

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254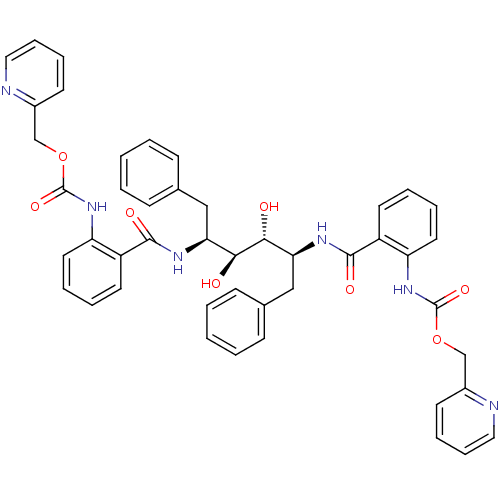 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285701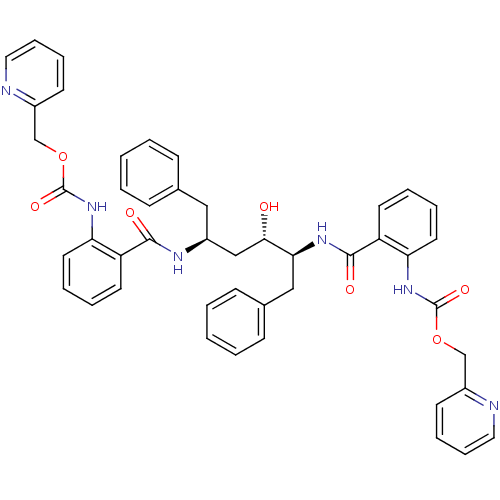 (Anthranilamide derivative | CHEMBL313767) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285698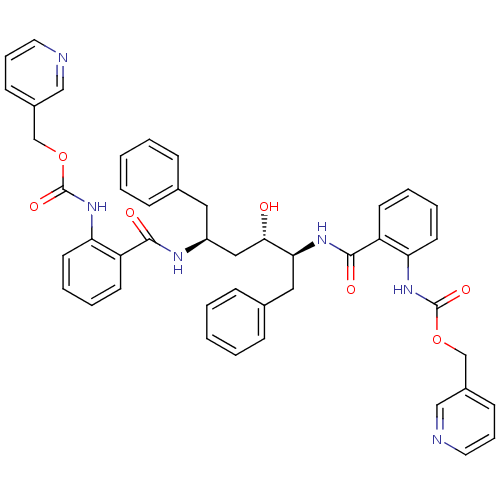 (Anthranilamide derivative | CHEMBL315500) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128931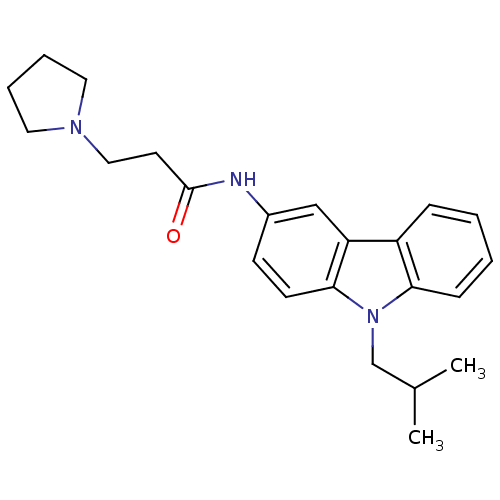 (CHEMBL61880 | N-(9-Isobutyl-9H-carbazol-3-yl)-3-py...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120502 (2-Amino-N-[(R)-2-(3a-benzyl-2-tert-butyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in HEK293 cell membranes measured after 120 mins | Eur J Med Chem 180: 673-689 (2019) Article DOI: 10.1016/j.ejmech.2019.07.050 BindingDB Entry DOI: 10.7270/Q2XK8JXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 cell membranes measured after 30 mins | Eur J Med Chem 180: 673-689 (2019) Article DOI: 10.1016/j.ejmech.2019.07.050 BindingDB Entry DOI: 10.7270/Q2XK8JXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285700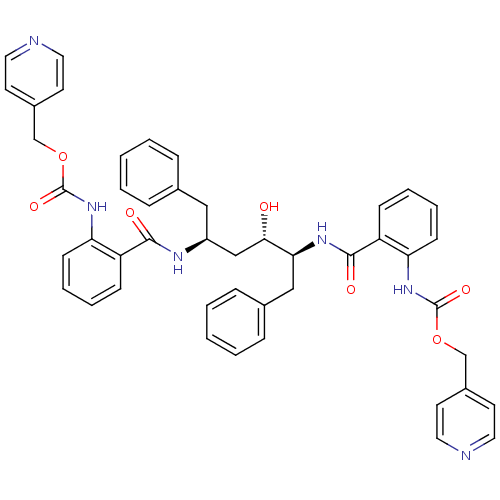 (Anthranilamide derivative | CHEMBL315742) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120504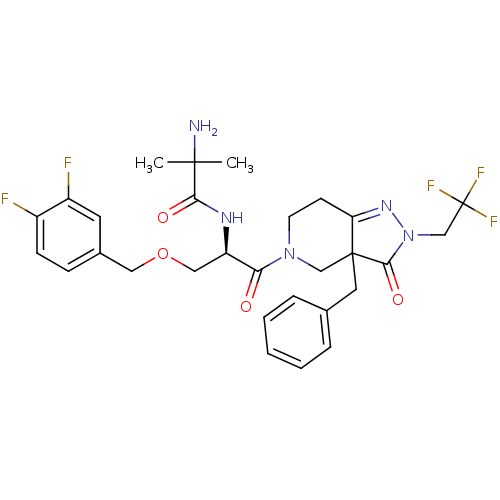 (2-Amino-N-[(R)-2-[3a-benzyl-3-oxo-2-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50121335 (CHEMBL3622099) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D4 receptor expressed in CHO cells | ACS Med Chem Lett 6: 882-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00131 BindingDB Entry DOI: 10.7270/Q2708376 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50583520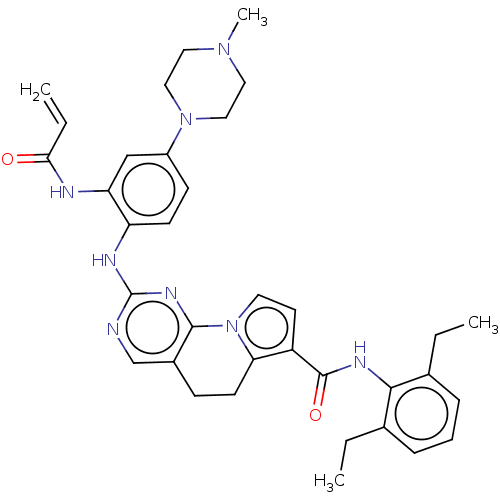 (CHEMBL5094006) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length N-terminal GST-fused MPS1 (1 to 857 residues) using histone H3 as substrate by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01165 BindingDB Entry DOI: 10.7270/Q2T157JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128927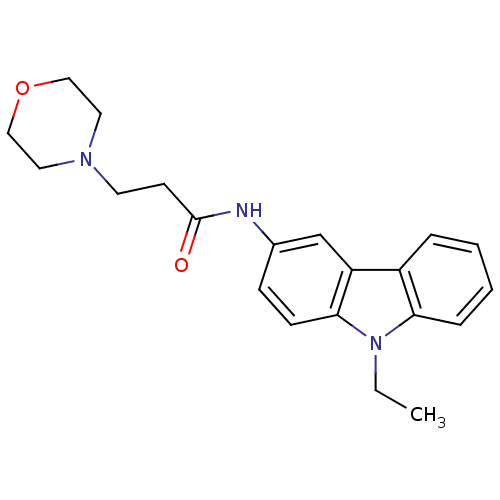 (CHEMBL59680 | N-(9-Ethyl-9H-carbazol-3-yl)-3-morph...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D2L receptor expressed in CHO cells | ACS Med Chem Lett 6: 882-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00131 BindingDB Entry DOI: 10.7270/Q2708376 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285696 (Anthranilamide derivative | CHEMBL85640) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123737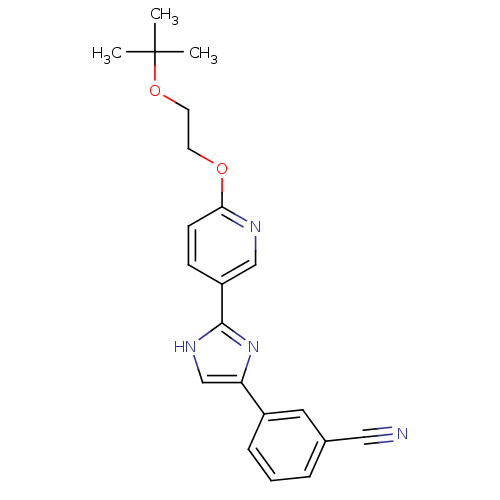 (3-{2-[6-(2-tert-Butoxy-ethoxy)-pyridin-3-yl]-3H-im...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound to the human Neuropeptide Y receptor type 5 was determined using [125I]- [PYY] as radioligand | J Med Chem 46: 670-3 (2003) Article DOI: 10.1021/jm025584p BindingDB Entry DOI: 10.7270/Q2V987DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285703 (Anthranilamide derivative | CHEMBL315928) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128943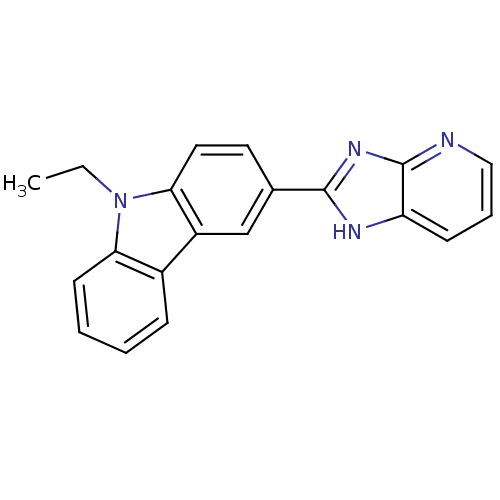 (9-Ethyl-3-(3H-imidazo[4,5-b]pyridin-2-yl)-9H-carba...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133761 (3-[5-(3-Trifluoromethyl-phenyl)-1H-imidazol-2-yl]-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50123737 (3-{2-[6-(2-tert-Butoxy-ethoxy)-pyridin-3-yl]-3H-im...