 Found 87 hits with Last Name = 'sohn' and Initial = 'jh'
Found 87 hits with Last Name = 'sohn' and Initial = 'jh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311254
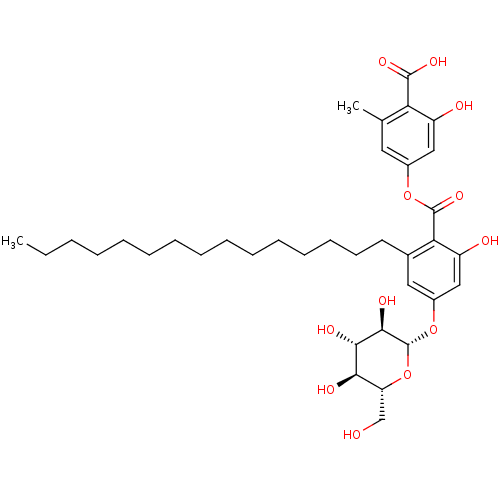
(2-hydroxy-4-(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| Show InChI InChI=1S/C36H52O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-18-25(47-36-33(42)32(41)31(40)28(21-37)48-36)20-27(39)30(23)35(45)46-24-17-22(2)29(34(43)44)26(38)19-24/h17-20,28,31-33,36-42H,3-16,21H2,1-2H3,(H,43,44)/t28-,31-,32+,33-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Competitive inhibition of PTP1B mediated pNPP hydrolysis by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50374277

(CHEMBL403094)Show SMILES Oc1ccc-2c(c1)[C@@H]1Oc3ccccc3[C@@]3(O)CC(=O)c4c(O)cc(O)c-2c4[C@@H]13 Show InChI InChI=1S/C23H16O6/c24-10-5-6-11-12(7-10)22-21-20-18(11)14(25)8-15(26)19(20)16(27)9-23(21,28)13-3-1-2-4-17(13)29-22/h1-8,21-22,24-26,28H,9H2/t21-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B by Lineweaver-Burke plot |
Bioorg Med Chem Lett 18: 772-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.036
BindingDB Entry DOI: 10.7270/Q25B03BB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM13373

((2S)-2-[(2S)-2-[(2S)-2-[(2R)-2-amino-3-sulfanylpro...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(O)=O |r| Show InChI InChI=1S/C22H34N4O5S2/c1-13(2)18(26-19(27)15(23)12-32)21(29)25-17(11-14-7-5-4-6-8-14)20(28)24-16(22(30)31)9-10-33-3/h4-8,13,15-18,32H,9-12,23H2,1-3H3,(H,24,28)(H,25,29)(H,26,27)(H,30,31)/t15-,16-,17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477454
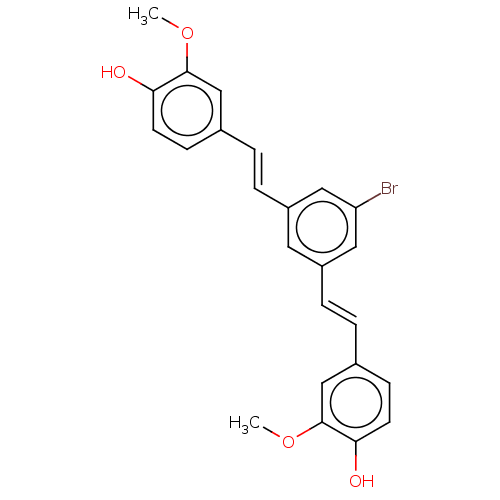
(CHEMBL245688)Show SMILES COc1cc(\C=C\c2cc(Br)cc(\C=C\c3ccc(O)c(OC)c3)c2)ccc1O Show InChI InChI=1S/C24H21BrO4/c1-28-23-14-16(7-9-21(23)26)3-5-18-11-19(13-20(25)12-18)6-4-17-8-10-22(27)24(15-17)29-2/h3-15,26-27H,1-2H3/b5-3+,6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311254

(2-hydroxy-4-(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| Show InChI InChI=1S/C36H52O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-18-25(47-36-33(42)32(41)31(40)28(21-37)48-36)20-27(39)30(23)35(45)46-24-17-22(2)29(34(43)44)26(38)19-24/h17-20,28,31-33,36-42H,3-16,21H2,1-2H3,(H,43,44)/t28-,31-,32+,33-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311256
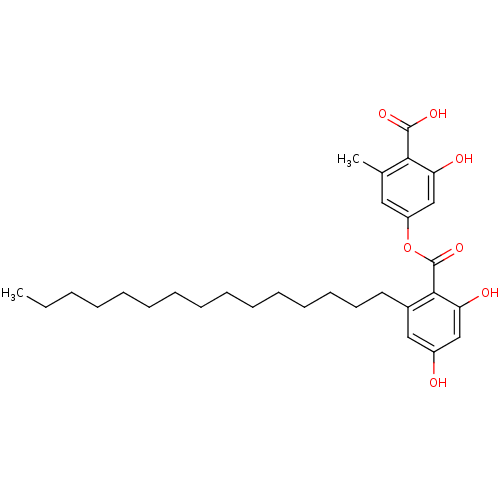
(4-(2,4-dihydroxy-6-pentadecylbenzoyloxy)-2-hydroxy...)Show SMILES CCCCCCCCCCCCCCCc1cc(O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 Show InChI InChI=1S/C30H42O7/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-22-18-23(31)19-25(32)28(22)30(36)37-24-17-21(2)27(29(34)35)26(33)20-24/h17-20,31-33H,3-16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477429
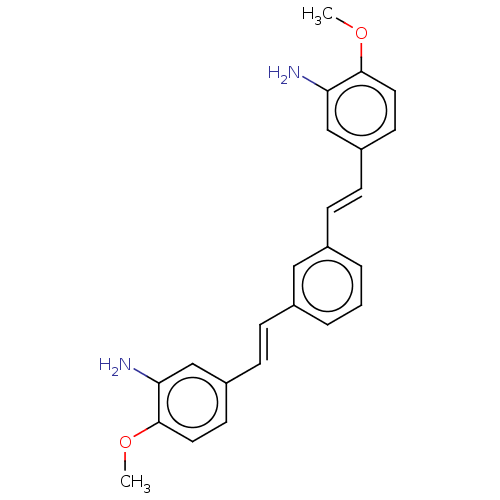
(CHEMBL248132)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(N)c3)c2)cc1N Show InChI InChI=1S/C24H24N2O2/c1-27-23-12-10-19(15-21(23)25)8-6-17-4-3-5-18(14-17)7-9-20-11-13-24(28-2)22(26)16-20/h3-16H,25-26H2,1-2H3/b8-6+,9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477447

