

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10597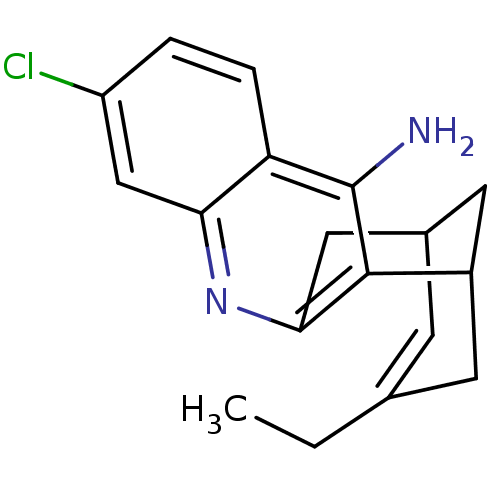 ((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262988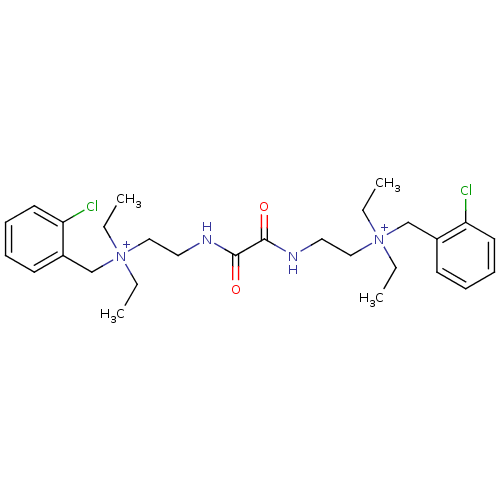 (CHEMBL1200541 | N-(2-chlorobenzyl)-2-(2-(2-((2-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50089616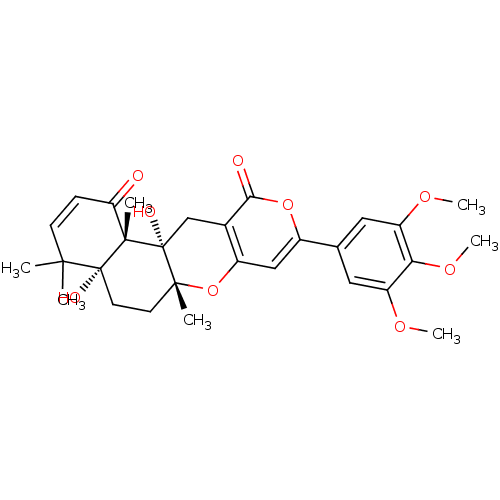 (4a,12a-Dihydroxy-4,4,6a,12b-tetramethyl-9-(3,4,5-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York Structural Biology Center Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase | ACS Med Chem Lett 4: 1091-6 (2013) Article DOI: 10.1021/ml400304w BindingDB Entry DOI: 10.7270/Q2VT1W2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50089616 (4a,12a-Dihydroxy-4,4,6a,12b-tetramethyl-9-(3,4,5-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | -50.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 4.60 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 18 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitinase B (Serratia marcescens) | BDBM10854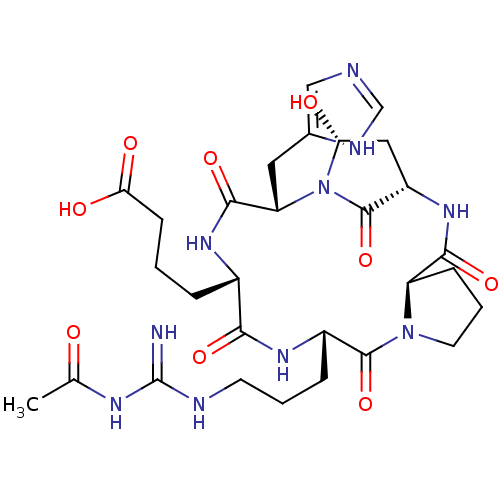 (4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem | Article PubMed | 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 65-76 (2005) Article DOI: 10.1016/j.chembiol.2004.10.013 BindingDB Entry DOI: 10.7270/Q23F4MV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241461 (3,7-bis(dimethylamino)phenothiazin-5-ium chloride ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 110 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM120262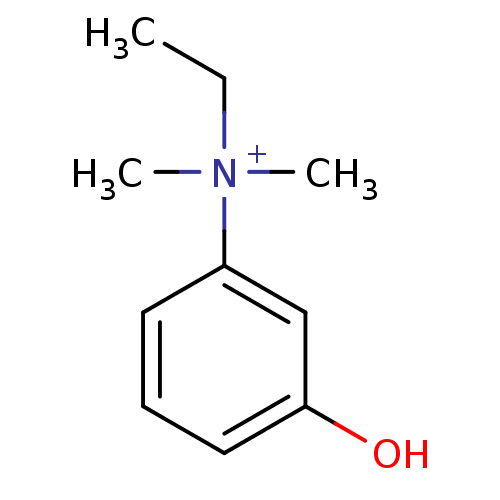 (EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 