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound to the rat Neuropeptide Y receptor type 5 was determined using [125I]- [Leu31,Pro34]PYY as radioligand | J Med Chem 46: 670-3 (2003) Article DOI: 10.1021/jm025584p BindingDB Entry DOI: 10.7270/Q2V987DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118337 (1-(benzo[b]thiophen-3-yl)-3-(4-(3,4-dihydro-2H-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285691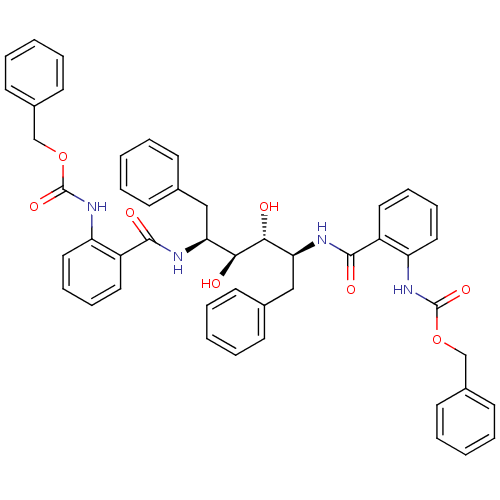 (Anthranilamide derivative | CHEMBL264622) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50415830 (CHEMBL1095256) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128938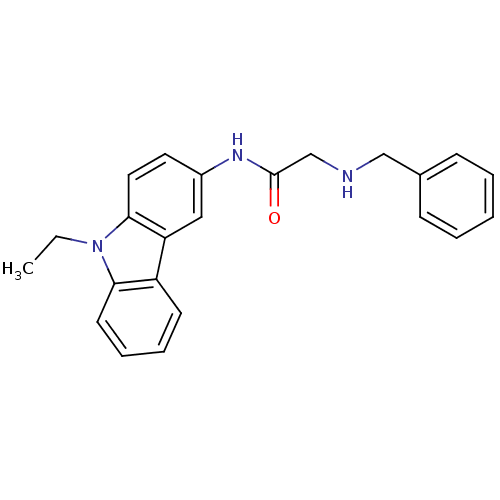 (2-Benzylamino-N-(9-ethyl-9H-carbazol-3-yl)-acetami...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118336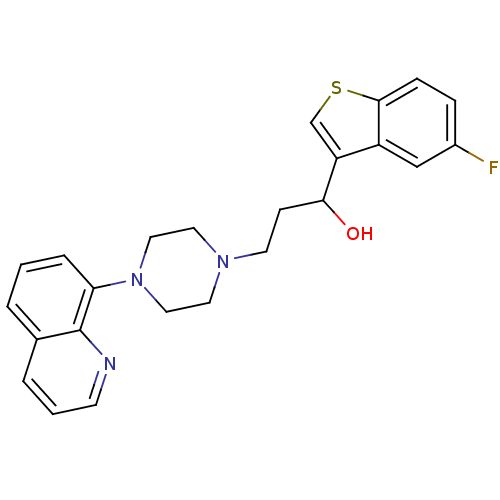 (1-(5-Fluoro-benzo[b]thiophen-3-yl)-3-(4-quinolin-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285695 (Anthranilamide derivative | CHEMBL86263) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118327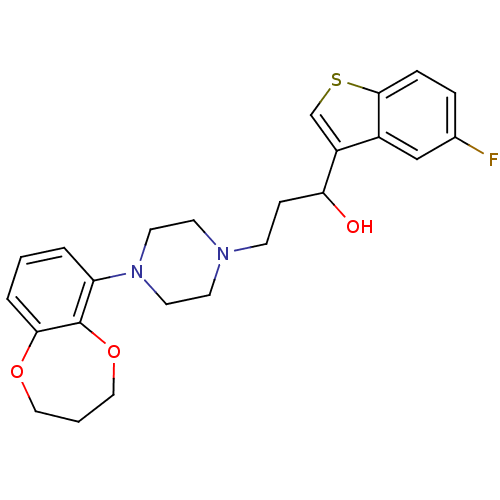 (3-(4-(3,4-dihydro-2H-benzo[b][1,4]dioxepin-6-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human D2S receptor expressed in CHOK1 cell membranes measured after 120 mins | Eur J Med Chem 180: 673-689 (2019) Article DOI: 10.1016/j.ejmech.2019.07.050 BindingDB Entry DOI: 10.7270/Q2XK8JXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492547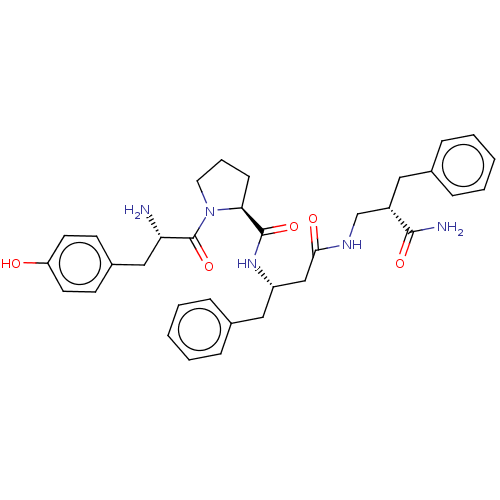 (CHEMBL2409169) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in CHO cells after 90 mins by liquid scintillation counting | ACS Med Chem Lett 4: 795-9 (2013) Article DOI: 10.1021/ml400189r BindingDB Entry DOI: 10.7270/Q20G3P2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128928 (CHEMBL301638 | N-(9-Isopropyl-9H-carbazol-3-yl)-3-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133775 (4-[5-(3,4-Dichloro-phenyl)-1H-imidazol-2-yl]-pyrid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552436 (CHEMBL4749654) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133780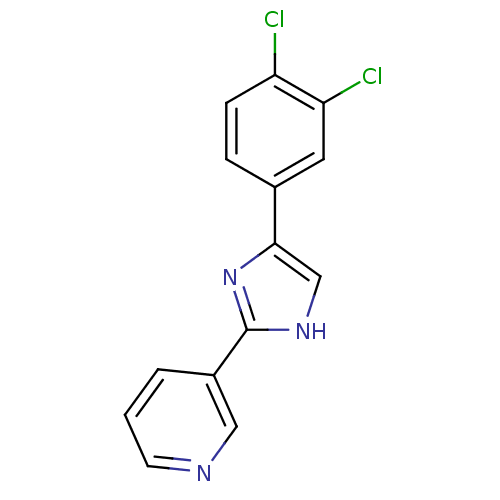 (3-[5-(3,4-Dichloro-phenyl)-1H-imidazol-2-yl]-pyrid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50081174 (CHEMBL3421968) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128764 BindingDB Entry DOI: 10.7270/Q2NZ8CPQ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128935 (CHEMBL294305 | N-(9-Ethyl-9H-carbazol-3-yl)-2-pyri...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133762 (3-[5-(3-Trifluoromethoxy-phenyl)-1H-imidazol-2-yl]...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120505 (2-Amino-N-{(R)-1-(2,4-difluoro-benzyloxymethyl)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102331 (1-(2,5-Dimethyl-thiophen-3-yl)-3-[4-(2-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | Eur J Med Chem 43: 364-72 (2008) Article DOI: 10.1016/j.ejmech.2007.03.036 BindingDB Entry DOI: 10.7270/Q2SQ91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D3 receptor expressed in CHO-K1 cells by radioligand competitive binding analysis | J Nat Prod 83: 127-133 (2020) Article DOI: 10.1021/acs.jnatprod.9b00921 BindingDB Entry DOI: 10.7270/Q28S4T7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D3 receptor expressed in CHO-K1 cells by radioligand competitive binding analysis | J Nat Prod 83: 127-133 (2020) Article DOI: 10.1021/acs.jnatprod.9b00921 BindingDB Entry DOI: 10.7270/Q28S4T7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in CHO cells after 90 mins by liquid scintillation counting | ACS Med Chem Lett 4: 795-9 (2013) Article DOI: 