(CHEMBL248327)Show SMILES CNc1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(NC)c3)n2)ccc1O Show InChI InChI=1S/C23H23N3O2/c1-24-20-14-16(8-12-22(20)27)6-10-18-4-3-5-19(26-18)11-7-17-9-13-23(28)21(15-17)25-2/h3-15,24-25,27-28H,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477441

(CHEMBL247674)Show SMILES CNc1ccc(\C=C\c2cccc(\C=C\c3ccc(NC)c(OC)c3)n2)cc1OC Show InChI InChI=1S/C25H27N3O2/c1-26-22-14-10-18(16-24(22)29-3)8-12-20-6-5-7-21(28-20)13-9-19-11-15-23(27-2)25(17-19)30-4/h5-17,26-27H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50114333

(3-Methyl-but-2-enoic acid 4-{(S)-2-[(S)-2-((R)-2-a...)Show SMILES [#6]-[#8]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8]-[#6](=O)\[#6]=[#6](\[#6])-[#6])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16])-[#6](-[#6])-[#6] Show InChI InChI=1S/C23H33N3O6S/c1-13(2)10-19(27)32-16-8-6-15(7-9-16)11-18(23(30)31-5)25-22(29)20(14(3)4)26-21(28)17(24)12-33/h6-10,14,17-18,20,33H,11-12,24H2,1-5H3,(H,25,29)(H,26,28)/t17-,18-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50311254

(2-hydroxy-4-(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| Show InChI InChI=1S/C36H52O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-18-25(47-36-33(42)32(41)31(40)28(21-37)48-36)20-27(39)30(23)35(45)46-24-17-22(2)29(34(43)44)26(38)19-24/h17-20,28,31-33,36-42H,3-16,21H2,1-2H3,(H,43,44)/t28-,31-,32+,33-,36-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311257

(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5S,6R)-3,4,5-t...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(O)=O |r| Show InChI InChI=1S/C28H46O9/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-19-16-20(17-21(30)23(19)27(34)35)36-28-26(33)25(32)24(31)22(18-29)37-28/h16-17,22,24-26,28-33H,2-15,18H2,1H3,(H,34,35)/t22-,24-,25+,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50105462
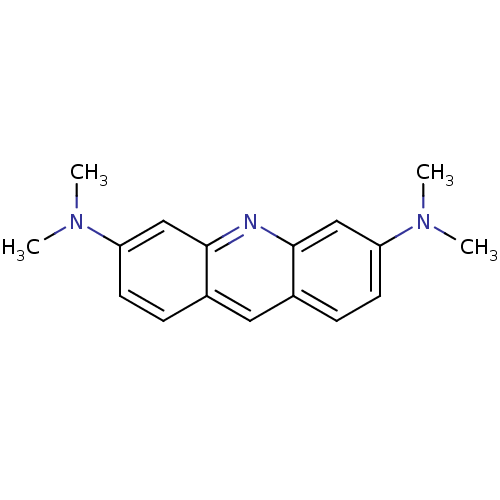
(CHEMBL81880 | N,N,N',N'-Tetramethyl-acridine-3,6-d...)Show InChI InChI=1S/C17H19N3/c1-19(2)14-7-5-12-9-13-6-8-15(20(3)4)11-17(13)18-16(12)10-14/h5-11H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477456
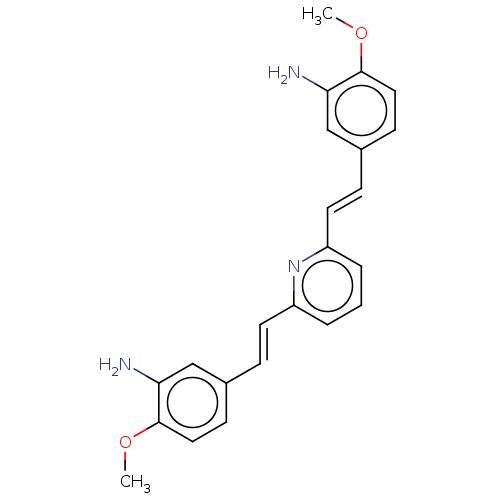
(CHEMBL392878)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(N)c3)n2)cc1N Show InChI InChI=1S/C23H23N3O2/c1-27-22-12-8-16(14-20(22)24)6-10-18-4-3-5-19(26-18)11-7-17-9-13-23(28-2)21(25)15-17/h3-15H,24-25H2,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477437
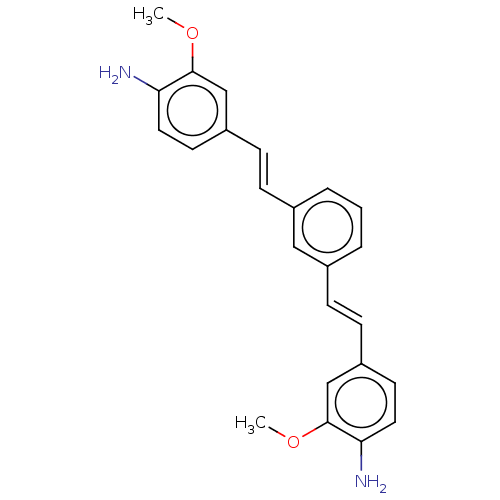
(CHEMBL247930)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N)c(OC)c3)c2)ccc1N Show InChI InChI=1S/C24H24N2O2/c1-27-23-15-19(10-12-21(23)25)8-6-17-4-3-5-18(14-17)7-9-20-11-13-22(26)24(16-20)28-2/h3-16H,25-26H2,1-2H3/b8-6+,9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477434

