 Found 897 hits with Last Name = 'shinde' and Initial = 'bu'
Found 897 hits with Last Name = 'shinde' and Initial = 'bu' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50323728
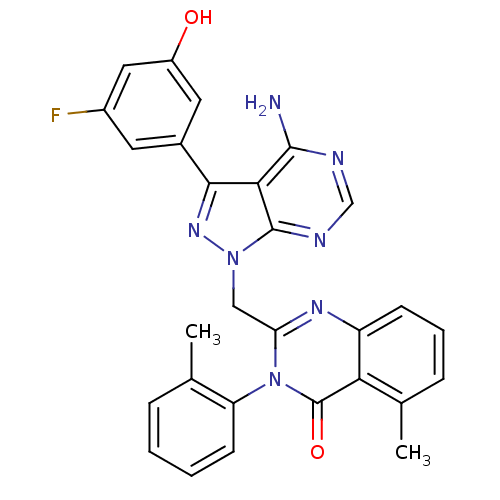
(2-((4-amino-3-(3-fluoro-5-hydroxyphenyl)-1H-pyrazo...)Show SMILES Cc1ccccc1-n1c(Cn2nc(-c3cc(O)cc(F)c3)c3c(N)ncnc23)nc2cccc(C)c2c1=O |(2.13,-14.38,;2.12,-12.84,;3.46,-12.06,;3.45,-10.52,;2.1,-9.75,;.77,-10.55,;.79,-12.08,;-.54,-12.85,;-.54,-14.41,;.81,-15.18,;.81,-16.72,;-.43,-17.63,;.05,-19.1,;-.74,-20.41,;-2.27,-20.38,;-3.06,-21.69,;-4.6,-21.66,;-2.32,-23.04,;-.78,-23.06,;-.03,-24.4,;.01,-21.74,;1.59,-19.09,;2.62,-20.22,;2.15,-21.69,;4.12,-19.9,;4.59,-18.43,;3.56,-17.3,;2.07,-17.63,;-1.87,-15.18,;-3.21,-14.41,;-4.55,-15.19,;-5.88,-14.42,;-5.87,-12.87,;-4.55,-12.1,;-4.56,-10.56,;-3.21,-12.87,;-1.88,-12.1,;-1.89,-10.56,)| Show InChI InChI=1S/C28H22FN7O2/c1-15-6-3-4-9-21(15)36-22(33-20-8-5-7-16(2)23(20)28(36)38)13-35-27-24(26(30)31-14-32-27)25(34-35)17-10-18(29)12-19(37)11-17/h3-12,14,37H,13H2,1-2H3,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50323728

