

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50442585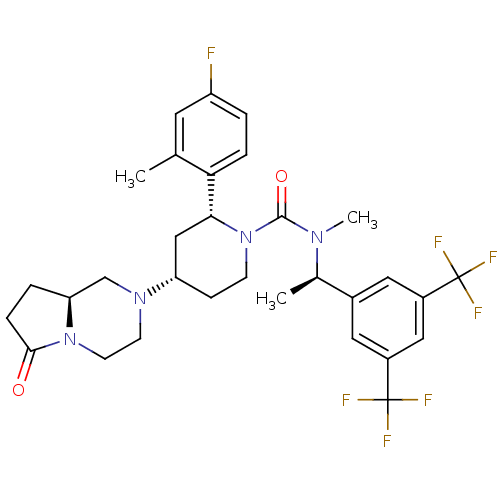 (GW823296X | ORVEPITANT) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]GR205171 from Mongolian gerbil brain NK1 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem 21: 6264-73 (2013) Article DOI: 10.1016/j.bmc.2013.09.001 BindingDB Entry DOI: 10.7270/Q2D79CWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50413549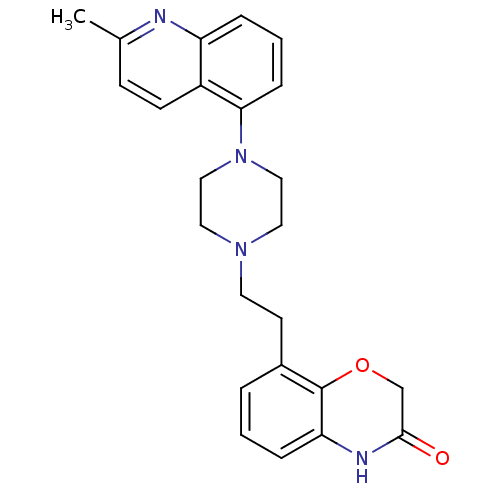 (CHEMBL513715) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50442585 (GW823296X | ORVEPITANT) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis | Bioorg Med Chem 21: 6264-73 (2013) Article DOI: 10.1016/j.bmc.2013.09.001 BindingDB Entry DOI: 10.7270/Q2D79CWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50442588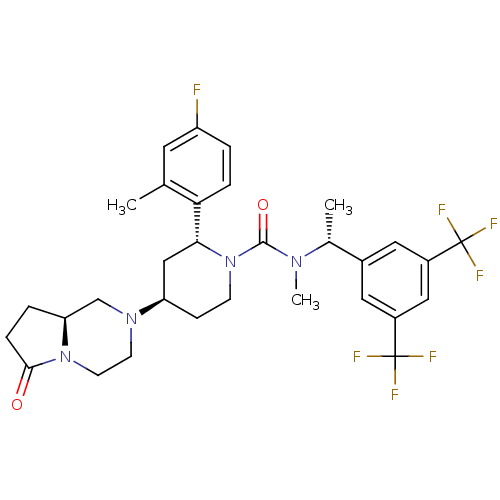 (CHEMBL2441373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis | Bioorg Med Chem 21: 6264-73 (2013) Article DOI: 10.1016/j.bmc.2013.09.001 BindingDB Entry DOI: 10.7270/Q2D79CWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat OX2R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417409 (CHEMBL1290487) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417420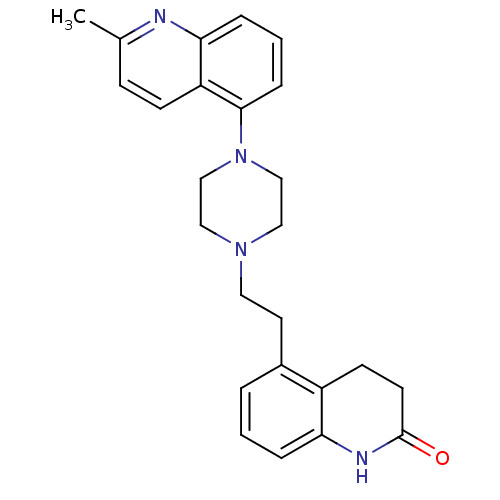 (CHEMBL1290486) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50413550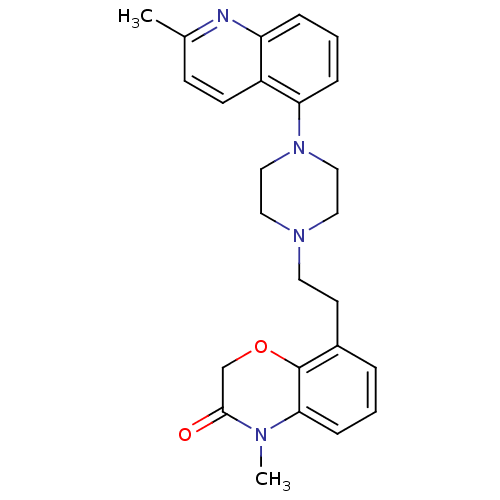 (CHEMBL469345) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Rattus norvegicus (Rat)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat OX1R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417411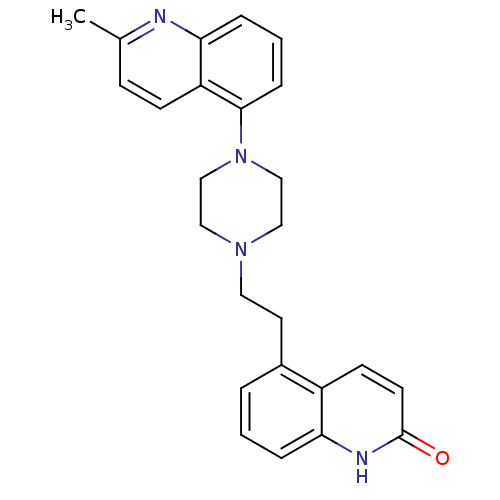 (CHEMBL1290715) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50419142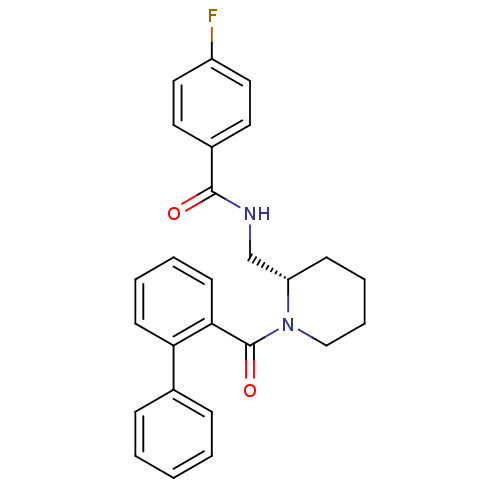 (CHEMBL1830963) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417424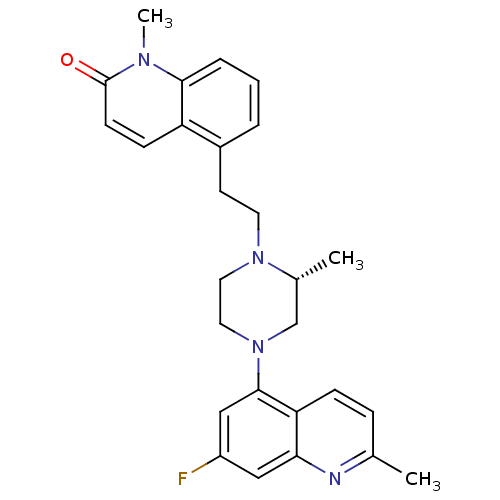 (CHEMBL1289394) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50442589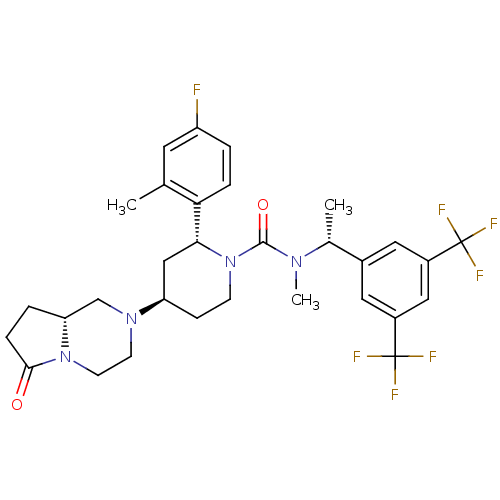 (CHEMBL2441371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis | Bioorg Med Chem 21: 6264-73 (2013) Article DOI: 10.1016/j.bmc.2013.09.001 BindingDB Entry DOI: 10.7270/Q2D79CWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50413550 (CHEMBL469345) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50419136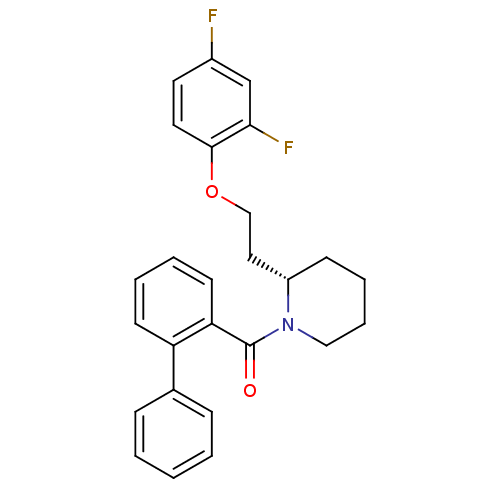 (CHEMBL1830961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50442590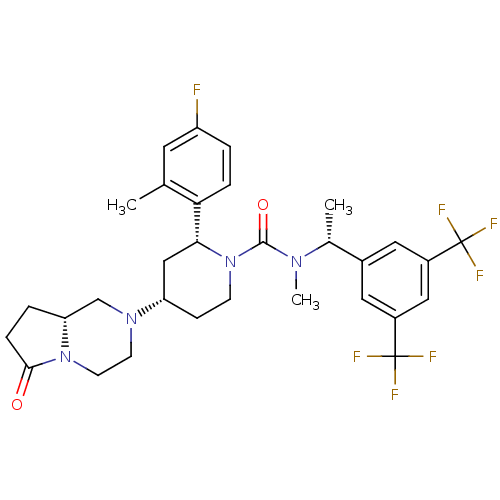 (CHEMBL2441372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis | Bioorg Med Chem 21: 6264-73 (2013) Article DOI: 10.1016/j.bmc.2013.09.001 BindingDB Entry DOI: 