

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233227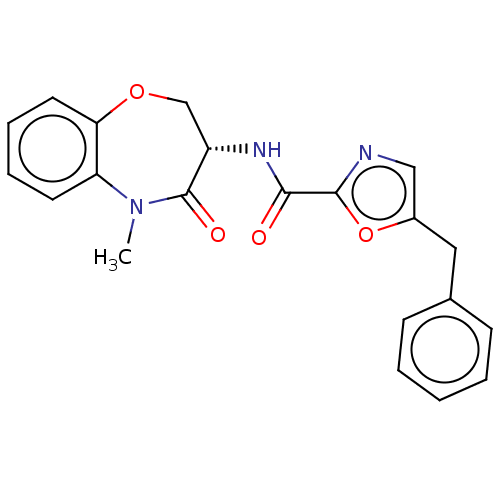 (CHEMBL4067372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50159697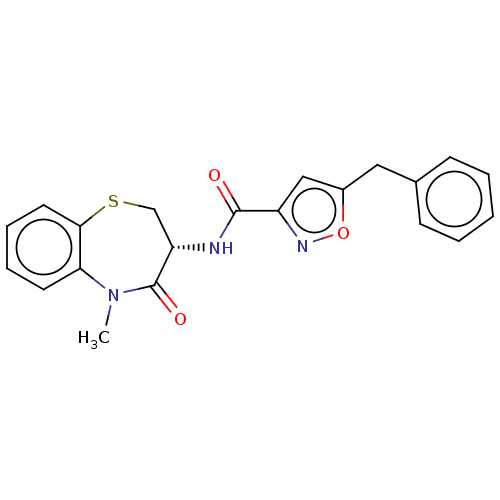 (CHEMBL3785745) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233256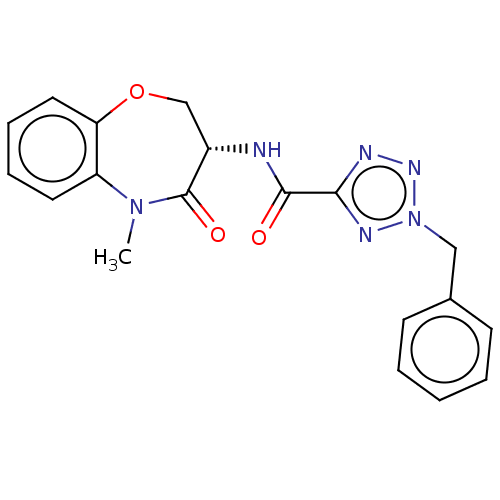 (CHEMBL4093220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50159696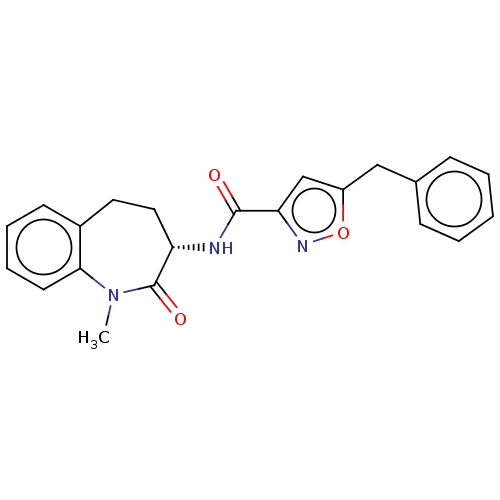 (CHEMBL3786997) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233244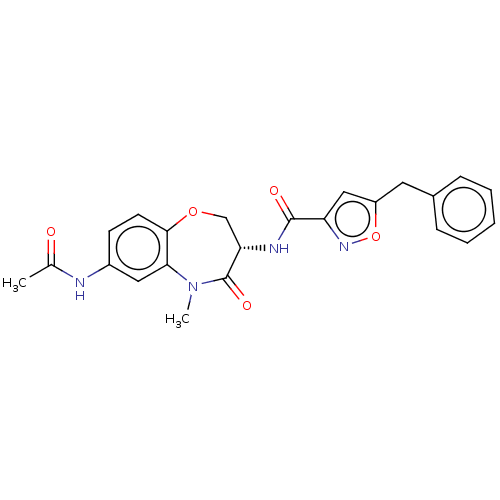 (CHEMBL4068954) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233248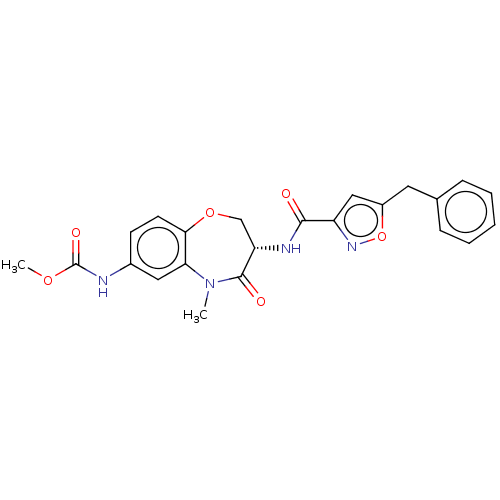 (CHEMBL4096224) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233225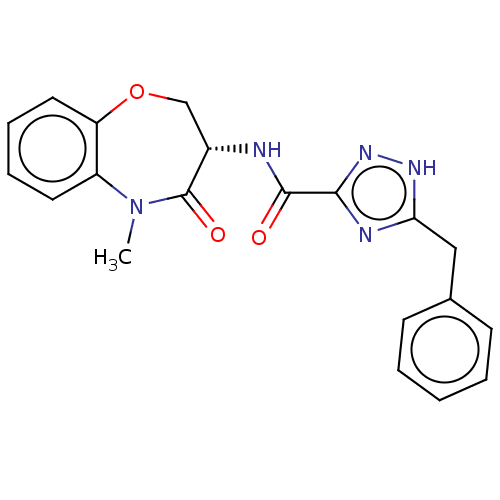 (CHEMBL4071864) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of RIP1 in human primary neutrophils assessed as reduction in TNFalpha/QCD-OPh/SMAC mimetic RMT 5265-induced necroptosis at 21 hrs post-st... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233228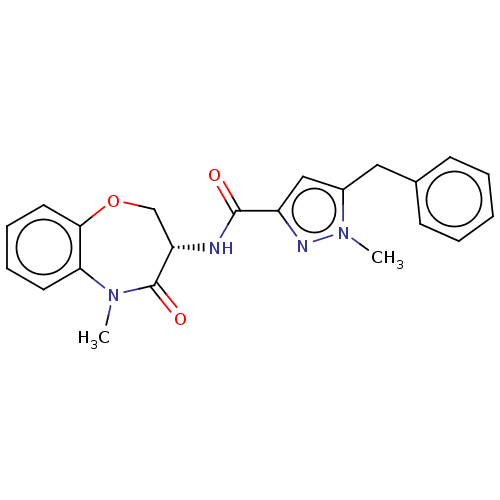 (CHEMBL4060644) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233255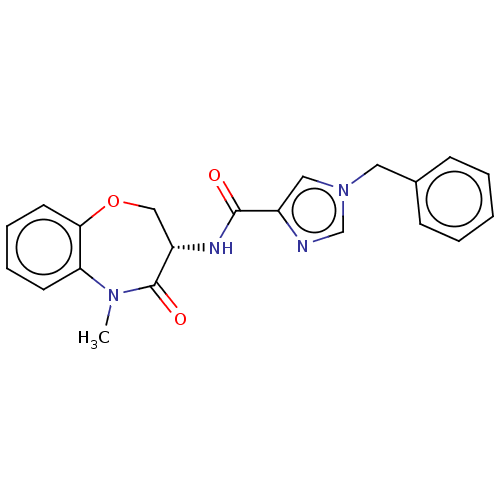 (CHEMBL4069318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233226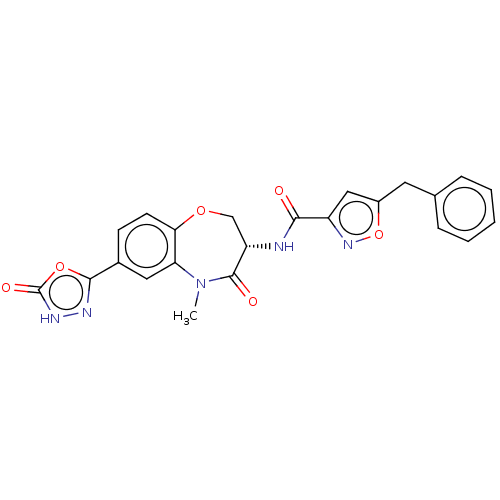 (CHEMBL4089558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233225 (CHEMBL4071864) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of RIP1 in human primary neutrophils assessed as reduction in TNFalpha/QCD-OPh/SMAC mimetic RMT 5265-MIP-1beta production at 21 hrs post-s... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233230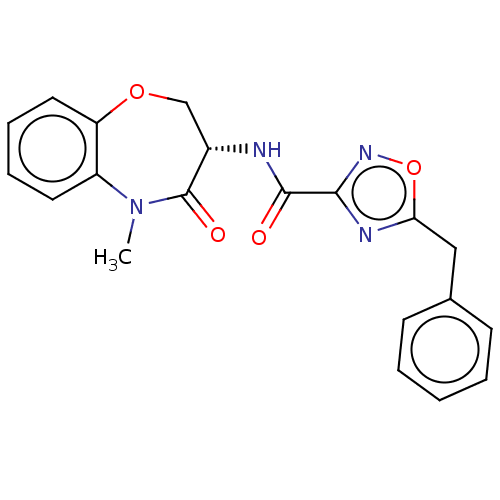 (CHEMBL4099004) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233240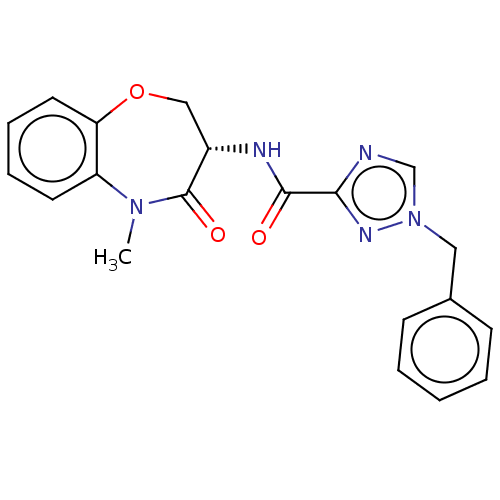 (CHEMBL4065561) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233249 (CHEMBL4081685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233229 (CHEMBL4088857) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50159508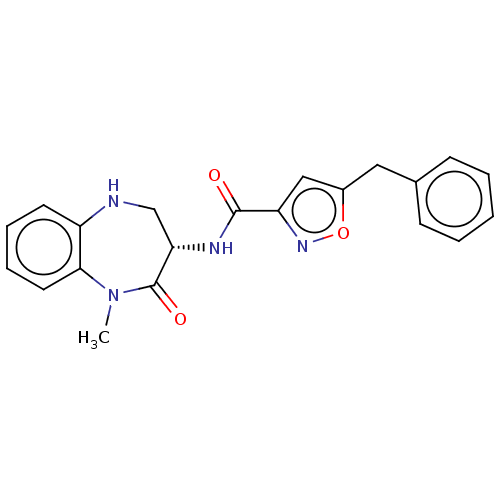 (CHEMBL3786078) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378590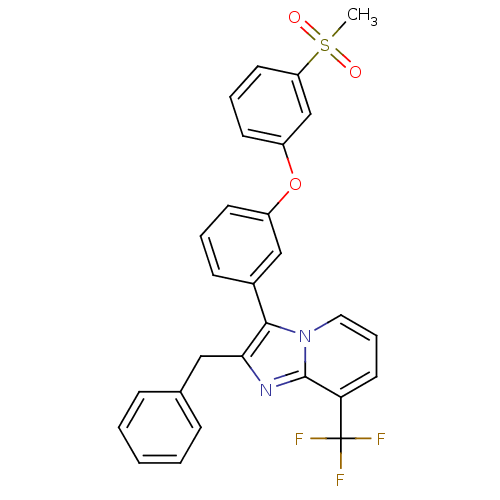 (CHEMBL611735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305075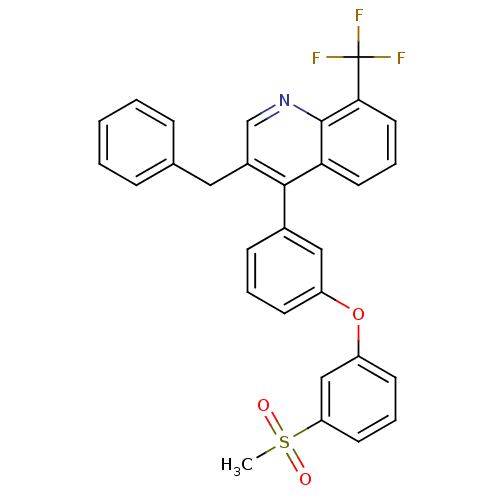 (3-benzyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233227 (CHEMBL4067372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necrosis after 24 hrs by Cell Titer-Glo luminescent cell via... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233225 (CHEMBL4071864) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233252 (CHEMBL4080978) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233250 (CHEMBL4099698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305072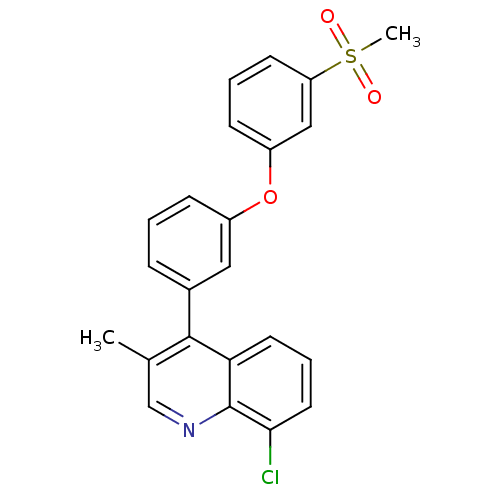 (8-chloro-3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305073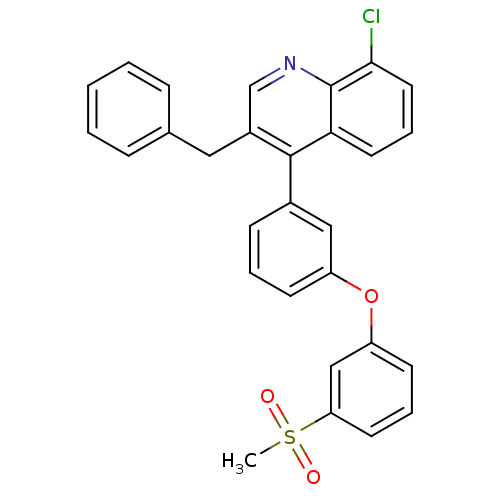 (3-benzyl-8-chloro-4-(3-(3-(methylsulfonyl)phenoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305064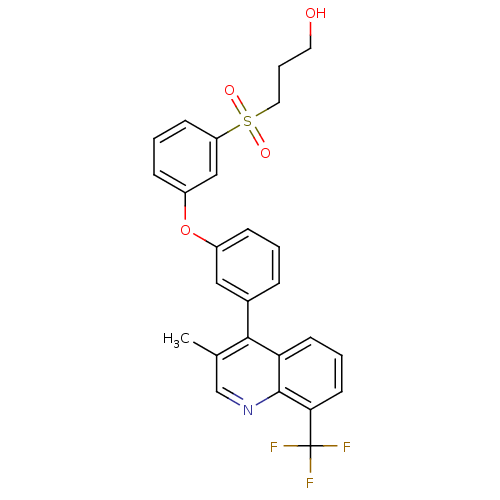 (3-(3-(3-(3-methyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233225 (CHEMBL4071864) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of RIP1 in human primary neutrophils assessed as reduction in TNFalpha/QCD-OPh/SMAC mimetic RMT 5265-induced necroptosis by measuring cell... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233240 (CHEMBL4065561) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necrosis after 24 hrs by Cell Titer-Glo luminescent cell via... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305070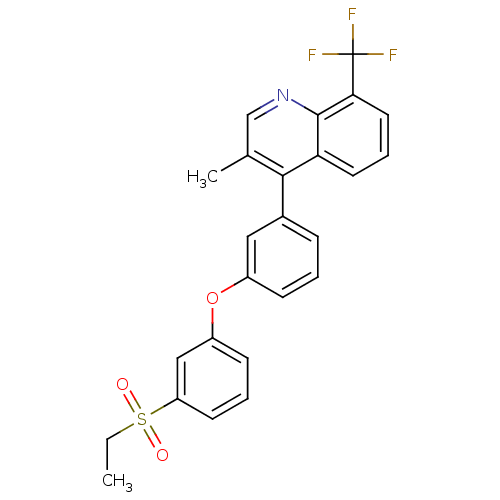 (4-(3-(3-(ethylsulfonyl)phenoxy)phenyl)-3-methyl-8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305068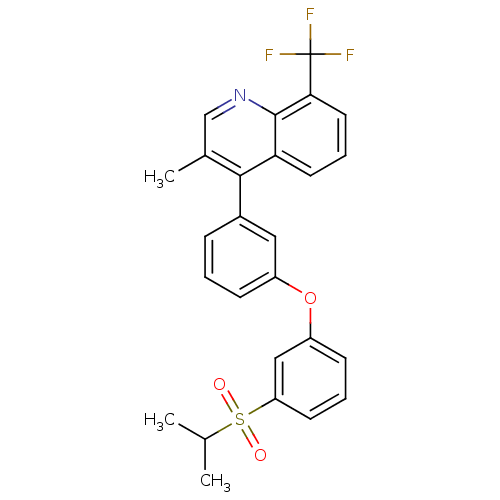 (4-(3-(3-(isopropylsulfonyl)phenoxy)phenyl)-3-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35107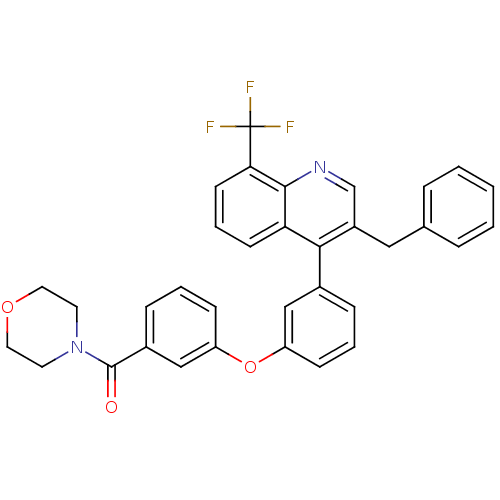 (biarylether amide quinoline, 4g) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 138 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 17: 1663-70 (2009) Article DOI: 10.1016/j.bmc.2008.12.048 BindingDB