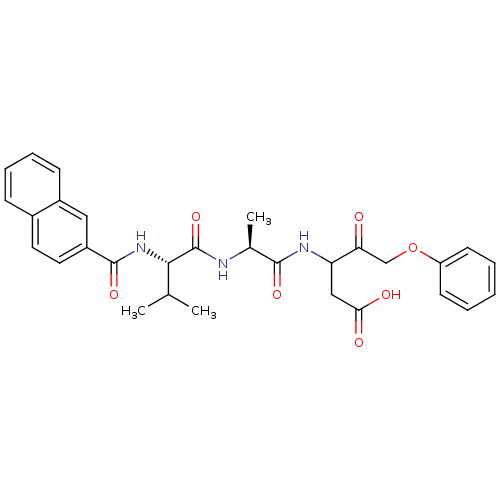
(CHEMBL26544 | Peptidic phenyl ketoether analogue)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C30H33N3O7/c1-18(2)27(33-29(38)22-14-13-20-9-7-8-10-21(20)15-22)30(39)31-19(3)28(37)32-24(16-26(35)36)25(34)17-40-23-11-5-4-6-12-23/h4-15,18-19,24,27H,16-17H2,1-3H3,(H,31,39)(H,32,37)(H,33,38)(H,35,36)/t19-,24?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289398

(CHEMBL553107 | Peptidic phenyl ketoether analogue)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N1CCC[C@H]1C(=O)NC(CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C32H35N3O7/c1-20(2)29(34-30(39)23-15-14-21-9-6-7-10-22(21)17-23)32(41)35-16-8-13-26(35)31(40)33-25(18-28(37)38)27(36)19-42-24-11-4-3-5-12-24/h3-7,9-12,14-15,17,20,25-26,29H,8,13,16,18-19H2,1-2H3,(H,33,40)(H,34,39)(H,37,38)/t25?,26-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289405

(CHEMBL2371932 | Peptidic phenyl ketoether analogue)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C30H38N4O9/c1-17(2)27(34-29(41)24(32-19(4)35)14-20-10-12-21(36)13-11-20)30(42)31-18(3)28(40)33-23(15-26(38)39)25(37)16-43-22-8-6-5-7-9-22/h5-13,17-18,23-24,27,36H,14-16H2,1-4H3,(H,31,42)(H,32,35)(H,33,40)(H,34,41)(H,38,39)/t18-,23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50084633
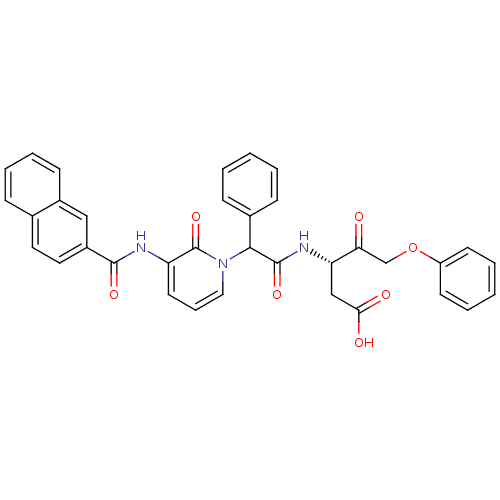
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)C(c1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C35H29N3O7/c39-30(22-45-27-14-5-2-6-15-27)29(21-31(40)41)37-34(43)32(24-11-3-1-4-12-24)38-19-9-16-28(35(38)44)36-33(42)26-18-17-23-10-7-8-13-25(23)20-26/h1-20,29,32H,21-22H2,(H,36,42)(H,37,43)(H,40,41)/t29-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289402