10.7270/Q2D79CWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50417419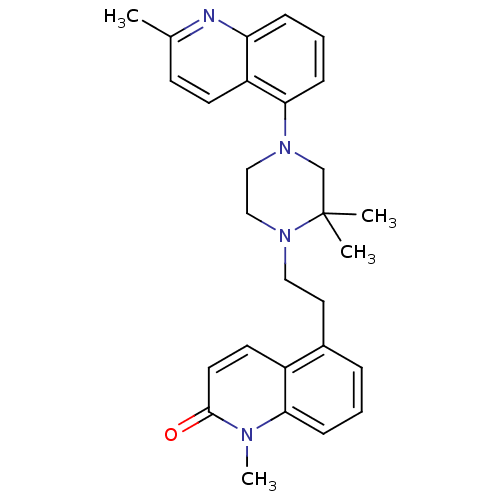 (CHEMBL1289618) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram in human SERT expressed in LLCPK cells by filtration assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50419142 (CHEMBL1830963) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50413549 (CHEMBL513715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417420 (CHEMBL1290486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417413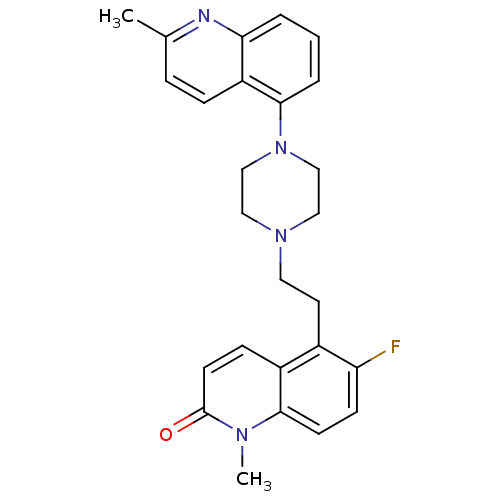 (CHEMBL1289163) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417413 (CHEMBL1289163) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50417425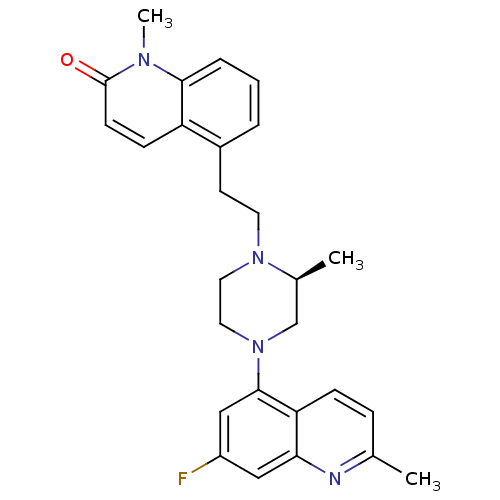 (CHEMBL1289510) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram in human SERT expressed in LLCPK cells by filtration assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50417417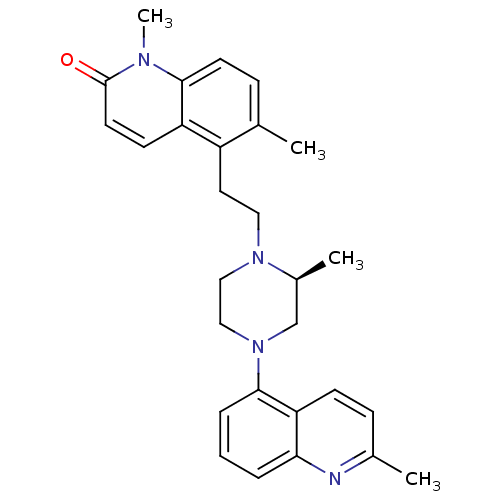 (CHEMBL1289509) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram in human SERT expressed in LLCPK cells by filtration assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417419 (CHEMBL1289618) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50417424 (CHEMBL1289394) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram in human