250 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM120263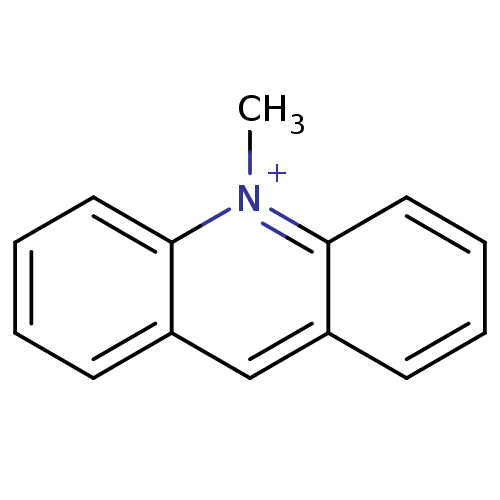 (N-methylacridinium) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 310 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50060582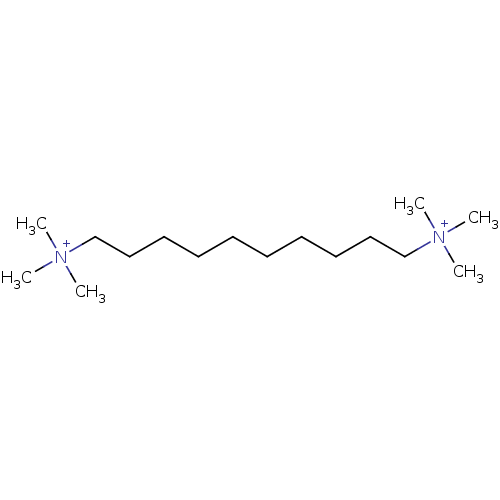 (1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 460 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50423877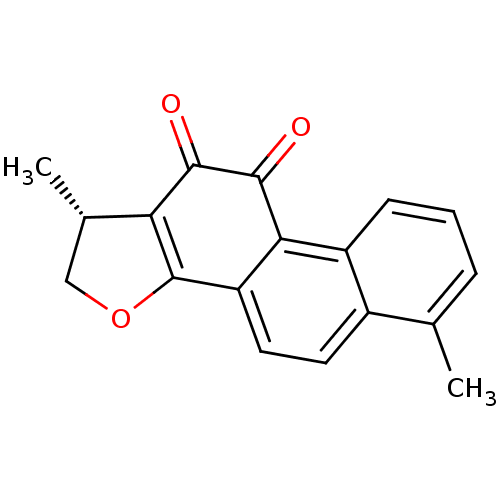 (DIHYDROTANSHINONE | Dihydrotanshinone I | acs.jmed...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York Structural Biology Center Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase | ACS Med Chem Lett 4: 1091-6 (2013) Article DOI: 10.1021/ml400304w BindingDB Entry DOI: 10.7270/Q2VT1W2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31904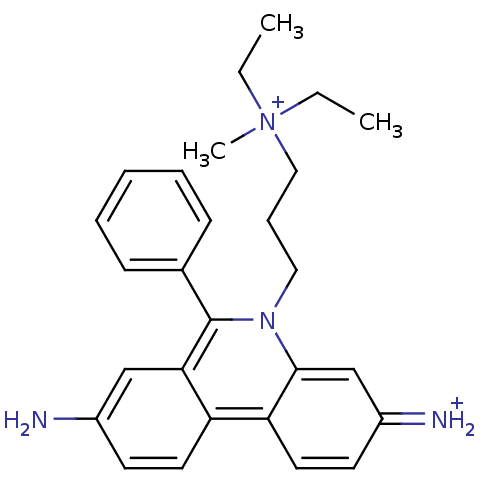 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50423877 (DIHYDROTANSHINONE | Dihydrotanshinone I | acs.jmed...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 750 | -35.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50100134 (2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM12342 (2H-chromen-2-one | CHEMBL6466 | Coumarin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.30E+3 | -28.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM120261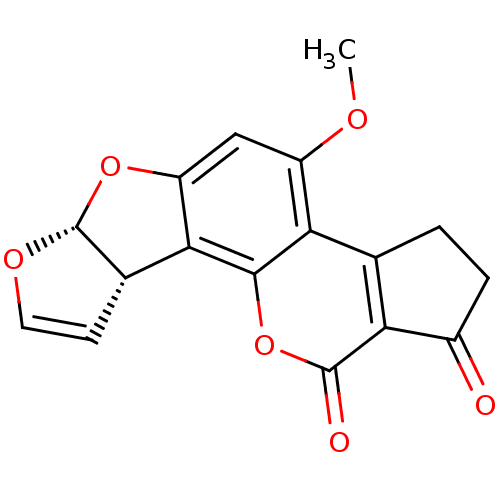 (Aflatoxin B1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 2.80E+4 | -26.