((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES CCC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C31H29N3O7/c1-2-26(30(39)33-25(18-28(36)37)27(35)19-41-23-11-4-3-5-12-23)34-16-8-13-24(31(34)40)32-29(38)22-15-14-20-9-6-7-10-21(20)17-22/h3-17,25-26H,2,18-19H2,1H3,(H,32,38)(H,33,39)(H,36,37)/t25-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289412
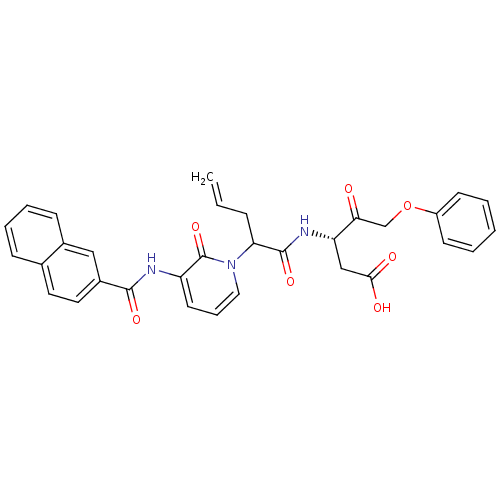
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)C(CC=C)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C32H29N3O7/c1-2-9-27(31(40)34-26(19-29(37)38)28(36)20-42-24-12-4-3-5-13-24)35-17-8-14-25(32(35)41)33-30(39)23-16-15-21-10-6-7-11-22(21)18-23/h2-8,10-18,26-27H,1,9,19-20H2,(H,33,39)(H,34,40)(H,37,38)/t26-,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50091577
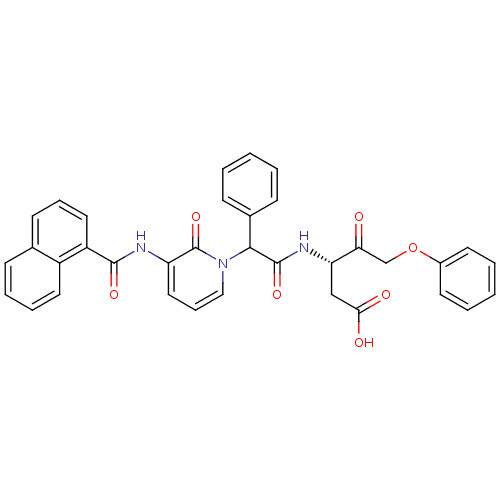
((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)C(c1ccccc1)n1cccc(NC(=O)c2cccc3ccccc23)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C35H29N3O7/c39-30(22-45-25-15-5-2-6-16-25)29(21-31(40)41)37-34(43)32(24-12-3-1-4-13-24)38-20-10-19-28(35(38)44)36-33(42)27-18-9-14-23-11-7-8-17-26(23)27/h1-20,29,32H,21-22H2,(H,36,42)(H,37,43)(H,40,41)/t29-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289393
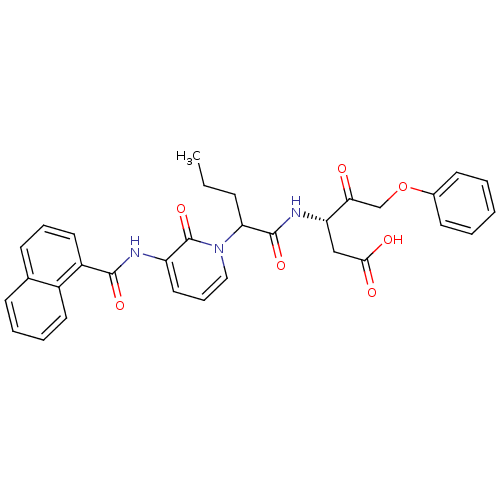
((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES CCCC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2cccc3ccccc23)c1=O Show InChI InChI=1S/C32H31N3O7/c1-2-10-27(31(40)34-26(19-29(37)38)28(36)20-42-22-13-4-3-5-14-22)35-18-9-17-25(32(35)41)33-30(39)24-16-8-12-21-11-6-7-15-23(21)24/h3-9,11-18,26-27H,2,10,19-20H2,1H3,(H,33,39)(H,34,40)(H,37,38)/t26-,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289407
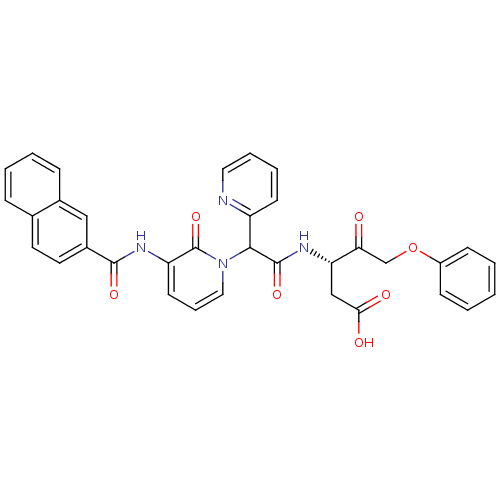
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)C(c1ccccn1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C34H28N4O7/c39-29(21-45-25-11-2-1-3-12-25)28(20-30(40)41)37-33(43)31(26-13-6-7-17-35-26)38-18-8-14-27(34(38)44)36-32(42)24-16-15-22-9-4-5-10-23(22)19-24/h1-19,28,31H,20-21H2,(H,36,42)(H,37,43)(H,40,41)/t28-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289388

((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES CCC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2cccc3ccccc23)c1=O Show InChI InChI=1S/C31H29N3O7/c1-2-26(30(39)33-25(18-28(36)37)27(35)19-41-21-12-4-3-5-13-21)34-17-9-16-24(31(34)40)32-29(38)23-15-8-11-20-10-6-7-14-22(20)23/h3-17,25-26H,2,18-19H2,1H3,(H,32,38)(H,33,39)(H,36,37)/t25-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289408
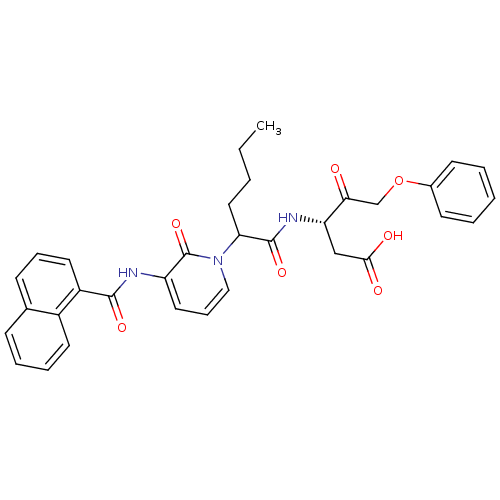
((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES CCCCC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2cccc3ccccc23)c1=O Show InChI InChI=1S/C33H33N3O7/c1-2-3-18-28(32(41)35-27(20-30(38)39)29(37)21-43-23-13-5-4-6-14-23)36-19-10-17-26(33(36)42)34-31(40)25-16-9-12-22-11-7-8-15-24(22)25/h4-17,19,27-28H,2-3,18,20-21H2,1H3,(H,34,40)(H,35,41)(H,38,39)/t27-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289400