(2-((4-amino-3-(3-fluoro-5-hydroxyphenyl)-1H-pyrazo...)Show SMILES Cc1ccccc1-n1c(Cn2nc(-c3cc(O)cc(F)c3)c3c(N)ncnc23)nc2cccc(C)c2c1=O |(2.13,-14.38,;2.12,-12.84,;3.46,-12.06,;3.45,-10.52,;2.1,-9.75,;.77,-10.55,;.79,-12.08,;-.54,-12.85,;-.54,-14.41,;.81,-15.18,;.81,-16.72,;-.43,-17.63,;.05,-19.1,;-.74,-20.41,;-2.27,-20.38,;-3.06,-21.69,;-4.6,-21.66,;-2.32,-23.04,;-.78,-23.06,;-.03,-24.4,;.01,-21.74,;1.59,-19.09,;2.62,-20.22,;2.15,-21.69,;4.12,-19.9,;4.59,-18.43,;3.56,-17.3,;2.07,-17.63,;-1.87,-15.18,;-3.21,-14.41,;-4.55,-15.19,;-5.88,-14.42,;-5.87,-12.87,;-4.55,-12.1,;-4.56,-10.56,;-3.21,-12.87,;-1.88,-12.1,;-1.89,-10.56,)| Show InChI InChI=1S/C28H22FN7O2/c1-15-6-3-4-9-21(15)36-22(33-20-8-5-7-16(2)23(20)28(36)38)13-35-27-24(26(30)31-14-32-27)25(34-35)17-10-18(29)12-19(37)11-17/h3-12,14,37H,13H2,1-2H3,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) in presence of [gamma-32P]ATP by phosphorimaging assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201717
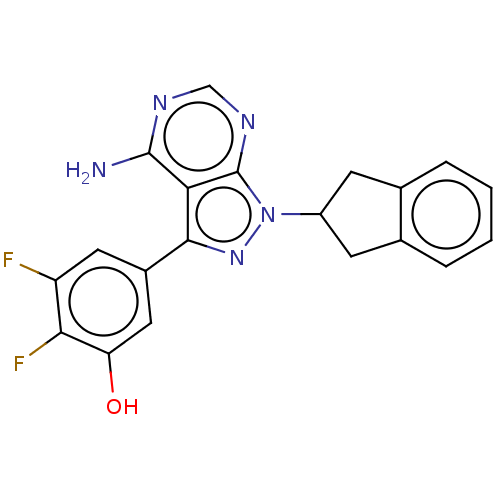
(CHEMBL3907591)Show SMILES Nc1ncnc2n(nc(-c3cc(O)c(F)c(F)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H15F2N5O/c21-14-7-12(8-15(28)17(14)22)18-16-19(23)24-9-25-20(16)27(26-18)13-5-10-3-1-2-4-11(10)6-13/h1-4,7-9,13,28H,5-6H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405402
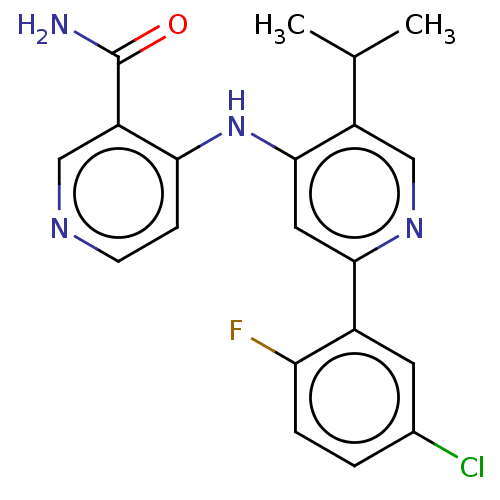
(CHEMBL5267945)Show SMILES COc1ccc(CCNCC(O)COc2ccc(COCc3ncc[nH]3)cc2)cc1OC Show InChI InChI=1S/C24H31N3O5/c1-29-22-8-5-18(13-23(22)30-2)9-10-25-14-20(28)16-32-21-6-3-19(4-7-21)15-31-17-24-26-11-12-27-24/h3-8,11-13,20,25,28H,9-10,14-17H2,1-2H3,(H,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405407

(CHEMBL5285361)Show SMILES COc1ccc(CCNC[C@H](O)COc2ccc(Cc3nc(c[nH]3)C(C)=O)cc2)cc1OC Show InChI InChI=1S/C25H31N3O5/c1-17(29)22-15-27-25(28-22)13-18-4-7-21(8-5-18)33-16-20(30)14-26-11-10-19-6-9-23(31-2)24(12-19)32-3/h4-9,12,15,20,26,30H,10-11,13-14,16H2,1-3H3,(H,27,28)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280370

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{1H-...)Show SMILES COc1ccc(Cn2ncc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C27H24ClFN6O/c1-16(2)21-12-30-24(20-10-18(28)6-9-23(20)29)11-25(21)34-26-22-13-33-35(27(22)32-15-31-26)14-17-4-7-19(36-3)8-5-17/h4-13,15-16H,14H2,1-3H3,(H,30,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280370