Entry DOI: 10.7270/Q2QR4VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305060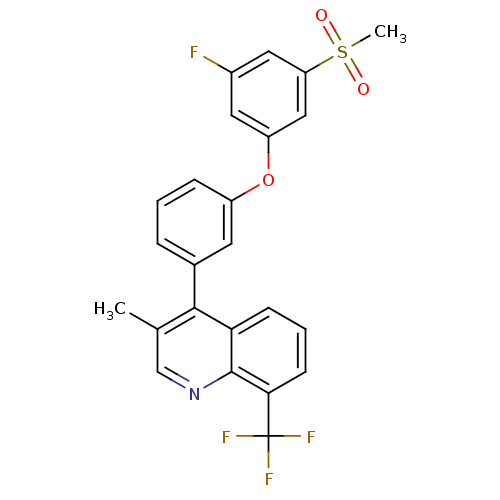 (4-(3-(3-fluoro-5-(methylsulfonyl)phenoxy)phenyl)-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233225 (CHEMBL4071864) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of RIP1 in human whole blood assessed as reduction in TNFalpha/QCD-OPh/zVAD FMK/SMAC mimetic RMT 5265-MIP-1beta production after 6 hrs by ... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50159697 (CHEMBL3785745) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necrosis after 24 hrs by Cell Titer-Glo luminescent cell via... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305075 (3-benzyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233241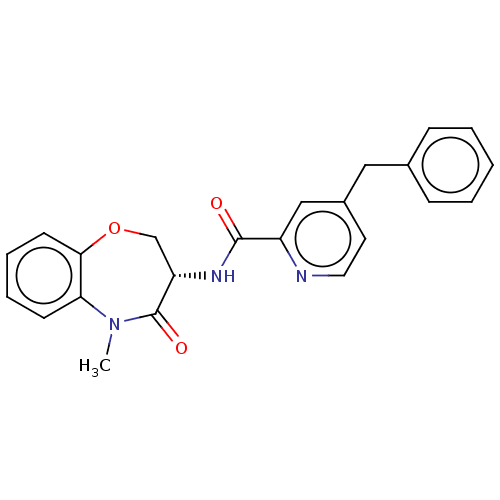 (CHEMBL4071080) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378593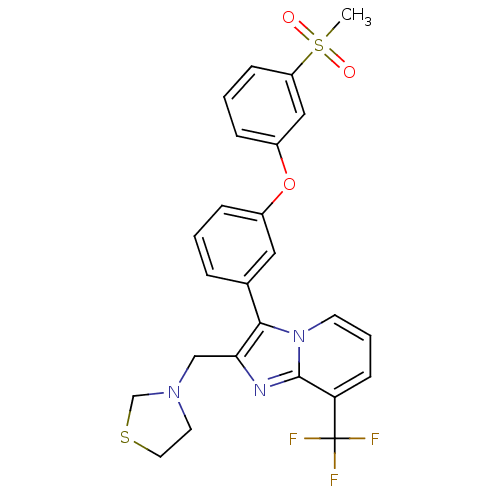 (CHEMBL612007) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000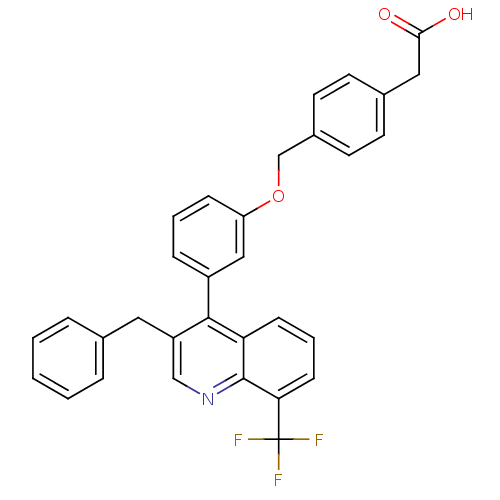 