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM10853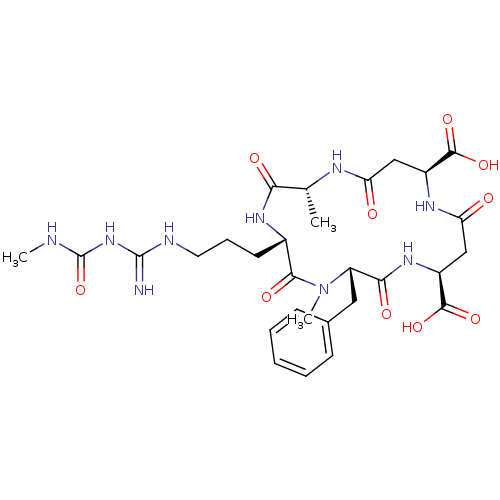 ((2R,5S,8S,11S,15S)-8-benzyl-2,7-dimethyl-5-[3-({[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.30E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 65-76 (2005) Article DOI: 10.1016/j.chembiol.2004.10.013 BindingDB Entry DOI: 10.7270/Q23F4MV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM10854 (4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 5.2 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 65-76 (2005) Article DOI: 10.1016/j.chembiol.2004.10.013 BindingDB Entry DOI: 10.7270/Q23F4MV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitinase A (Serratia marcescens) | BDBM50173286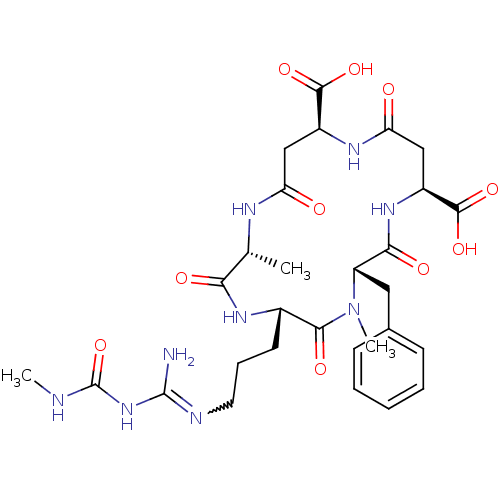 (5-[3-[amino-(methylcarbamoylamino)methylidene]amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiA | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase A (Serratia marcescens) | BDBM50257241 (CHEMBL506684 | N-Ac-D-Ala-Arg{N-omega-(N-methylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiA | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50089857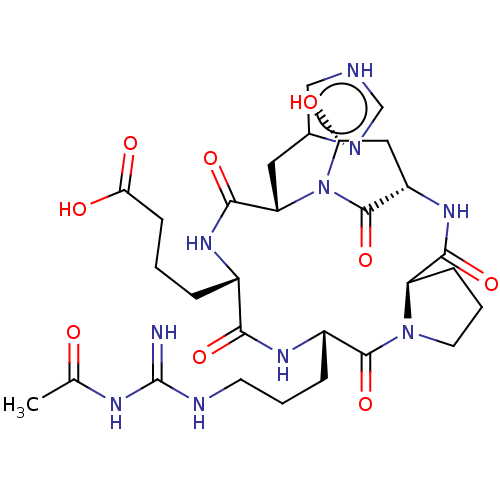 (Argadin) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50089847 (CHEMBL3577620) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a... | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50331851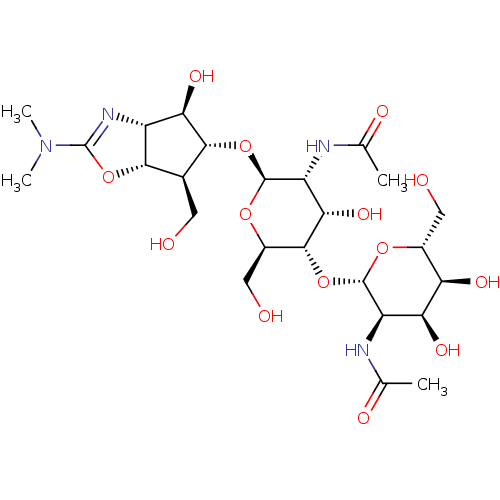 (Allosamidin | CHEMBL1230997) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a... | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase A (Serratia marcescens) | BDBM50257244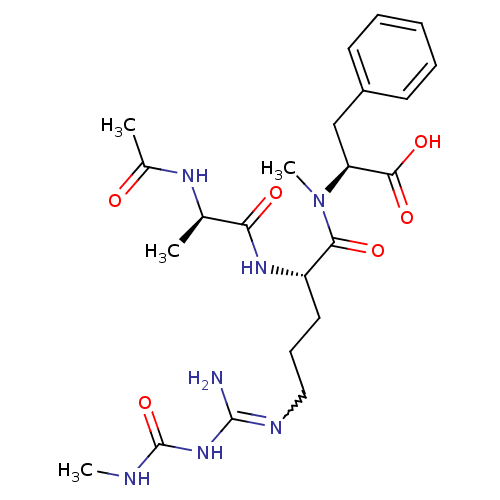 (CHEMBL522670 | N-Ac-D-Ala-Arg{N-omega-(N-methylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiA | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50257241 (CHEMBL506684 | N-Ac-D-Ala-Arg{N-omega-(N-methylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiB | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50257240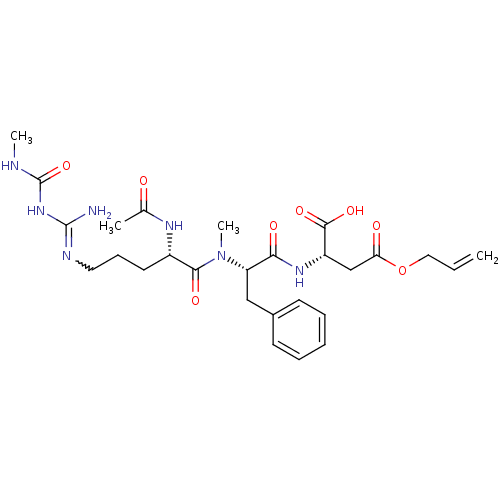 (CHEMBL450552 | N-Ac-Arg{N-omega-(N-methylcarbanoyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiB | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase A (Serratia marcescens) | BDBM50257240 (CHEMBL450552 | N-Ac-Arg{N-omega-(N-methylcarbanoyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiA | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50089854 (CHEMBL3577613) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a... | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50294763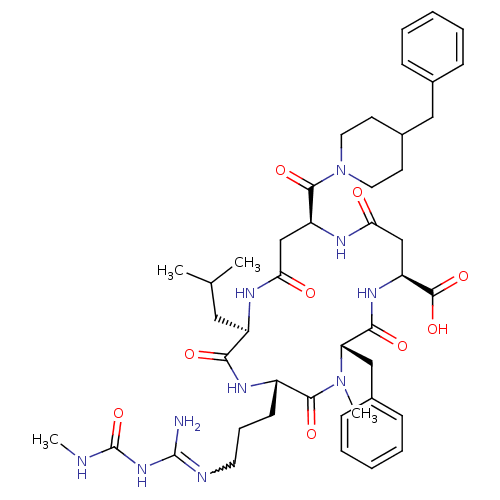 ((2R,5S,8S,11S,15S)-8-benzyl-15-(4-benzylpiperidine...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase B | Bioorg Med Chem Lett 19: 2630-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.013 BindingDB Entry DOI: 10.7270/Q2PG1RSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50089846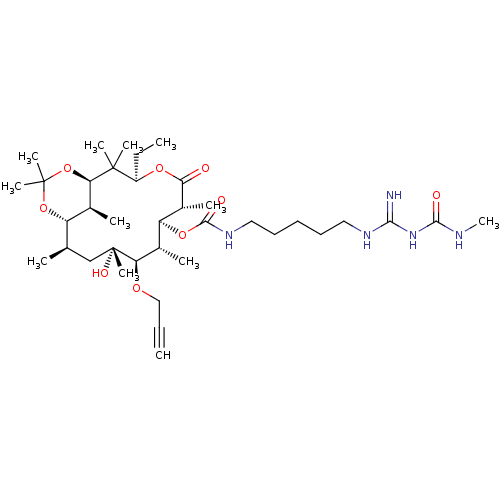 (CHEMBL3577621) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 374 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a... | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endochitinase B1 (Aspergillus fumigatus) | BDBM10854 (4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 65-76 (2005) Article DOI: 10.1016/j.chembiol.2004.10.013 BindingDB Entry DOI: 10.7270/Q23F4MV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitinase A (Serratia marcescens) | BDBM50257243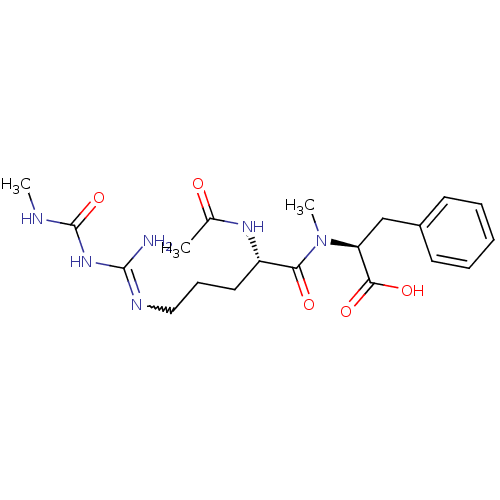 ((2S)-2-[[(2S)-2-acetamido-5-[[N-(methylcarbamoyl)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiA | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50257242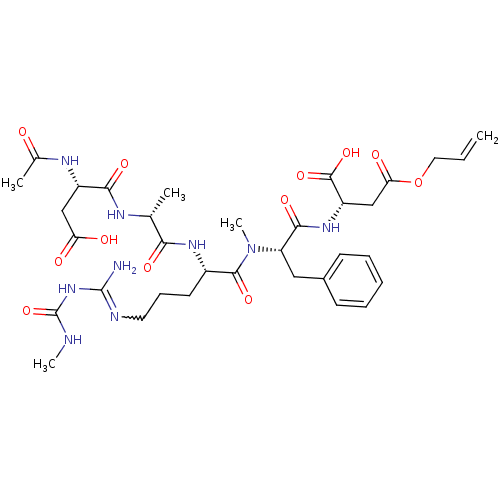 (CHEMBL454279 | N-Ac-Asp-D-Ala-Arg{N-omega-(N-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiB | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50423877 (DIHYDROTANSHINONE | Dihydrotanshinone I | acs.jmed...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
New York Structural Biology Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) | ACS Med Chem Lett 4: 1091-6 (2013) Article DOI: 10.1021/ml400304w BindingDB Entry DOI: 10.7270/Q2VT1W2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitinase A (Serratia marcescens) | BDBM50257242 (CHEMBL454279 | N-Ac-Asp-D-Ala-Arg{N-omega-(N-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiA | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endochitinase B1 (Aspergillus fumigatus) | BDBM10853 ((2R,5S,8S,11S,15S)-8-benzyl-2,7-dimethyl-5-[3-({[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 65-76 (2005) Article DOI: 10.1016/j.chembiol.2004.10.013 BindingDB Entry DOI: 10.7270/Q23F4MV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50294757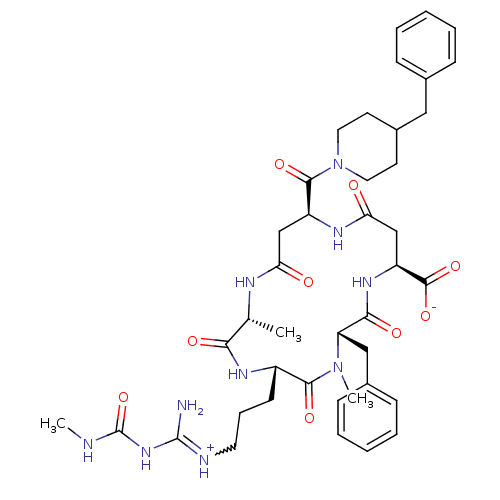 ((2R,5S,8S,11S,15S)-8-benzyl-15-(4-benzylpiperidine...