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{1H-...)Show SMILES COc1ccc(Cn2ncc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C27H24ClFN6O/c1-16(2)21-12-30-24(20-10-18(28)6-9-23(20)29)11-25(21)34-26-22-13-33-35(27(22)32-15-31-26)14-17-4-7-19(36-3)8-5-17/h4-13,15-16H,14H2,1-3H3,(H,30,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280369
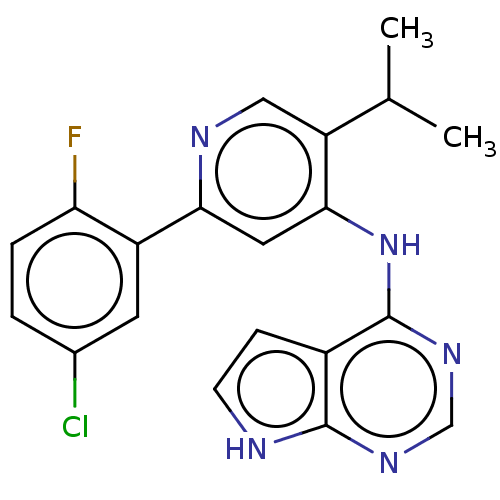
(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280370

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{1H-...)Show SMILES COc1ccc(Cn2ncc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C27H24ClFN6O/c1-16(2)21-12-30-24(20-10-18(28)6-9-23(20)29)11-25(21)34-26-22-13-33-35(27(22)32-15-31-26)14-17-4-7-19(36-3)8-5-17/h4-13,15-16H,14H2,1-3H3,(H,30,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280370

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{1H-...)Show SMILES COc1ccc(Cn2ncc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C27H24ClFN6O/c1-16(2)21-12-30-24(20-10-18(28)6-9-23(20)29)11-25(21)34-26-22-13-33-35(27(22)32-15-31-26)14-17-4-7-19(36-3)8-5-17/h4-13,15-16H,14H2,1-3H3,(H,30,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201728
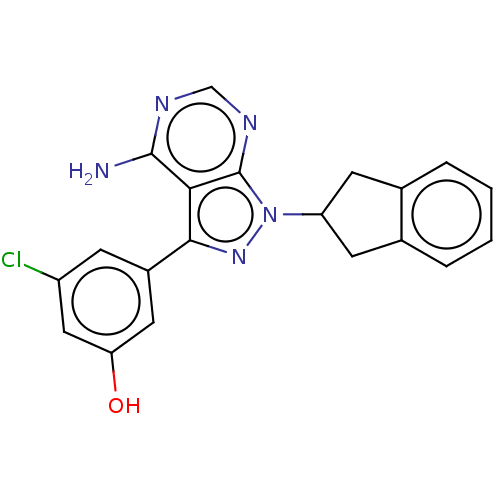
(CHEMBL3914552)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(Cl)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16ClN5O/c21-14-5-13(8-16(27)9-14)18-17-19(22)23-10-24-20(17)26(25-18)15-6-11-3-1-2-4-12(11)7-15/h1-5,8-10,15,27H,6-7H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280365
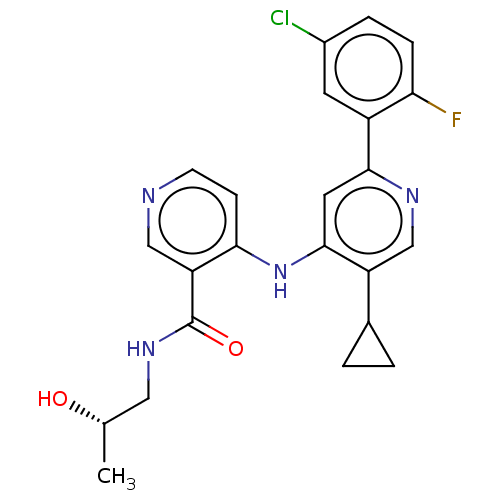
(4-{[2-(5-chloro-2-fluorophenyl)-5-cyclopropylpyrid...)Show SMILES C[C@H](O)CNC(=O)c1cnccc1Nc1cc(ncc1C1CC1)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H22ClFN4O2/c1-13(30)10-28-23(31)18-11-26-7-6-20(18)29-22-9-21(27-12-17(22)14-2-3-14)16-8-15(24)4-5-19(16)25/h4-9,11-14,30H,2-3,10H2,1H3,(H,28,31)(H,26,27,29)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280365