((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES CC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2cccc3ccccc23)c1=O Show InChI InChI=1S/C30H27N3O7/c1-19(28(37)32-25(17-27(35)36)26(34)18-40-21-11-3-2-4-12-21)33-16-8-15-24(30(33)39)31-29(38)23-14-7-10-20-9-5-6-13-22(20)23/h2-16,19,25H,17-18H2,1H3,(H,31,38)(H,32,37)(H,35,36)/t19?,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289403
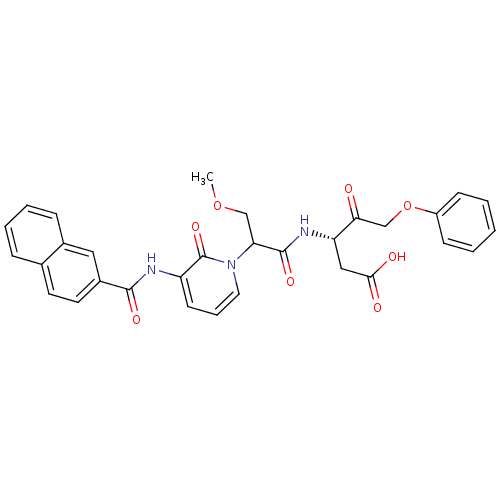
((S)-3-(3-Methoxy-2-{3-[(naphthalene-2-carbonyl)-am...)Show SMILES COCC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C31H29N3O8/c1-41-18-26(30(39)33-25(17-28(36)37)27(35)19-42-23-10-3-2-4-11-23)34-15-7-12-24(31(34)40)32-29(38)22-14-13-20-8-5-6-9-21(20)16-22/h2-16,25-26H,17-19H2,1H3,(H,32,38)(H,33,39)(H,36,37)/t25-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289387
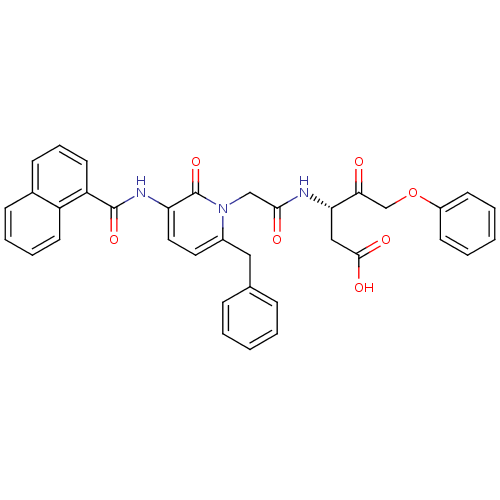
((S)-3-(2-{6-Benzyl-3-[(naphthalene-1-carbonyl)-ami...)Show SMILES OC(=O)C[C@H](NC(=O)Cn1c(Cc2ccccc2)ccc(NC(=O)c2cccc3ccccc23)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C36H31N3O7/c40-32(23-46-27-14-5-2-6-15-27)31(21-34(42)43)37-33(41)22-39-26(20-24-10-3-1-4-11-24)18-19-30(36(39)45)38-35(44)29-17-9-13-25-12-7-8-16-28(25)29/h1-19,31H,20-23H2,(H,37,41)(H,38,44)(H,42,43)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289409

((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES CC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C30H27N3O7/c1-19(28(37)32-25(17-27(35)36)26(34)18-40-23-10-3-2-4-11-23)33-15-7-12-24(30(33)39)31-29(38)22-14-13-20-8-5-6-9-21(20)16-22/h2-16,19,25H,17-18H2,1H3,(H,31,38)(H,32,37)(H,35,36)/t19?,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289395

((S)-3-(2-{6-Butyl-3-[(naphthalene-1-carbonyl)-amin...)Show SMILES CCCCc1ccc(NC(=O)c2cccc3ccccc23)c(=O)n1CC(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C33H33N3O7/c1-2-3-12-23-17-18-27(35-32(41)26-16-9-11-22-10-7-8-15-25(22)26)33(42)36(23)20-30(38)34-28(19-31(39)40)29(37)21-43-24-13-5-4-6-14-24/h4-11,13-18,28H,2-3,12,19-21H2,1H3,(H,34,38)(H,35,41)(H,39,40)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289411
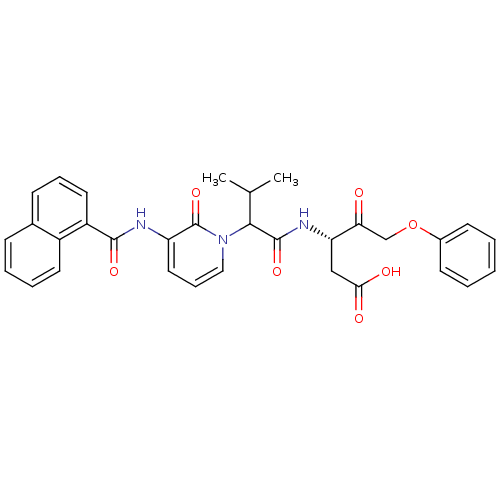
((S)-3-(3-Methyl-2-{3-[(naphthalene-1-carbonyl)-ami...)Show SMILES CC(C)C(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2cccc3ccccc23)c1=O Show InChI InChI=1S/C32H31N3O7/c1-20(2)29(31(40)34-26(18-28(37)38)27(36)19-42-22-12-4-3-5-13-22)35-17-9-16-25(32(35)41)33-30(39)24-15-8-11-21-10-6-7-14-23(21)24/h3-17,20,26,29H,18-19H2,1-2H3,(H,33,39)(H,34,40)(H,37,38)/t26-,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289404
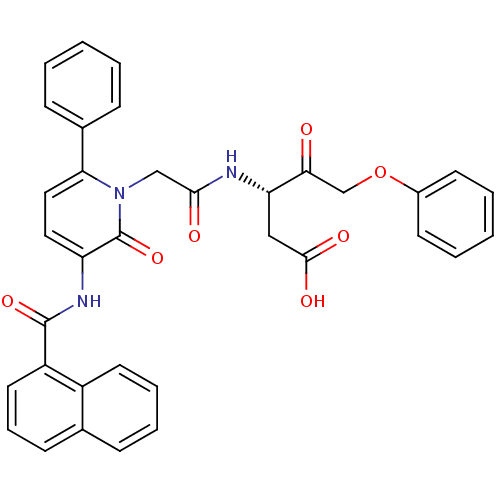
((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)Cn1c(ccc(NC(=O)c2cccc3ccccc23)c1=O)-c1ccccc1)C(=O)COc1ccccc1 Show InChI InChI=1S/C35H29N3O7/c39-31(22-45-25-14-5-2-6-15-25)29(20-33(41)42)36-32(40)21-38-30(24-11-3-1-4-12-24)19-18-28(35(38)44)37-34(43)27-17-9-13-23-10-7-8-16-26(23)27/h1-19,29H,20-22H2,(H,36,40)(H,37,43)(H,41,42)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289390

((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)Cn1cccc(NC(=O)c2cccc3ccccc23)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C29H25N3O7/c33-25(18-39-20-10-2-1-3-11-20)24(16-27(35)36)30-26(34)17-32-15-7-14-23(29(32)38)31-28(37)22-13-6-9-19-8-4-5-12-21(19)22/h1-15,24H,16-18H2,(H,30,34)(H,31,37)(H,35,36)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289399