(CHEMBL247931)Show SMILES CNc1ccc(\C=C\c2cccc(\C=C\c3ccc(NC)c(OC)c3)c2)cc1OC Show InChI InChI=1S/C26H28N2O2/c1-27-23-14-12-21(17-25(23)29-3)10-8-19-6-5-7-20(16-19)9-11-22-13-15-24(28-2)26(18-22)30-4/h5-18,27-28H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477439

(CHEMBL247524)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N)c(OC)c3)n2)ccc1N Show InChI InChI=1S/C23H23N3O2/c1-27-22-14-16(8-12-20(22)24)6-10-18-4-3-5-19(26-18)11-7-17-9-13-21(25)23(15-17)28-2/h3-15H,24-25H2,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477428

(CHEMBL248131)Show SMILES CN(C)c1ccc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(O)c3)c2)cc1O Show InChI InChI=1S/C26H28N2O2/c1-27(2)23-14-12-21(17-25(23)29)10-8-19-6-5-7-20(16-19)9-11-22-13-15-24(28(3)4)26(30)18-22/h5-18,29-30H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477426

(CHEMBL391205)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(OC)c3)n2)ccc1N(C)C Show InChI InChI=1S/C27H31N3O2/c1-29(2)24-16-12-20(18-26(24)31-5)10-14-22-8-7-9-23(28-22)15-11-21-13-17-25(30(3)4)27(19-21)32-6/h7-19H,1-6H3/b14-10+,15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477435
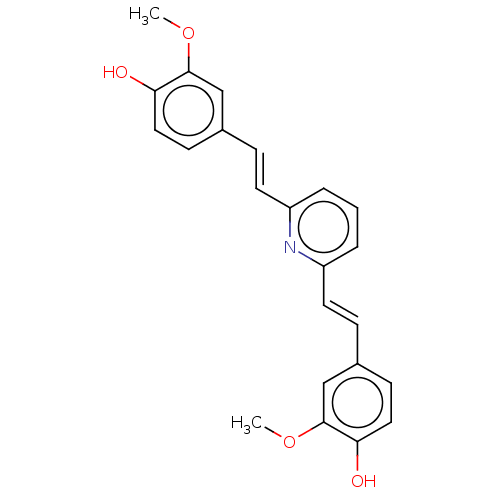
(CHEMBL247328)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(OC)c3)n2)ccc1O Show InChI InChI=1S/C23H21NO4/c1-27-22-14-16(8-12-20(22)25)6-10-18-4-3-5-19(24-18)11-7-17-9-13-21(26)23(15-17)28-2/h3-15,25-26H,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50067040
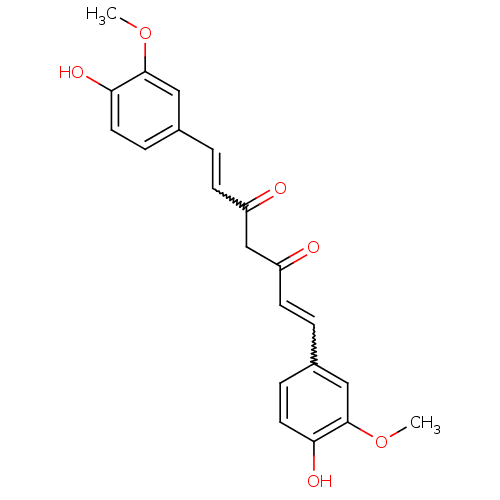
(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50114335
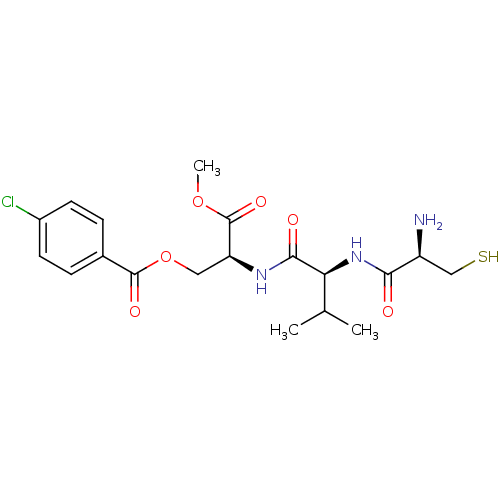
(4-Chloro-benzoic acid (S)-2-[(S)-2-((R)-2-amino-3-...)Show SMILES COC(=O)[C@H](COC(=O)c1ccc(Cl)cc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C Show InChI InChI=1S/C19H26ClN3O6S/c1-10(2)15(23-16(24)13(21)9-30)17(25)22-14(19(27)28-3)8-29-18(26)11-4-6-12(20)7-5-11/h4-7,10,13-15,30H,8-9,21H2,1-3H3,(H,22,25)(H,23,24)/t13-,14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50114334