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase B | Bioorg Med Chem Lett 19: 2630-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.013 BindingDB Entry DOI: 10.7270/Q2PG1RSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50089844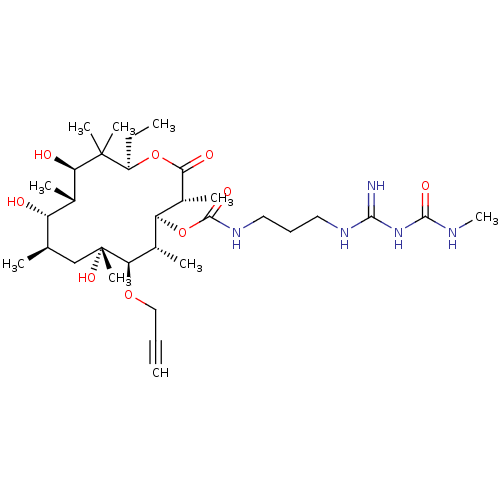 (CHEMBL3577623) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a... | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50089845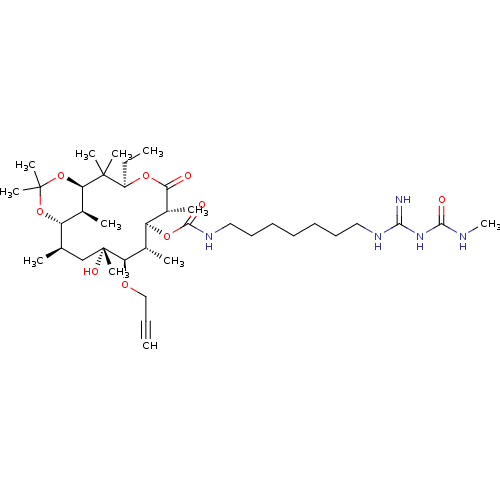 (CHEMBL3577622) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a... | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50294759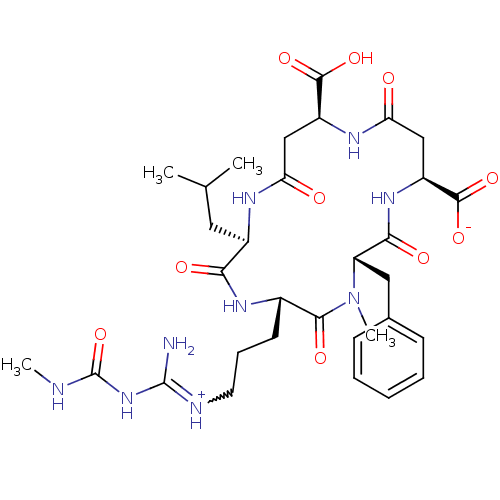 ((2R,5S,8S,11S,15S)-8-benzyl-15-carboxy-5-(3-(imini...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase B | Bioorg Med Chem Lett 19: 2630-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.013 BindingDB Entry DOI: 10.7270/Q2PG1RSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50257244 (CHEMBL522670 | N-Ac-D-Ala-Arg{N-omega-(N-methylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiB | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50089853 (CHEMBL3577614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a... | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50257243 ((2S)-2-[[(2S)-2-acetamido-5-[[N-(methylcarbamoyl)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase B | Bioorg Med Chem 18: 5835-44 (2010) Article DOI: 10.1016/j.bmc.2010.06.093 BindingDB Entry DOI: 10.7270/Q2FQ9WTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50257243 ((2S)-2-[[(2S)-2-acetamido-5-[[N-(methylcarbamoyl)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiB | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50438378 (CHEMBL2413400) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of His-tagged recombinant human