((S)-3-(2-{6-Ethyl-3-[(naphthalene-1-carbonyl)-amin...)Show SMILES CCc1ccc(NC(=O)c2cccc3ccccc23)c(=O)n1CC(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C31H29N3O7/c1-2-21-15-16-25(33-30(39)24-14-8-10-20-9-6-7-13-23(20)24)31(40)34(21)18-28(36)32-26(17-29(37)38)27(35)19-41-22-11-4-3-5-12-22/h3-16,26H,2,17-19H2,1H3,(H,32,36)(H,33,39)(H,37,38)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289414

((S)-3-(2-{6-Hexyl-3-[(naphthalene-1-carbonyl)-amin...)Show SMILES CCCCCCc1ccc(NC(=O)c2cccc3ccccc23)c(=O)n1CC(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C35H37N3O7/c1-2-3-4-6-14-25-19-20-29(37-34(43)28-18-11-13-24-12-9-10-17-27(24)28)35(44)38(25)22-32(40)36-30(21-33(41)42)31(39)23-45-26-15-7-5-8-16-26/h5,7-13,15-20,30H,2-4,6,14,21-23H2,1H3,(H,36,40)(H,37,43)(H,41,42)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289415

((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)Cn1cccc(NC(=O)c2ccc3ccccc3c2)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C29H25N3O7/c33-25(18-39-22-9-2-1-3-10-22)24(16-27(35)36)30-26(34)17-32-14-6-11-23(29(32)38)31-28(37)21-13-12-19-7-4-5-8-20(19)15-21/h1-15,24H,16-18H2,(H,30,34)(H,31,37)(H,35,36)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289394
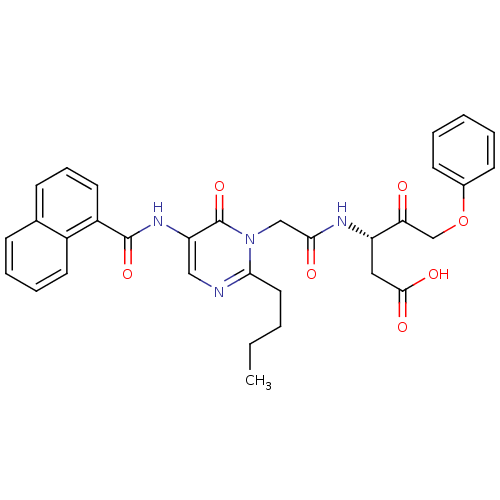
((S)-3-(2-{2-Butyl-5-[(naphthalene-1-carbonyl)-amin...)Show SMILES CCCCc1ncc(NC(=O)c2cccc3ccccc23)c(=O)n1CC(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C32H32N4O7/c1-2-3-16-28-33-18-26(35-31(41)24-15-9-11-21-10-7-8-14-23(21)24)32(42)36(28)19-29(38)34-25(17-30(39)40)27(37)20-43-22-12-5-4-6-13-22/h4-15,18,25H,2-3,16-17,19-20H2,1H3,(H,34,38)(H,35,41)(H,39,40)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289401

((S)-3-(2-{5-[(Naphthalene-1-carbonyl)-amino]-6-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)Cn1c(ncc(NC(=O)c2cccc3ccccc23)c1=O)-c1ccccc1)C(=O)COc1ccccc1 Show InChI InChI=1S/C34H28N4O7/c39-29(21-45-24-14-5-2-6-15-24)27(18-31(41)42)36-30(40)20-38-32(23-11-3-1-4-12-23)35-19-28(34(38)44)37-33(43)26-17-9-13-22-10-7-8-16-25(22)26/h1-17,19,27H,18,20-21H2,(H,36,40)(H,37,43)(H,41,42)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289386