(4-{[2-(5-chloro-2-fluorophenyl)-5-cyclopropylpyrid...)Show SMILES C[C@H](O)CNC(=O)c1cnccc1Nc1cc(ncc1C1CC1)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H22ClFN4O2/c1-13(30)10-28-23(31)18-11-26-7-6-20(18)29-22-9-21(27-12-17(22)14-2-3-14)16-8-15(24)4-5-19(16)25/h4-9,11-14,30H,2-3,10H2,1H3,(H,28,31)(H,26,27,29)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280365

(4-{[2-(5-chloro-2-fluorophenyl)-5-cyclopropylpyrid...)Show SMILES C[C@H](O)CNC(=O)c1cnccc1Nc1cc(ncc1C1CC1)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H22ClFN4O2/c1-13(30)10-28-23(31)18-11-26-7-6-20(18)29-22-9-21(27-12-17(22)14-2-3-14)16-8-15(24)4-5-19(16)25/h4-9,11-14,30H,2-3,10H2,1H3,(H,28,31)(H,26,27,29)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280365

(4-{[2-(5-chloro-2-fluorophenyl)-5-cyclopropylpyrid...)Show SMILES C[C@H](O)CNC(=O)c1cnccc1Nc1cc(ncc1C1CC1)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H22ClFN4O2/c1-13(30)10-28-23(31)18-11-26-7-6-20(18)29-22-9-21(27-12-17(22)14-2-3-14)16-8-15(24)4-5-19(16)25/h4-9,11-14,30H,2-3,10H2,1H3,(H,28,31)(H,26,27,29)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405385
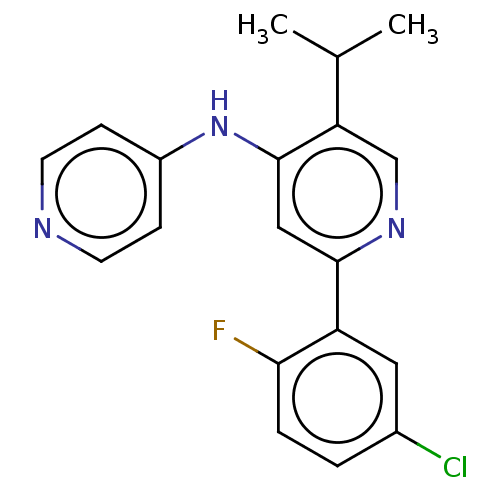
(CHEMBL5285746)Show SMILES CC(CCc1ccccc1)NCC(O)c1cc(C(N)=O)c2[nH]ccc2c1 Show InChI InChI=1S/C21H25N3O2/c1-14(7-8-15-5-3-2-4-6-15)24-13-19(25)17-11-16-9-10-23-20(16)18(12-17)21(22)26/h2-6,9-12,14,19,23-25H,7-8,13H2,1H3,(H2,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280352
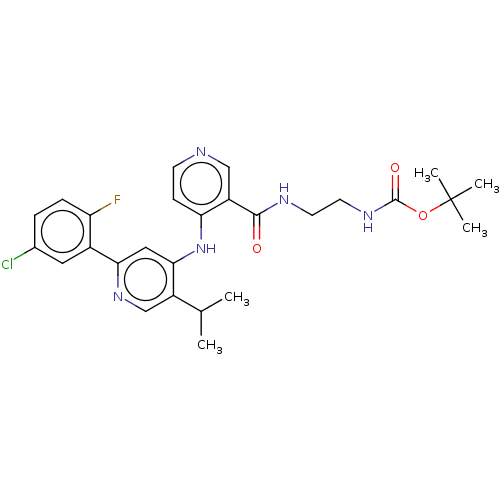
(N-(2-aminoethyl)-4-[[2-(5-chloro-2-fluoro-phenyl)-...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCNC(=O)OC(C)(C)C)-c1cc(Cl)ccc1F Show InChI InChI=1S/C27H31ClFN5O3/c1-16(2)19-15-33-23(18-12-17(28)6-7-21(18)29)13-24(19)34-22-8-9-30-14-20(22)25(35)31-10-11-32-26(36)37-27(3,4)5/h6-9,12-16H,10-11H2,1-5H3,(H,31,35)(H,32,36)(H,30,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM514529
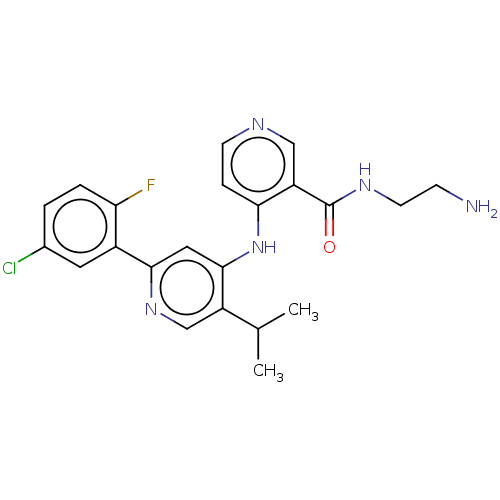
(N-(2-aminoethyl)-4-[[2-(5-chloro-2-fluoro-phenyl)-...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCN)-c1cc(Cl)ccc1F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280352