acidic mammalian chitinase expressed in Escherichia coli Rosetta-gami 2 (DE3) using 4MU-(GlcNAc)2 as subst... | Bioorg Med Chem 21: 3214-20 (2013) Article DOI: 10.1016/j.bmc.2013.03.047 BindingDB Entry DOI: 10.7270/Q2SX6FMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50173286 (5-[3-[amino-(methylcarbamoylamino)methylidene]amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human acidic mammalian chitinase | Bioorg Med Chem 17: 6270-8 (2009) Article DOI: 10.1016/j.bmc.2009.07.045 BindingDB Entry DOI: 10.7270/Q2TB17TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM10853 ((2R,5S,8S,11S,15S)-8-benzyl-2,7-dimethyl-5-[3-({[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | 5.2 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 65-76 (2005) Article DOI: 10.1016/j.chembiol.2004.10.013 BindingDB Entry DOI: 10.7270/Q23F4MV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50294762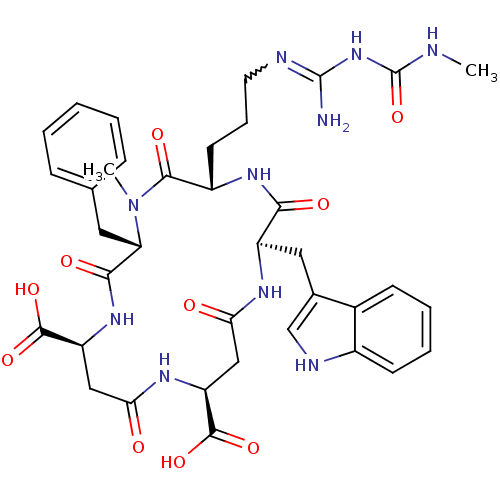 ((2R,5S,8S,11S,15S)-2-((1H-indol-3-yl)methyl)-8-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase B | Bioorg Med Chem Lett 19: 2630-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.013 BindingDB Entry DOI: 10.7270/Q2PG1RSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50294758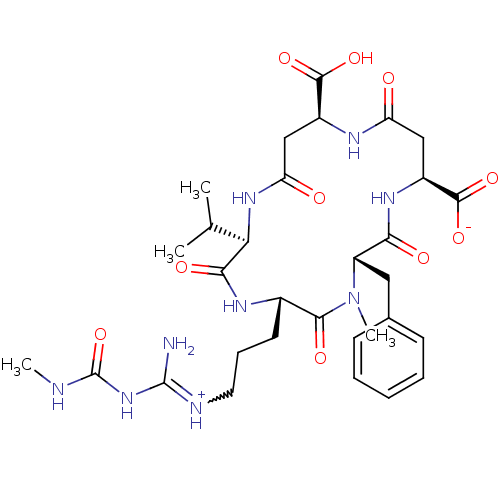 ((2R,5S,8S,11S,15S)-8-benzyl-15-carboxy-5-(3-(imini...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase B | Bioorg Med Chem Lett 19: 2630-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.013 BindingDB Entry DOI: 10.7270/Q2PG1RSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 107 total ) | Next | Last >> |