((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES CCCc1ccc(NC(=O)c2cccc3ccccc23)c(=O)n1CC(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C32H31N3O7/c1-2-9-22-16-17-26(34-31(40)25-15-8-11-21-10-6-7-14-24(21)25)32(41)35(22)19-29(37)33-27(18-30(38)39)28(36)20-42-23-12-4-3-5-13-23/h3-8,10-17,27H,2,9,18-20H2,1H3,(H,33,37)(H,34,40)(H,38,39)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289396
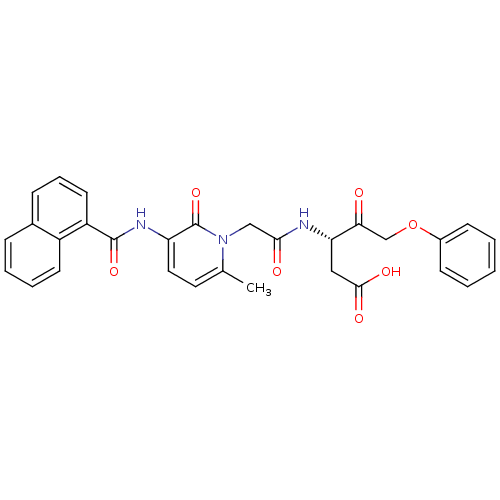
((S)-3-(2-{6-Methyl-3-[(naphthalene-1-carbonyl)-ami...)Show SMILES Cc1ccc(NC(=O)c2cccc3ccccc23)c(=O)n1CC(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C30H27N3O7/c1-19-14-15-24(32-29(38)23-13-7-9-20-8-5-6-12-22(20)23)30(39)33(19)17-27(35)31-25(16-28(36)37)26(34)18-40-21-10-3-2-4-11-21/h2-15,25H,16-18H2,1H3,(H,31,35)(H,32,38)(H,36,37)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289413
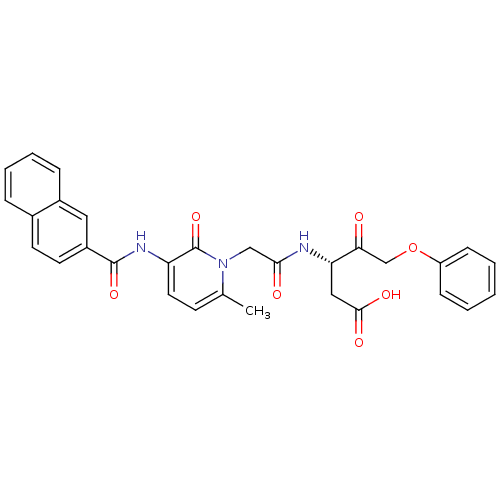
((S)-3-(2-{6-Methyl-3-[(naphthalene-2-carbonyl)-ami...)Show SMILES Cc1ccc(NC(=O)c2ccc3ccccc3c2)c(=O)n1CC(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C30H27N3O7/c1-19-11-14-24(32-29(38)22-13-12-20-7-5-6-8-21(20)15-22)30(39)33(19)17-27(35)31-25(16-28(36)37)26(34)18-40-23-9-3-2-4-10-23/h2-15,25H,16-18H2,1H3,(H,31,35)(H,32,38)(H,36,37)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246925
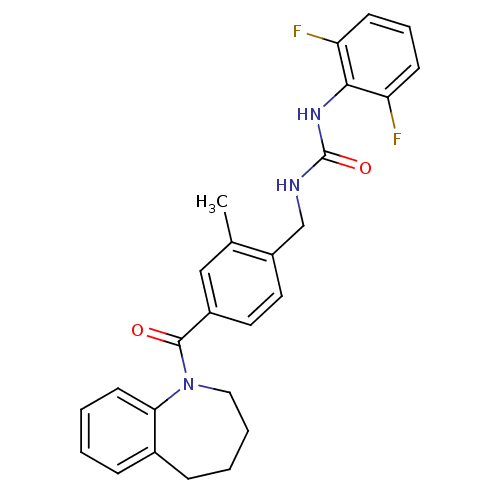
(1-(4-[3-(2,6-Difluorophenyl)ureidomethyl]-3-methyl...)Show SMILES Cc1cc(ccc1CNC(=O)Nc1c(F)cccc1F)C(=O)N1CCCCc2ccccc12 Show InChI InChI=1S/C26H25F2N3O2/c1-17-15-19(25(32)31-14-5-4-8-18-7-2-3-11-23(18)31)12-13-20(17)16-29-26(33)30-24-21(27)9-6-10-22(24)28/h2-3,6-7,9-13,15H,4-5,8,14,16H2,1H3,(H2,29,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human vasopressin V2 receptor expressed in HEK293 cells by radioligand binding assay |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289391
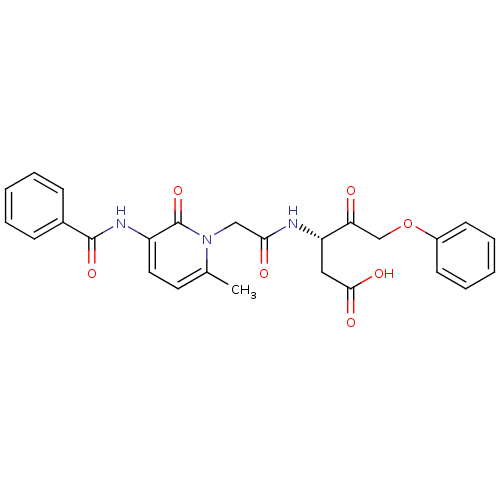
((S)-3-[2-(3-Benzoylamino-6-methyl-2-oxo-2H-pyridin...)Show SMILES Cc1ccc(NC(=O)c2ccccc2)c(=O)n1CC(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C26H25N3O7/c1-17-12-13-20(28-25(34)18-8-4-2-5-9-18)26(35)29(17)15-23(31)27-21(14-24(32)33)22(30)16-36-19-10-6-3-7-11-19/h2-13,21H,14-16H2,1H3,(H,27,31)(H,28,34)(H,32,33)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289406

((S)-3-[2-(3-Benzyloxycarbonylamino-6-methyl-2-oxo-...)Show SMILES Cc1ccc(NC(=O)OCc2ccccc2)c(=O)n1CC(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C27H27N3O8/c1-18-12-13-21(29-27(36)38-16-19-8-4-2-5-9-19)26(35)30(18)15-24(32)28-22(14-25(33)34)23(31)17-37-20-10-6-3-7-11-20/h2-13,22H,14-17H2,1H3,(H,28,32)(H,29,36)(H,33,34)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246575
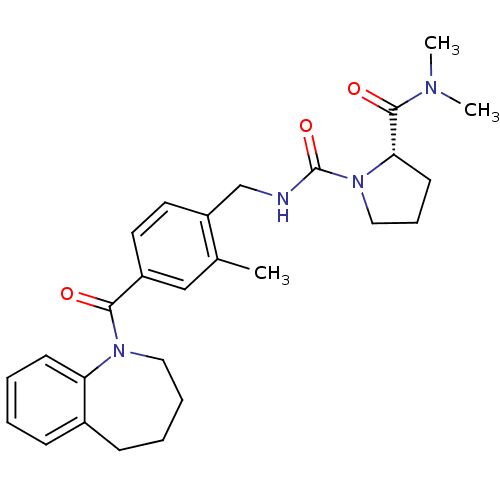
(1-(2-Methyl-4-(2,3,4,5-tetrahydro-1-benzazepin-1-y...)Show SMILES CN(C)C(=O)[C@@H]1CCCN1C(=O)NCc1ccc(cc1C)C(=O)N1CCCCc2ccccc12 |r| Show InChI InChI=1S/C27H34N4O3/c1-19-17-21(25(32)30-15-7-6-10-20-9-4-5-11-23(20)30)13-14-22(19)18-28-27(34)31-16-8-12-24(31)26(33)29(2)3/h4-5,9,11,13-14,17,24H,6-8,10,12,15-16,18H2,1-3H3,(H,28,34)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human vasopressin V2 receptor expressed in HEK293 cells by radioligand binding assay |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289389
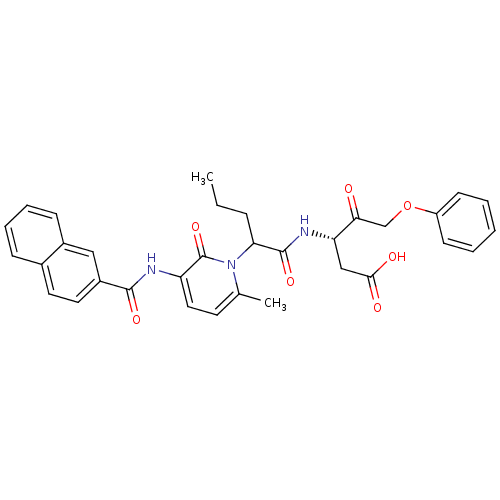
((S)-3-(2-{6-Methyl-3-[(naphthalene-2-carbonyl)-ami...)Show SMILES CCCC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1c(C)ccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C33H33N3O7/c1-3-9-28(32(41)35-27(19-30(38)39)29(37)20-43-25-12-5-4-6-13-25)36-21(2)14-17-26(33(36)42)34-31(40)24-16-15-22-10-7-8-11-23(22)18-24/h4-8,10-18,27-28H,3,9,19-20H2,1-2H3,(H,34,40)(H,35,41)(H,38,39)/t27-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289392