SERT expressed in LLCPK cells by filtration assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50417420 (CHEMBL1290486) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1B receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417422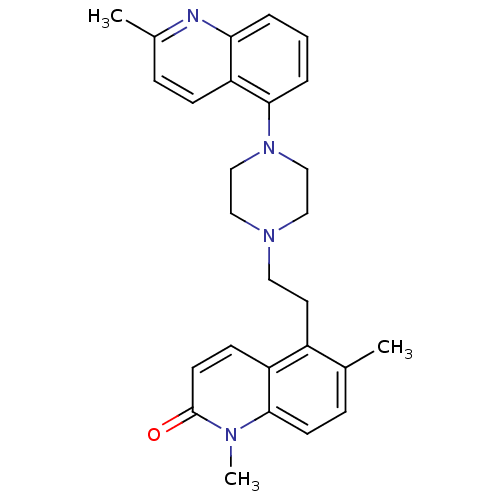 (CHEMBL1289048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417421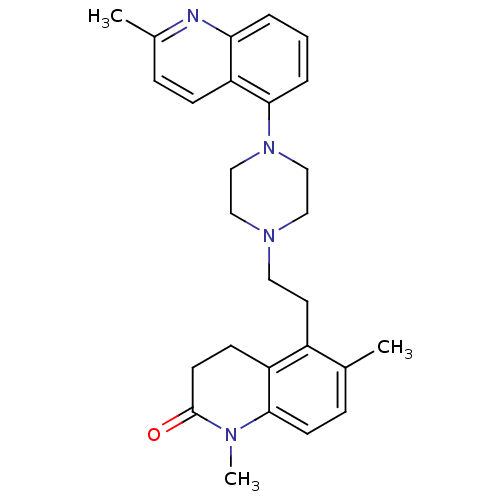 (CHEMBL1290597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417409 (CHEMBL1290487) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417411 (CHEMBL1290715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]almorexant from recombinant human OX1R expressed in CHO cells | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50419136 (CHEMBL1830961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50417409 (CHEMBL1290487) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1B receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417410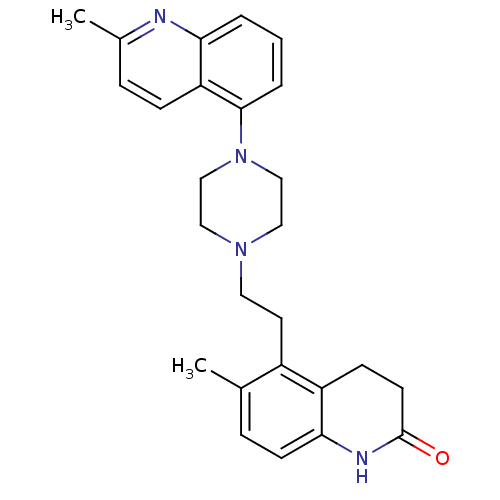 (CHEMBL1290596) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50417424 (CHEMBL1289394) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1B receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50417427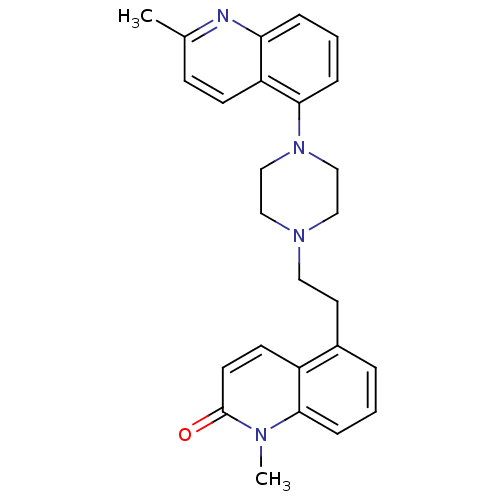 (CHEMBL1290716) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1B receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50419131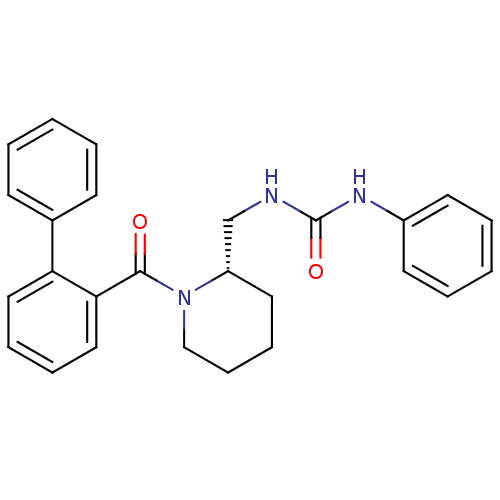 (CHEMBL1830968) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417424 (CHEMBL1289394) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50413549 (CHEMBL513715) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1B receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417421 (CHEMBL1290597) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417425 (CHEMBL1289510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50417418 (CHEMBL1289617) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram in human SERT expressed in LLCPK cells by filtration assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417412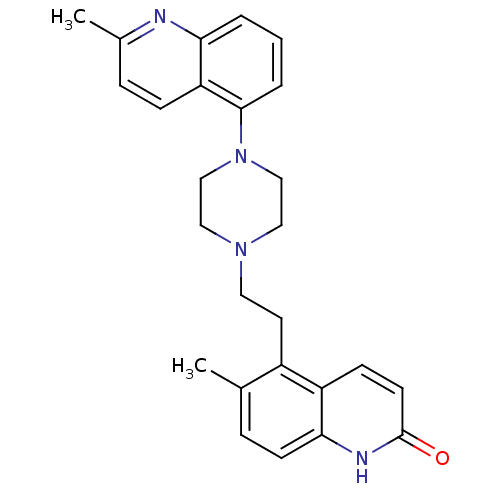 (CHEMBL1289047) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50417426 (CHEMBL1289721) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram in human SERT expressed in LLCPK cells by filtration assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417426 (CHEMBL1289721) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417425 (CHEMBL1289510) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50417415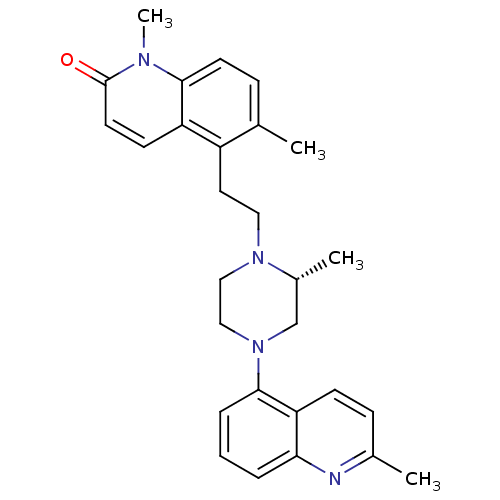 (CHEMBL1289277) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 776 total ) | Next | Last >> |