(3-Methyl-butyric acid 4-{(S)-2-[(S)-2-((R)-2-amino...)Show SMILES COC(=O)[C@H](Cc1ccc(OC(=O)CC(C)C)cc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C Show InChI InChI=1S/C23H35N3O6S/c1-13(2)10-19(27)32-16-8-6-15(7-9-16)11-18(23(30)31-5)25-22(29)20(14(3)4)26-21(28)17(24)12-33/h6-9,13-14,17-18,20,33H,10-12,24H2,1-5H3,(H,25,29)(H,26,28)/t17-,18-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50294526
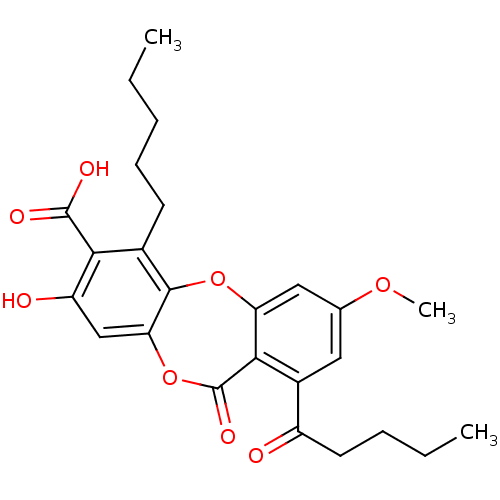
(CHEMBL551842 | cid_73157 | lobaric acid)Show SMILES CCCCCc1c2Oc3cc(OC)cc(C(=O)CCCC)c3C(=O)Oc2cc(O)c1C(O)=O Show InChI InChI=1S/C25H28O8/c1-4-6-8-9-15-21(24(28)29)18(27)13-20-23(15)32-19-12-14(31-3)11-16(17(26)10-7-5-2)22(19)25(30)33-20/h11-13,27H,4-10H2,1-3H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 19: 2801-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.108
BindingDB Entry DOI: 10.7270/Q20P10ZF |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477444
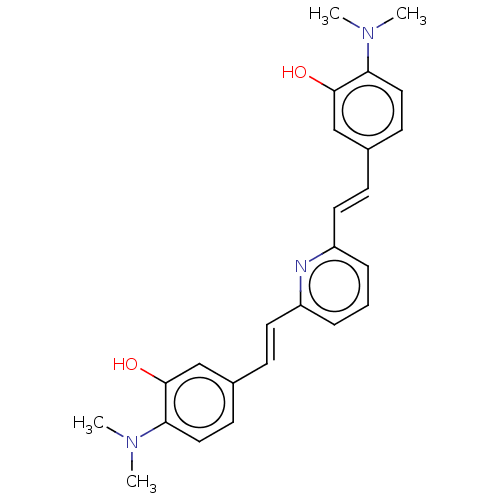
(CHEMBL247927)Show SMILES CN(C)c1ccc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(O)c3)n2)cc1O Show InChI InChI=1S/C25H27N3O2/c1-27(2)22-14-10-18(16-24(22)29)8-12-20-6-5-7-21(26-20)13-9-19-11-15-23(28(3)4)25(30)17-19/h5-17,29-30H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477446
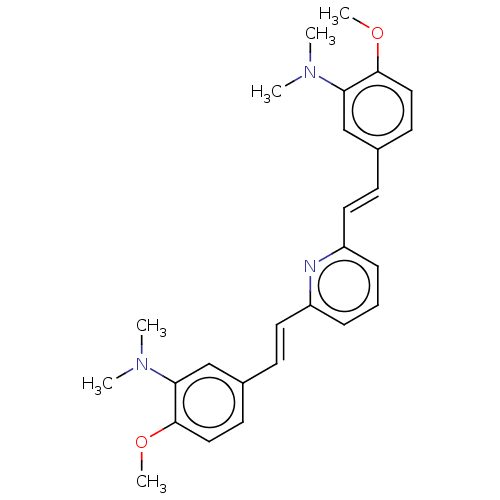
(CHEMBL394713)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(c3)N(C)C)n2)cc1N(C)C Show InChI InChI=1S/C27H31N3O2/c1-29(2)24-18-20(12-16-26(24)31-5)10-14-22-8-7-9-23(28-22)15-11-21-13-17-27(32-6)25(19-21)30(3)4/h7-19H,1-6H3/b14-10+,15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50114332