((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES CCCC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1c(ccc(NC(=O)c2cccc3ccccc23)c1=O)-c1ccccc1 Show InChI InChI=1S/C38H35N3O7/c1-2-12-33(37(46)40-31(23-35(43)44)34(42)24-48-27-17-7-4-8-18-27)41-32(26-14-5-3-6-15-26)22-21-30(38(41)47)39-36(45)29-20-11-16-25-13-9-10-19-28(25)29/h3-11,13-22,31,33H,2,12,23-24H2,1H3,(H,39,45)(H,40,46)(H,43,44)/t31-,33?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50246890
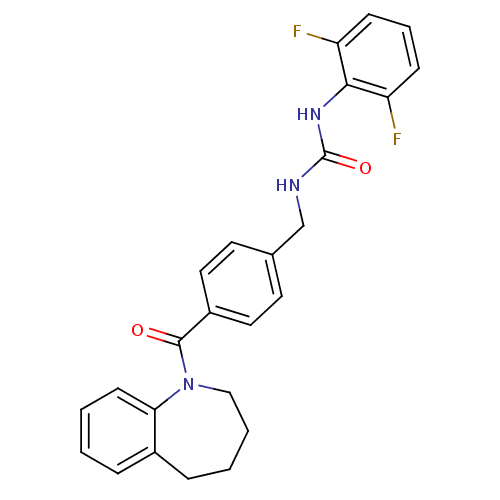
(1-(4-[3-(2,6-Difluorophenyl)ureidomethyl]benzoyl)-...)Show SMILES Fc1cccc(F)c1NC(=O)NCc1ccc(cc1)C(=O)N1CCCCc2ccccc12 Show InChI InChI=1S/C25H23F2N3O2/c26-20-8-5-9-21(27)23(20)29-25(32)28-16-17-11-13-19(14-12-17)24(31)30-15-4-3-7-18-6-1-2-10-22(18)30/h1-2,5-6,8-14H,3-4,7,15-16H2,(H2,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to oxytocin receptor (unknown origin) |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50246925

(1-(4-[3-(2,6-Difluorophenyl)ureidomethyl]-3-methyl...)Show SMILES Cc1cc(ccc1CNC(=O)Nc1c(F)cccc1F)C(=O)N1CCCCc2ccccc12 Show InChI InChI=1S/C26H25F2N3O2/c1-17-15-19(25(32)31-14-5-4-8-18-7-2-3-11-23(18)31)12-13-20(17)16-29-26(33)30-24-21(27)9-6-10-22(24)28/h2-3,6-7,9-13,15H,4-5,8,14,16H2,1H3,(H2,29,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to oxytocin receptor (unknown origin) |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50246575

(1-(2-Methyl-4-(2,3,4,5-tetrahydro-1-benzazepin-1-y...)Show SMILES CN(C)C(=O)[C@@H]1CCCN1C(=O)NCc1ccc(cc1C)C(=O)N1CCCCc2ccccc12 |r| Show InChI InChI=1S/C27H34N4O3/c1-19-17-21(25(32)30-15-7-6-10-20-9-4-5-11-23(20)30)13-14-22(19)18-28-27(34)31-16-8-12-24(31)26(33)29(2)3/h4-5,9,11,13-14,17,24H,6-8,10,12,15-16,18H2,1-3H3,(H,28,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to oxytocin receptor (unknown origin) |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246608
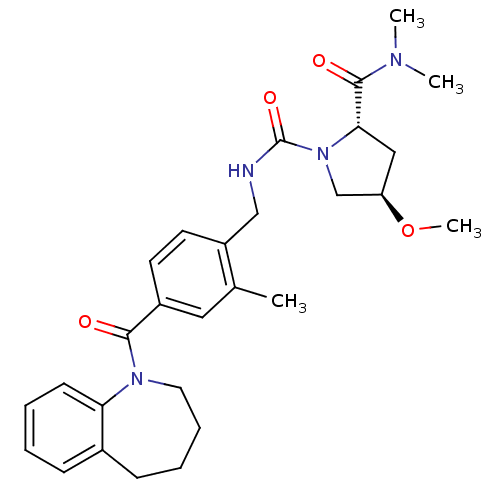
((4R)-4-Methoxy-1-(2-methyl-4-(2,3,4,5-tetrahydro-1...)Show SMILES CO[C@@H]1C[C@H](N(C1)C(=O)NCc1ccc(cc1C)C(=O)N1CCCCc2ccccc12)C(=O)N(C)C |r| Show InChI InChI=1S/C28H36N4O4/c1-19-15-21(26(33)31-14-8-7-10-20-9-5-6-11-24(20)31)12-13-22(19)17-29-28(35)32-18-23(36-4)16-25(32)27(34)30(2)3/h5-6,9,11-13,15,23,25H,7-8,10,14,16-18H2,1-4H3,(H,29,35)/t23-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246609