(N-(2-aminoethyl)-4-[[2-(5-chloro-2-fluoro-phenyl)-...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCNC(=O)OC(C)(C)C)-c1cc(Cl)ccc1F Show InChI InChI=1S/C27H31ClFN5O3/c1-16(2)19-15-33-23(18-12-17(28)6-7-21(18)29)13-24(19)34-22-8-9-30-14-20(22)25(35)31-10-11-32-26(36)37-27(3,4)5/h6-9,12-16H,10-11H2,1-5H3,(H,31,35)(H,32,36)(H,30,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280352

(N-(2-aminoethyl)-4-[[2-(5-chloro-2-fluoro-phenyl)-...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCNC(=O)OC(C)(C)C)-c1cc(Cl)ccc1F Show InChI InChI=1S/C27H31ClFN5O3/c1-16(2)19-15-33-23(18-12-17(28)6-7-21(18)29)13-24(19)34-22-8-9-30-14-20(22)25(35)31-10-11-32-26(36)37-27(3,4)5/h6-9,12-16H,10-11H2,1-5H3,(H,31,35)(H,32,36)(H,30,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM514529

(N-(2-aminoethyl)-4-[[2-(5-chloro-2-fluoro-phenyl)-...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCN)-c1cc(Cl)ccc1F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50201716

(CHEMBL3942550)Show SMILES Nc1ncnc2n(nc(-c3ccc(F)c(O)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16FN5O/c21-15-6-5-13(9-16(15)27)18-17-19(22)23-10-24-20(17)26(25-18)14-7-11-3-1-2-4-12(11)8-14/h1-6,9-10,14,27H,7-8H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405403