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | 93 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 17: 1663-70 (2009) Article DOI: 10.1016/j.bmc.2008.12.048 BindingDB Entry DOI: 10.7270/Q2QR4VGV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | 71 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35094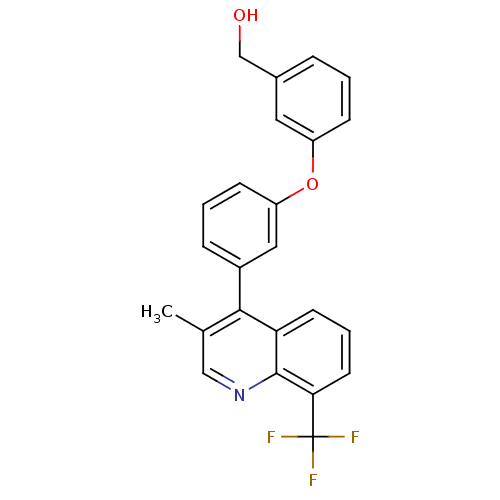 (biarylether alcohol quinoline, 5f) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | 233 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305067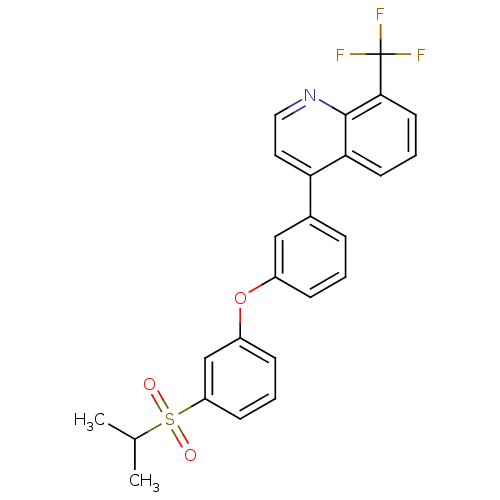 (4-(3-(3-(isopropylsulfonyl)phenoxy)phenyl)-8-(trif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233256 (CHEMBL4093220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necrosis after 24 hrs by Cell Titer-Glo luminescent cell via... | J Med Chem 60: 1247-1261 (2017) Article DOI: 10.1021/acs.jmedchem.6b01751 BindingDB Entry DOI: 10.7270/Q2SQ92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35099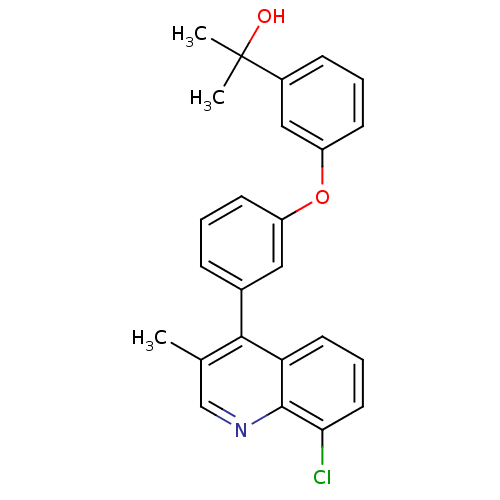 (biarylether alcohol quinoline, 