(Benzoic acid (S)-2-[(S)-2-((R)-2-amino-3-mercapto-...)Show SMILES COC(=O)[C@H](COC(=O)c1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C Show InChI InChI=1S/C19H27N3O6S/c1-11(2)15(22-16(23)13(20)10-29)17(24)21-14(19(26)27-3)9-28-18(25)12-7-5-4-6-8-12/h4-8,11,13-15,29H,9-10,20H2,1-3H3,(H,21,24)(H,22,23)/t13-,14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477450
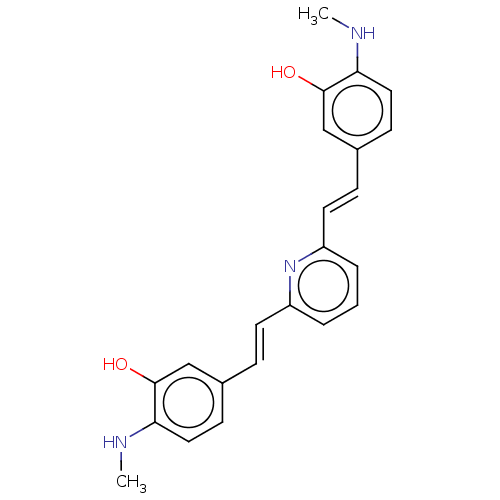
(CHEMBL247725)Show SMILES CNc1ccc(\C=C\c2cccc(\C=C\c3ccc(NC)c(O)c3)n2)cc1O Show InChI InChI=1S/C23H23N3O2/c1-24-20-12-8-16(14-22(20)27)6-10-18-4-3-5-19(26-18)11-7-17-9-13-21(25-2)23(28)15-17/h3-15,24-25,27-28H,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50114324

(4-Methyl-benzoic acid (S)-2-[(S)-2-((R)-2-amino-3-...)Show SMILES COC(=O)[C@H](COC(=O)c1ccc(C)cc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C Show InChI InChI=1S/C20H29N3O6S/c1-11(2)16(23-17(24)14(21)10-30)18(25)22-15(20(27)28-4)9-29-19(26)13-7-5-12(3)6-8-13/h5-8,11,14-16,30H,9-10,21H2,1-4H3,(H,22,25)(H,23,24)/t14-,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477448
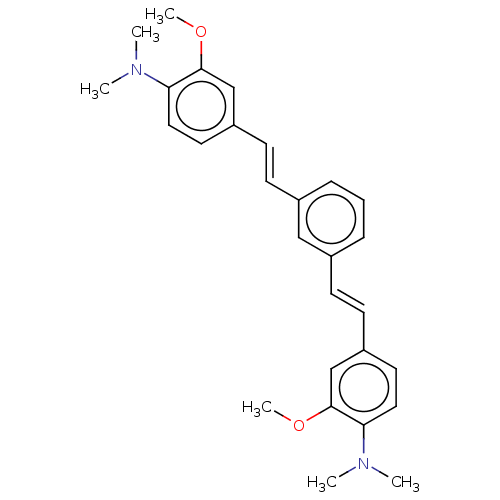
(CHEMBL247932)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(OC)c3)c2)ccc1N(C)C Show InChI InChI=1S/C28H32N2O2/c1-29(2)25-16-14-23(19-27(25)31-5)12-10-21-8-7-9-22(18-21)11-13-24-15-17-26(30(3)4)28(20-24)32-6/h7-20H,1-6H3/b12-10+,13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477431
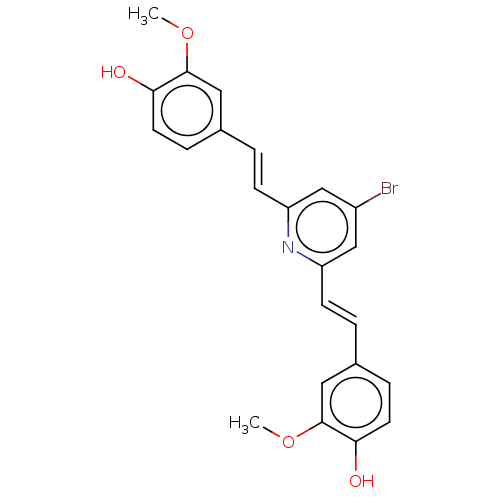
(CHEMBL427878)Show SMILES COc1cc(\C=C\c2cc(Br)cc(\C=C\c3ccc(O)c(OC)c3)n2)ccc1O Show InChI InChI=1S/C23H20BrNO4/c1-28-22-11-15(5-9-20(22)26)3-7-18-13-17(24)14-19(25-18)8-4-16-6-10-21(27)23(12-16)29-2/h3-14,26-27H,1-2H3/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477453
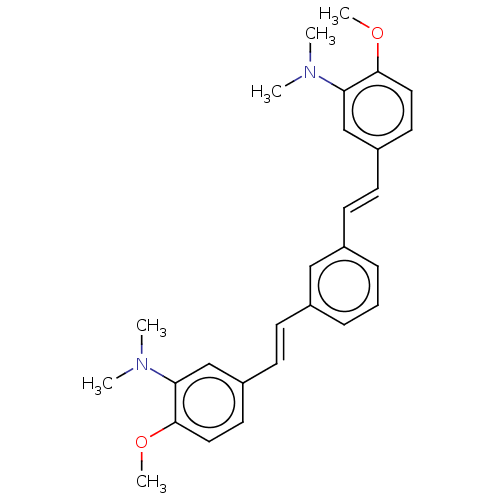
(CHEMBL248337)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(c3)N(C)C)c2)cc1N(C)C Show InChI InChI=1S/C28H32N2O2/c1-29(2)25-19-23(14-16-27(25)31-5)12-10-21-8-7-9-22(18-21)11-13-24-15-17-28(32-6)26(20-24)30(3)4/h7-20H,1-6H3/b12-10+,13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477455
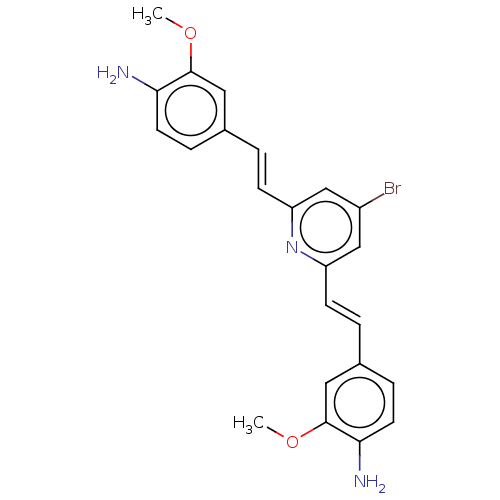
(CHEMBL247929)Show SMILES COc1cc(\C=C\c2cc(Br)cc(\C=C\c3ccc(N)c(OC)c3)n2)ccc1N Show InChI InChI=1S/C23H22BrN3O2/c1-28-22-11-15(5-9-20(22)25)3-7-18-13-17(24)14-19(27-18)8-4-16-6-10-21(26)23(12-16)29-2/h3-14H,25-26H2,1-2H3/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477436
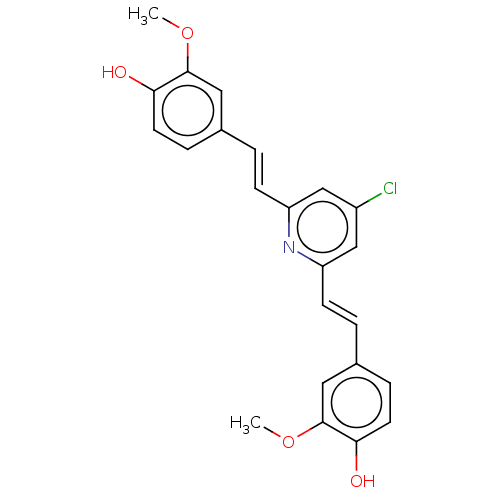
(CHEMBL247530)Show SMILES COc1cc(\C=C\c2cc(Cl)cc(\C=C\c3ccc(O)c(OC)c3)n2)ccc1O Show InChI InChI=1S/C23H20ClNO4/c1-28-22-11-15(5-9-20(22)26)3-7-18-13-17(24)14-19(25-18)8-4-16-6-10-21(27)23(12-16)29-2/h3-14,26-27H,1-2H3/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477438