(CHEMBL5274165)Show InChI InChI=1S/C16H22BrN3O2/c1-11(2)18-8-13(21)10-22-14-5-3-12(4-6-14)7-16-19-9-15(17)20-16/h3-6,9,11,13,18,21H,7-8,10H2,1-2H3,(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201721
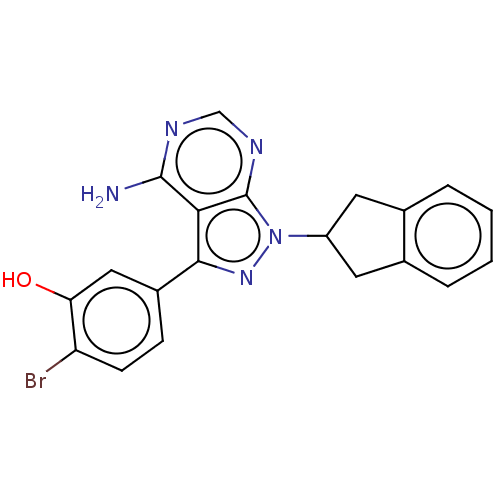
(CHEMBL3964073)Show SMILES Nc1ncnc2n(nc(-c3ccc(Br)c(O)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16BrN5O/c21-15-6-5-13(9-16(15)27)18-17-19(22)23-10-24-20(17)26(25-18)14-7-11-3-1-2-4-12(11)8-14/h1-6,9-10,14,27H,7-8H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201720
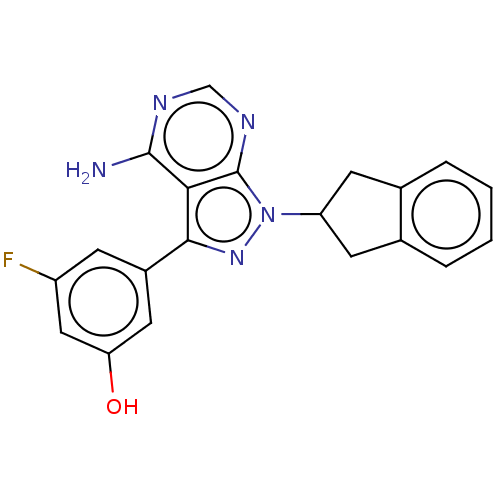
(CHEMBL3896635)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(F)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H16FN5O/c21-14-5-13(8-16(27)9-14)18-17-19(22)23-10-24-20(17)26(25-18)15-6-11-3-1-2-4-12(11)7-15/h1-5,8-10,15,27H,6-7H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50388180
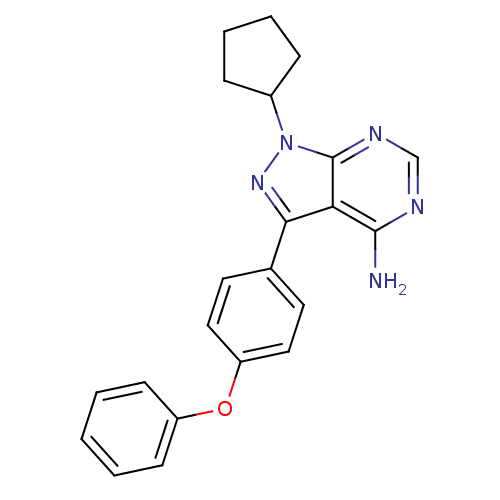
(CHEMBL2057912)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)C1CCCC1 Show InChI InChI=1S/C22H21N5O/c23-21-19-20(26-27(16-6-4-5-7-16)22(19)25-14-24-21)15-10-12-18(13-11-15)28-17-8-2-1-3-9-17/h1-3,8-14,16H,4-7H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280340

((S)-4-(2-(5-chloro-2-fluorophenyl)-5-isopropylpyri...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)N[C@@H](C)CO)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H24ClFN4O2/c1-13(2)17-11-27-21(16-8-15(24)4-5-19(16)25)9-22(17)29-20-6-7-26-10-18(20)23(31)28-14(3)12-30/h4-11,13-14,30H,12H2,1-3H3,(H,28,31)(H,26,27,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280340

((S)-4-(2-(5-chloro-2-fluorophenyl)-5-isopropylpyri...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)N[C@@H](C)CO)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H24ClFN4O2/c1-13(2)17-11-27-21(16-8-15(24)4-5-19(16)25)9-22(17)29-20-6-7-26-10-18(20)23(31)28-14(3)12-30/h4-11,13-14,30H,12H2,1-3H3,(H,28,31)(H,26,27,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280340

((S)-4-(2-(5-chloro-2-fluorophenyl)-5-isopropylpyri...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)N[C@@H](C)CO)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H24ClFN4O2/c1-13(2)17-11-27-21(16-8-15(24)4-5-19(16)25)9-22(17)29-20-6-7-26-10-18(20)23(31)28-14(3)12-30/h4-11,13-14,30H,12H2,1-3H3,(H,28,31)(H,26,27,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280340