5i) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | 108 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305069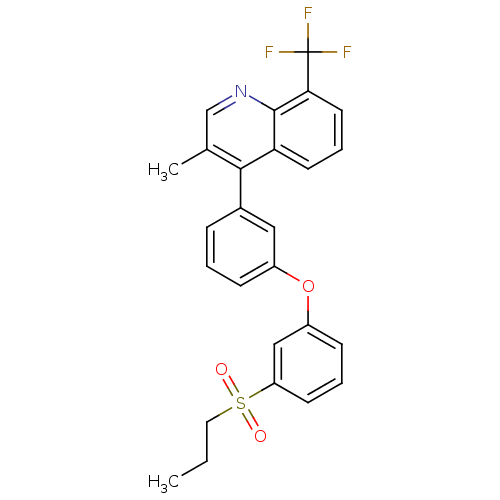 (3-methyl-4-(3-(3-(propylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35105 (biarylether amide quinoline, 4e) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | 98 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 17: 1663-70 (2009) Article DOI: 10.1016/j.bmc.2008.12.048 BindingDB Entry DOI: 10.7270/Q2QR4VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35104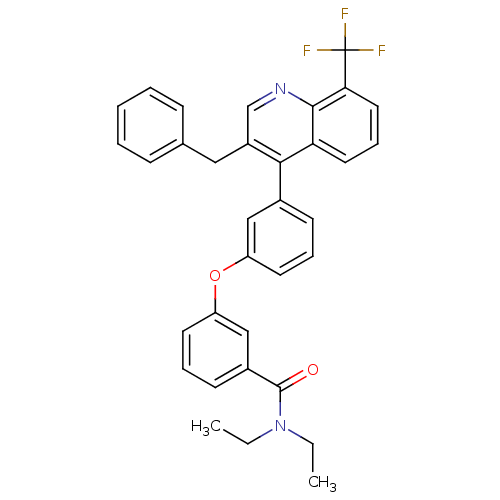 (biarylether amide quinoline, 4d) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | 144 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 17: 1663-70 (2009) Article DOI: 10.1016/j.bmc.2008.12.048 BindingDB Entry DOI: 10.7270/Q2QR4VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305074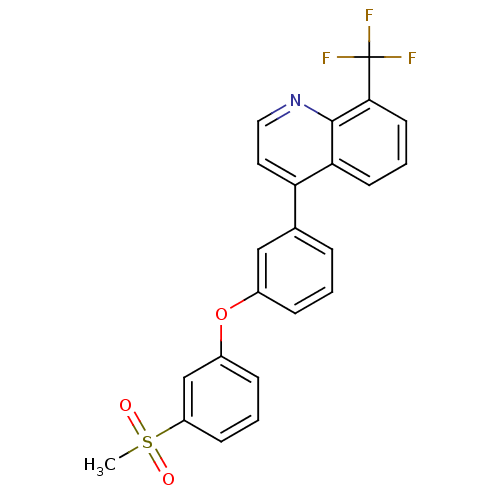 (4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8-(trifluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 341 total ) | Next | Last >> |