(CHEMBL248122)Show SMILES CNc1cc(\C=C\c2cccc(\C=C\c3ccc(OC)c(NC)c3)n2)ccc1OC Show InChI InChI=1S/C25H27N3O2/c1-26-22-16-18(10-14-24(22)29-3)8-12-20-6-5-7-21(28-20)13-9-19-11-15-25(30-4)23(17-19)27-2/h5-17,26-27H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50114330
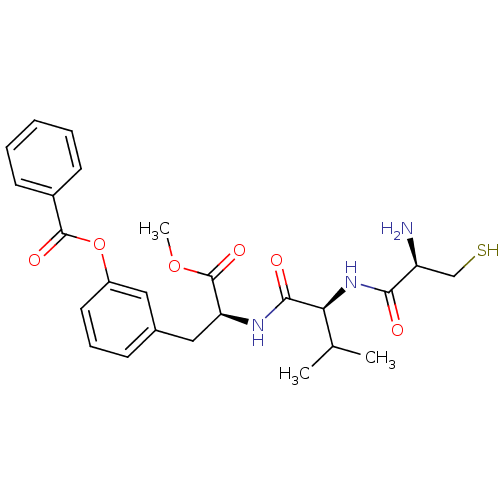
(Benzoic acid 3-{(S)-2-[(S)-2-((R)-2-amino-3-mercap...)Show SMILES COC(=O)[C@H](Cc1cccc(OC(=O)c2ccccc2)c1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C Show InChI InChI=1S/C25H31N3O6S/c1-15(2)21(28-22(29)19(26)14-35)23(30)27-20(25(32)33-3)13-16-8-7-11-18(12-16)34-24(31)17-9-5-4-6-10-17/h4-12,15,19-21,35H,13-14,26H2,1-3H3,(H,27,30)(H,28,29)/t19-,20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477433