((S)-4-(2-(5-chloro-2-fluorophenyl)-5-isopropylpyri...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)N[C@@H](C)CO)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H24ClFN4O2/c1-13(2)17-11-27-21(16-8-15(24)4-5-19(16)25)9-22(17)29-20-6-7-26-10-18(20)23(31)28-14(3)12-30/h4-11,13-14,30H,12H2,1-3H3,(H,28,31)(H,26,27,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201723
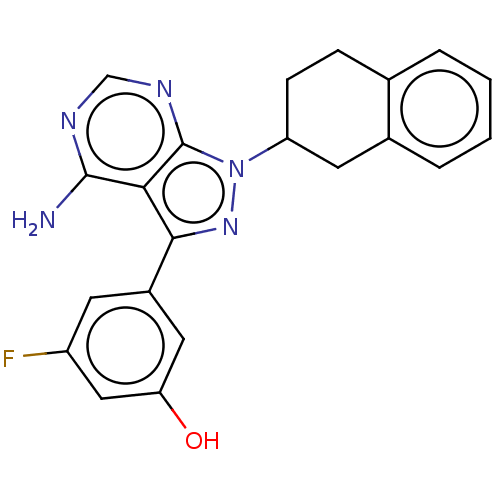
(CHEMBL3905578)Show SMILES Nc1ncnc2n(nc(-c3cc(O)cc(F)c3)c12)C1CCc2ccccc2C1 Show InChI InChI=1S/C21H18FN5O/c22-15-7-14(9-17(28)10-15)19-18-20(23)24-11-25-21(18)27(26-19)16-6-5-12-3-1-2-4-13(12)8-16/h1-4,7,9-11,16,28H,5-6,8H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280360
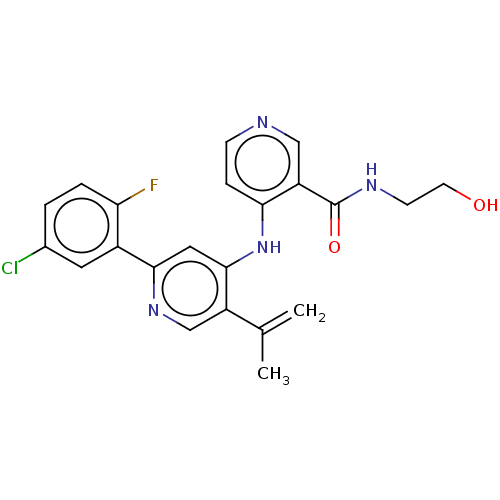
(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropenyl-4-p...)Show SMILES CC(=C)c1cnc(cc1Nc1ccncc1C(=O)NCCO)-c1cc(Cl)ccc1F Show InChI InChI=1S/C22H20ClFN4O2/c1-13(2)16-12-27-20(15-9-14(23)3-4-18(15)24)10-21(16)28-19-5-6-25-11-17(19)22(30)26-7-8-29/h3-6,9-12,29H,1,7-8H2,2H3,(H,26,30)(H,25,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280360

(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropenyl-4-p...)Show SMILES CC(=C)c1cnc(cc1Nc1ccncc1C(=O)NCCO)-c1cc(Cl)ccc1F Show InChI InChI=1S/C22H20ClFN4O2/c1-13(2)16-12-27-20(15-9-14(23)3-4-18(15)24)10-21(16)28-19-5-6-25-11-17(19)22(30)26-7-8-29/h3-6,9-12,29H,1,7-8H2,2H3,(H,26,30)(H,25,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280360

(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropenyl-4-p...)Show SMILES CC(=C)c1cnc(cc1Nc1ccncc1C(=O)NCCO)-c1cc(Cl)ccc1F Show InChI InChI=1S/C22H20ClFN4O2/c1-13(2)16-12-27-20(15-9-14(23)3-4-18(15)24)10-21(16)28-19-5-6-25-11-17(19)22(30)26-7-8-29/h3-6,9-12,29H,1,7-8H2,2H3,(H,26,30)(H,25,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280360

(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropenyl-4-p...)Show SMILES CC(=C)c1cnc(cc1Nc1ccncc1C(=O)NCCO)-c1cc(Cl)ccc1F Show InChI InChI=1S/C22H20ClFN4O2/c1-13(2)16-12-27-20(15-9-14(23)3-4-18(15)24)10-21(16)28-19-5-6-25-11-17(19)22(30)26-7-8-29/h3-6,9-12,29H,1,7-8H2,2H3,(H,26,30)(H,25,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data