10.1021/ml400189r BindingDB Entry DOI: 10.7270/Q20G3P2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492550 (CHEMBL2409168) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in CHO cells after 90 mins by liquid scintillation counting | ACS Med Chem Lett 4: 795-9 (2013) Article DOI: 10.1021/ml400189r BindingDB Entry DOI: 10.7270/Q20G3P2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118335 (3-(4-(2,3-dihydrobenzo[b][1,4]dioxin-5-yl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2S receptor expressed in CHO-K1 cells by radioligand competitive binding analysis | J Nat Prod 83: 127-133 (2020) Article DOI: 10.1021/acs.jnatprod.9b00921 BindingDB Entry DOI: 10.7270/Q28S4T7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human D2S receptor expressed in CHO-K1 cells by radioligand competitive binding analysis | J Nat Prod 83: 127-133 (2020) Article DOI: 10.1021/acs.jnatprod.9b00921 BindingDB Entry DOI: 10.7270/Q28S4T7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552434 (CHEMBL4747180) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102377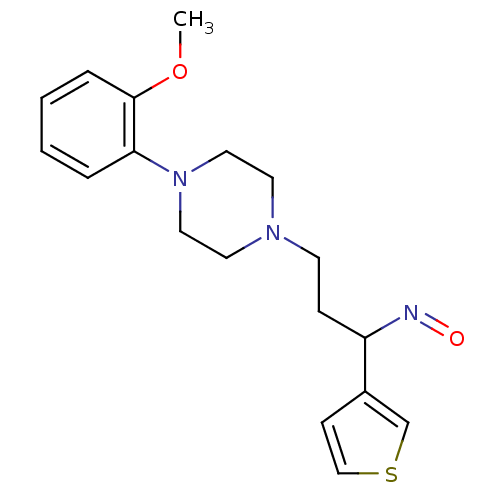 (3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-(thiophen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102377 (3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-(thiophen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | Eur J Med Chem 43: 364-72 (2008) Article DOI: 10.1016/j.ejmech.2007.03.036 BindingDB Entry DOI: 10.7270/Q2SQ91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | ACS Med Chem Lett 6: 882-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00131 BindingDB Entry DOI: 10.7270/Q2708376 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1995 total ) | Next | Last >> |