(CHEMBL248133)Show SMILES CNc1cc(\C=C\c2cccc(\C=C\c3ccc(OC)c(NC)c3)c2)ccc1OC Show InChI InChI=1S/C26H28N2O2/c1-27-23-17-21(12-14-25(23)29-3)10-8-19-6-5-7-20(16-19)9-11-22-13-15-26(30-4)24(18-22)28-2/h5-18,27-28H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477451
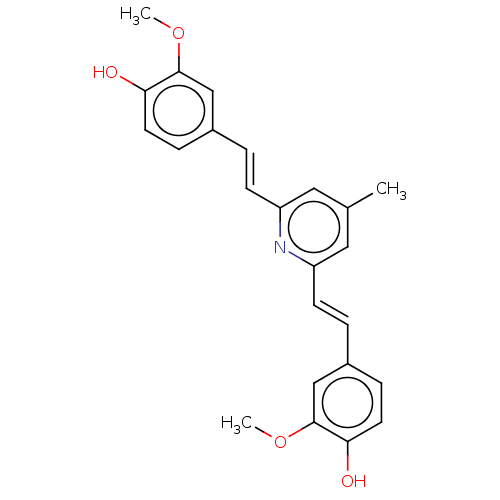
(CHEMBL247727)Show SMILES COc1cc(\C=C\c2cc(C)cc(\C=C\c3ccc(O)c(OC)c3)n2)ccc1O Show InChI InChI=1S/C24H23NO4/c1-16-12-19(8-4-17-6-10-21(26)23(14-17)28-2)25-20(13-16)9-5-18-7-11-22(27)24(15-18)29-3/h4-15,26-27H,1-3H3/b8-4+,9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477445
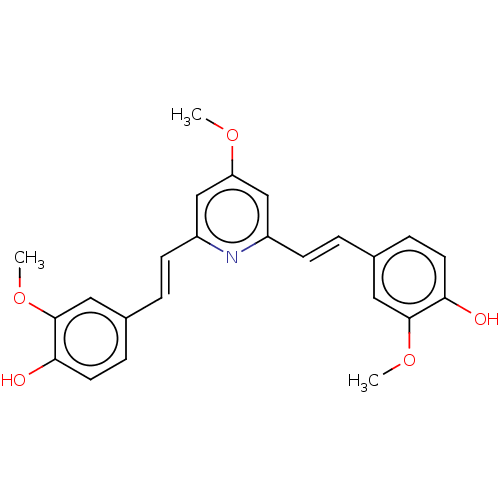
(CHEMBL247726)Show SMILES COc1cc(\C=C\c2ccc(O)c(OC)c2)nc(\C=C\c2ccc(O)c(OC)c2)c1 Show InChI InChI=1S/C24H23NO5/c1-28-20-14-18(8-4-16-6-10-21(26)23(12-16)29-2)25-19(15-20)9-5-17-7-11-22(27)24(13-17)30-3/h4-15,26-27H,1-3H3/b8-4+,9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50114326

(4-Methoxy-benzoic acid (S)-2-[(S)-2-((R)-2-amino-3...)Show SMILES COC(=O)[C@H](COC(=O)c1ccc(OC)cc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C Show InChI InChI=1S/C20H29N3O7S/c1-11(2)16(23-17(24)14(21)10-31)18(25)22-15(20(27)29-4)9-30-19(26)12-5-7-13(28-3)8-6-12/h5-8,11,14-16,31H,9-10,21H2,1-4H3,(H,22,25)(H,23,24)/t14-,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477452
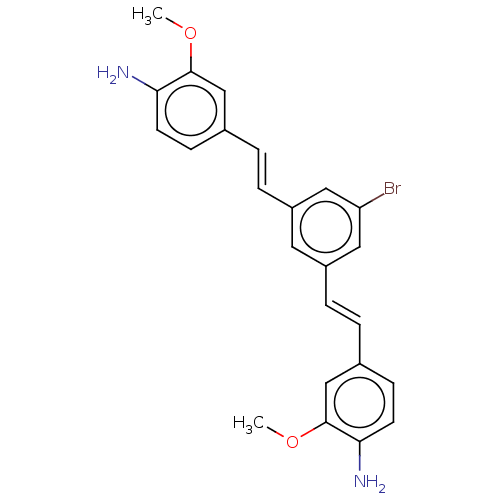
(CHEMBL396917)Show SMILES COc1cc(\C=C\c2cc(Br)cc(\C=C\c3ccc(N)c(OC)c3)c2)ccc1N Show InChI InChI=1S/C24H23BrN2O2/c1-28-23-14-16(7-9-21(23)26)3-5-18-11-19(13-20(25)12-18)6-4-17-8-10-22(27)24(15-17)29-2/h3-15H,26-27H2,1-2H3/b5-3+,6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477432

(CHEMBL247728)Show SMILES COc1cc(\C=C\c2cc(cc(\C=C\c3ccc(O)c(OC)c3)n2)N(C)C)ccc1O Show InChI InChI=1S/C25H26N2O4/c1-27(2)21-15-19(9-5-17-7-11-22(28)24(13-17)30-3)26-20(16-21)10-6-18-8-12-23(29)25(14-18)31-4/h5-16,28-29H,1-4H3/b9-5+,10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477443