((4R)-4-Ethoxy-1-(2-methyl-4-(2,3,4,5-tetrahydro-1-...)Show SMILES CCO[C@@H]1C[C@H](N(C1)C(=O)NCc1ccc(cc1C)C(=O)N1CCCCc2ccccc12)C(=O)N(C)C |r| Show InChI InChI=1S/C29H38N4O4/c1-5-37-24-17-26(28(35)31(3)4)33(19-24)29(36)30-18-23-14-13-22(16-20(23)2)27(34)32-15-9-8-11-21-10-6-7-12-25(21)32/h6-7,10,12-14,16,24,26H,5,8-9,11,15,17-19H2,1-4H3,(H,30,36)/t24-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246610
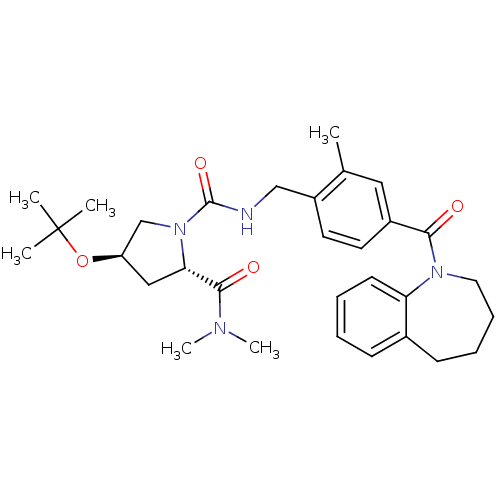
((4R)-4-tert-Butoxyoxy-1-(2-methyl-4-(2,3,4,5-tetra...)Show SMILES CN(C)C(=O)[C@@H]1C[C@H](CN1C(=O)NCc1ccc(cc1C)C(=O)N1CCCCc2ccccc12)OC(C)(C)C |r| Show InChI InChI=1S/C31H42N4O4/c1-21-17-23(28(36)34-16-10-9-12-22-11-7-8-13-26(22)34)14-15-24(21)19-32-30(38)35-20-25(39-31(2,3)4)18-27(35)29(37)33(5)6/h7-8,11,13-15,17,25,27H,9-10,12,16,18-20H2,1-6H3,(H,32,38)/t25-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246611
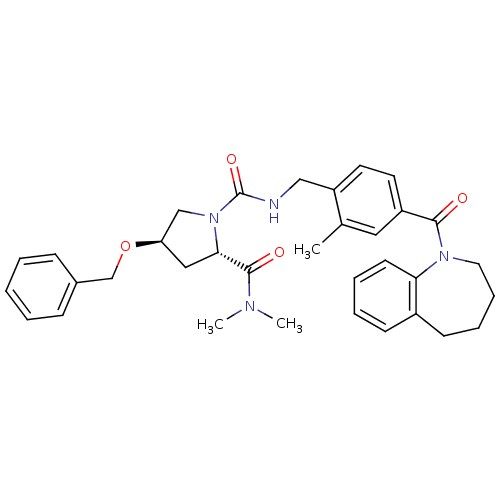
((4R)-4-Benzyloxy-1-(2-methyl-4-(2,3,4,5-tetrahydro...)Show SMILES CN(C)C(=O)[C@@H]1C[C@H](CN1C(=O)NCc1ccc(cc1C)C(=O)N1CCCCc2ccccc12)OCc1ccccc1 |r| Show InChI InChI=1S/C34H40N4O4/c1-24-19-27(32(39)37-18-10-9-14-26-13-7-8-15-30(26)37)16-17-28(24)21-35-34(41)38-22-29(20-31(38)33(40)36(2)3)42-23-25-11-5-4-6-12-25/h4-8,11-13,15-17,19,29,31H,9-10,14,18,20-23H2,1-3H3,(H,35,41)/t29-,31+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246612

(1-(2-Methyl-4-(2,3,4,5-tetrahydro-1-benzazepin-1-y...)Show SMILES CN(C)C(=O)[C@@H]1CC(=O)CN1C(=O)NCc1ccc(cc1C)C(=O)N1CCCCc2ccccc12 |r| Show InChI InChI=1S/C27H32N4O4/c1-18-14-20(25(33)30-13-7-6-9-19-8-4-5-10-23(19)30)11-12-21(18)16-28-27(35)31-17-22(32)15-24(31)26(34)29(2)3/h4-5,8,10-12,14,24H,6-7,9,13,15-17H2,1-3H3,(H,28,35)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246642

(4,4-Difluoro-1-(2-methyl-4-(2,3,4,5-tetrahydro-1-b...)Show SMILES CN(C)C(=O)[C@@H]1CC(CN1C(=O)NCc1ccc(cc1C)C(=O)N1CCCCc2ccccc12)C(F)F |r| Show InChI InChI=1S/C28H34F2N4O3/c1-18-14-20(26(35)33-13-7-6-9-19-8-4-5-10-23(19)33)11-12-21(18)16-31-28(37)34-17-22(25(29)30)15-24(34)27(36)32(2)3/h4-5,8,10-12,14,22,24-25H,6-7,9,13,15-17H2,1-3H3,(H,31,37)/t22?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246643

(4,4-Dimethoxy-1-(2-methyl-4-(2,3,4,5-tetrahydro-1-...)Show SMILES COC(OC)C1C[C@H](N(C1)C(=O)NCc1ccc(cc1C)C(=O)N1CCCCc2ccccc12)C(=O)N(C)C |r| Show InChI InChI=1S/C30H40N4O5/c1-20-16-22(27(35)33-15-9-8-11-21-10-6-7-12-25(21)33)13-14-23(20)18-31-30(37)34-19-24(29(38-4)39-5)17-26(34)28(36)32(2)3/h6-7,10,12-14,16,24,26,29H,8-9,11,15,17-19H2,1-5H3,(H,31,37)/t24?,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246644