(CHEMBL427877)Show SMILES CN(C)c1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(c3)N(C)C)n2)ccc1O Show InChI InChI=1S/C25H27N3O2/c1-27(2)22-16-18(10-14-24(22)29)8-12-20-6-5-7-21(26-20)13-9-19-11-15-25(30)23(17-19)28(3)4/h5-17,29-30H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50294527
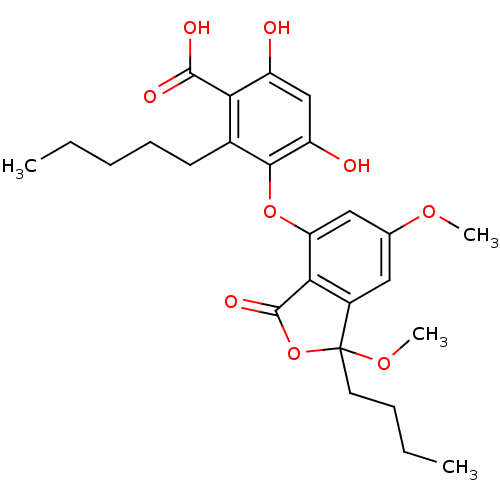
(3-(1-butyl-1,6-dimethoxy-3-oxo-1,3-dihydroisobenzo...)Show SMILES CCCCCc1c(Oc2cc(OC)cc3c2C(=O)OC3(CCCC)OC)c(O)cc(O)c1C(O)=O Show InChI InChI=1S/C26H32O9/c1-5-7-9-10-16-21(24(29)30)18(27)14-19(28)23(16)34-20-13-15(32-3)12-17-22(20)25(31)35-26(17,33-4)11-8-6-2/h12-14,27-28H,5-11H2,1-4H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 19: 2801-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.108
BindingDB Entry DOI: 10.7270/Q20P10ZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477425
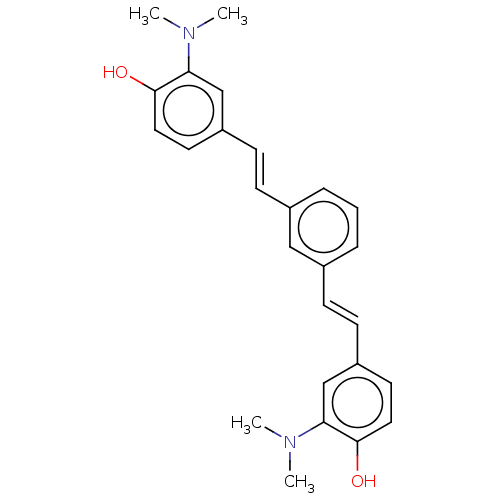
(CHEMBL248338)Show SMILES CN(C)c1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(c3)N(C)C)c2)ccc1O Show InChI InChI=1S/C26H28N2O2/c1-27(2)23-17-21(12-14-25(23)29)10-8-19-6-5-7-20(16-19)9-11-22-13-15-26(30)24(18-22)28(3)4/h5-18,29-30H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50294530
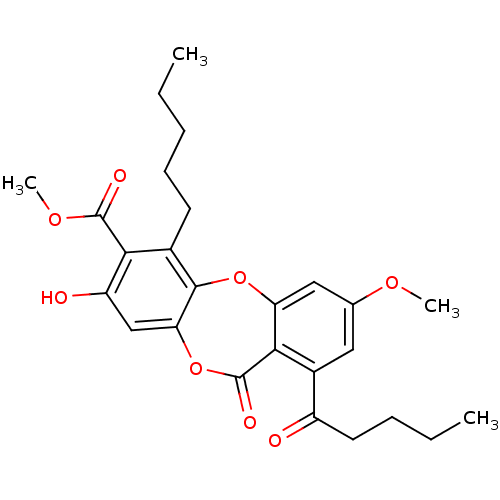
(CHEMBL559457 | methyl 8-hydroxy-3-methoxy-11-oxo-1...)Show SMILES CCCCCc1c2Oc3cc(OC)cc(C(=O)CCCC)c3C(=O)Oc2cc(O)c1C(=O)OC Show InChI InChI=1S/C26H30O8/c1-5-7-9-10-16-22(25(29)32-4)19(28)14-21-24(16)33-20-13-15(31-3)12-17(18(27)11-8-6-2)23(20)26(30)34-21/h12-14,28H,5-11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 19: 2801-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.108
BindingDB Entry DOI: 10.7270/Q20P10ZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 19: 2801-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.108
BindingDB Entry DOI: 10.7270/Q20P10ZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50374277

(CHEMBL403094)Show SMILES Oc1ccc-2c(c1)[C@@H]1Oc3ccccc3[C@@]3(O)CC(=O)c4c(O)cc(O)c-2c4[C@@H]13 Show InChI InChI=1S/C23H16O6/c24-10-5-6-11-12(7-10)22-21-20-18(11)14(25)8-15(26)19(20)16(27)9-23(21,28)13-3-1-2-4-17(13)29-22/h1-8,21-22,24-26,28H,9H2/t21-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 18: 772-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.036
BindingDB Entry DOI: 10.7270/Q25B03BB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50374279

(OHIOENSIN A)Show SMILES Oc1ccc-2c(c1)[C@@H]1Oc3ccccc3[C@H]3CC(=O)c4c(O)cc(O)c-2c4[C@@H]13 |r| Show InChI InChI=1S/C23H16O5/c24-10-5-6-12-14(7-10)23-20-13(11-3-1-2-4-18(11)28-23)8-15(25)21-17(27)9-16(26)19(12)22(20)21/h1-7,9,13,20,23-24,26-27H,8H2/t13-,20+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 18: 772-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.036
BindingDB Entry DOI: 10.7270/Q25B03BB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data