(1-(4-(5-Hydroxy-2,3,4,5-tetrahydro-1-benzazepin-1-...)Show SMILES CN(C)C(=O)[C@@H]1CCCN1C(=O)NCc1ccc(cc1C)C(=O)N1CCCC(O)c2ccccc12 |r| Show InChI InChI=1S/C27H34N4O4/c1-18-16-19(25(33)30-14-7-11-24(32)21-8-4-5-9-22(21)30)12-13-20(18)17-28-27(35)31-15-6-10-23(31)26(34)29(2)3/h4-5,8-9,12-13,16,23-24,32H,6-7,10-11,14-15,17H2,1-3H3,(H,28,35)/t23-,24?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246645
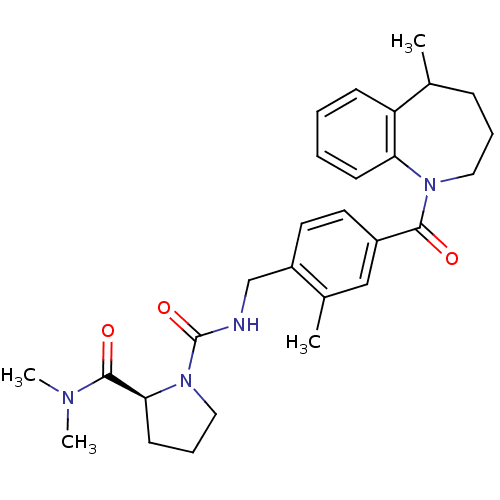
(1-(2-Methyl-4-(5-methyl-2,3,4,5-tetrahydro-1-benza...)Show SMILES CC1CCCN(C(=O)c2ccc(CNC(=O)N3CCC[C@H]3C(=O)N(C)C)c(C)c2)c2ccccc12 |r| Show InChI InChI=1S/C28H36N4O3/c1-19-9-7-15-31(24-11-6-5-10-23(19)24)26(33)21-13-14-22(20(2)17-21)18-29-28(35)32-16-8-12-25(32)27(34)30(3)4/h5-6,10-11,13-14,17,19,25H,7-9,12,15-16,18H2,1-4H3,(H,29,35)/t19?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246719
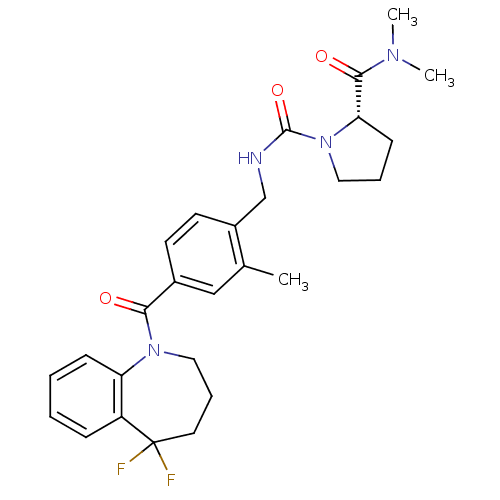
(1-(4-(5,5-Difluoro-2,3,4,5-tetrahydro-1-benzazepin...)Show SMILES CN(C)C(=O)[C@@H]1CCCN1C(=O)NCc1ccc(cc1C)C(=O)N1CCCC(F)(F)c2ccccc12 |r| Show InChI InChI=1S/C27H32F2N4O3/c1-18-16-19(24(34)32-15-7-13-27(28,29)21-8-4-5-9-22(21)32)11-12-20(18)17-30-26(36)33-14-6-10-23(33)25(35)31(2)3/h4-5,8-9,11-12,16,23H,6-7,10,13-15,17H2,1-3H3,(H,30,36)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246720
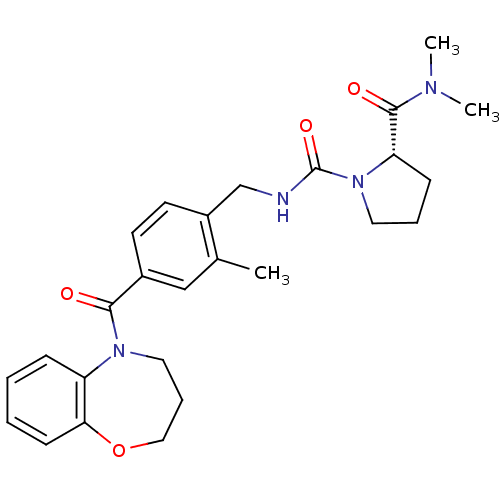
(1-(2-Methyl-4-(2,3,4,5-tetrahydro-1,5-benzoxazepin...)Show SMILES CN(C)C(=O)[C@@H]1CCCN1C(=O)NCc1ccc(cc1C)C(=O)N1CCCOc2ccccc12 |r| Show InChI InChI=1S/C26H32N4O4/c1-18-16-19(24(31)29-14-7-15-34-23-10-5-4-8-21(23)29)11-12-20(18)17-27-26(33)30-13-6-9-22(30)25(32)28(2)3/h4-5,8,10-12,16,22H,6-7,9,13-15,17H2,1-3H3,(H,27,33)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246721

(1-(4-(10,11-Dihydro-5H-pyrrolo[2,1-c][1,4]benzodia...)Show SMILES CN(C)C(=O)[C@@H]1CCCN1C(=O)NCc1ccc(cc1C)C(=O)N1Cc2cccn2Cc2ccccc12 |r| Show InChI InChI=1S/C29H33N5O3/c1-20-16-21(12-13-22(20)17-30-29(37)33-15-7-11-26(33)28(36)31(2)3)27(35)34-19-24-9-6-14-32(24)18-23-8-4-5-10-25(23)34/h4-6,8-10,12-14,16,26H,7,11,15,17-19H2,1-3H3,(H,30,37)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50246722

(1-(4-(6,7-Dihydro-5H-dibenzo[b,d]azepin-5-ylcarbon...)Show SMILES CN(C)C(=O)[C@@H]1CCCN1C(=O)NCc1ccc(cc1C)C(=O)N1CCc2ccccc2-c2ccccc12 |r| Show InChI InChI=1S/C31H34N4O3/c1-21-19-23(14-15-24(21)20-32-31(38)35-17-8-13-28(35)30(37)33(2)3)29(36)34-18-16-22-9-4-5-10-25(22)26-11-6-7-12-27(26)34/h4-7,9-12,14-15,19,28H,8,13,16-18,20H2,1-3H3,(H,32,38)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
Vantia Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level by CRE-luciferase report... |
J Med Chem 51: 8124-34 (2008)
Article DOI: 10.1021/jm8008162
BindingDB Entry DOI: 10.7270/Q28W3D6V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data