 Found 394 hits with Last Name = 'spencer' and Initial = 'a'
Found 394 hits with Last Name = 'spencer' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50115622
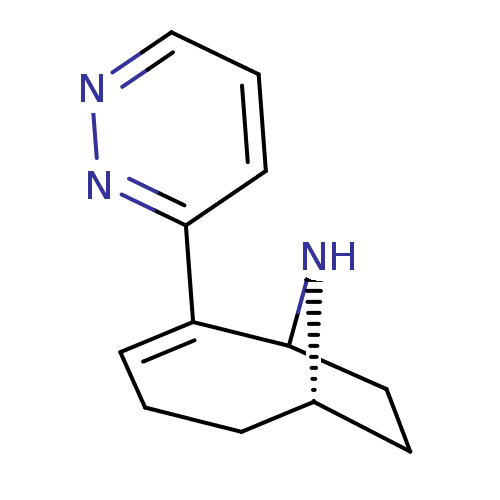
(2-Pyridazin-3-yl-9-aza-bicyclo[4.2.1]non-2-ene | C...)Show SMILES C1CC2N[C@H]1CCC=C2c1cccnn1 |c:8,THB:9:8:3:1.0| Show InChI InChI=1S/C12H15N3/c1-3-9-6-7-11(14-9)10(4-1)12-5-2-8-13-15-12/h2,4-5,8-9,11,14H,1,3,6-7H2/t9-,11?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 68.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7 |
J Med Chem 45: 3235-45 (2002)
BindingDB Entry DOI: 10.7270/Q22N530X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50110260

(2-Pyridin-3-yl-9-aza-bicyclo[4.2.1]non-2-ene | 2-P...)Show SMILES C1CC2N[C@H]1CCC=C2c1cccnc1 |c:8,THB:9:8:3:1.0| Show InChI InChI=1S/C13H16N2/c1-4-11-6-7-13(15-11)12(5-1)10-3-2-8-14-9-10/h2-3,5,8-9,11,13,15H,1,4,6-7H2/t11-,13?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7 |
J Med Chem 45: 3235-45 (2002)
BindingDB Entry DOI: 10.7270/Q22N530X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7 |
J Med Chem 45: 3235-45 (2002)
BindingDB Entry DOI: 10.7270/Q22N530X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342570

((E)-4-bromo-3-(2-oxo-2-(pyridin-3-yl)ethylidene)in...)Show InChI InChI=1S/C15H9BrN2O2/c16-11-4-1-5-12-14(11)10(15(20)18-12)7-13(19)9-3-2-6-17-8-9/h1-8H,(H,18,20)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using ZGBC as a substrate after 30 to 60 mins by fluorometric assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342570

((E)-4-bromo-3-(2-oxo-2-(pyridin-3-yl)ethylidene)in...)Show InChI InChI=1S/C15H9BrN2O2/c16-11-4-1-5-12-14(11)10(15(20)18-12)7-13(19)9-3-2-6-17-8-9/h1-8H,(H,18,20)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342568

((E)-1-benzyl-4-chloro-3-(2-oxo-2-(pyridin-3-yl)eth...)Show SMILES Clc1cccc2N(Cc3ccccc3)C(=O)\C(=C\C(=O)c3cccnc3)c12 Show InChI InChI=1S/C22H15ClN2O2/c23-18-9-4-10-19-21(18)17(12-20(26)16-8-5-11-24-13-16)22(27)25(19)14-15-6-2-1-3-7-15/h1-13H,14H2/b17-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342571
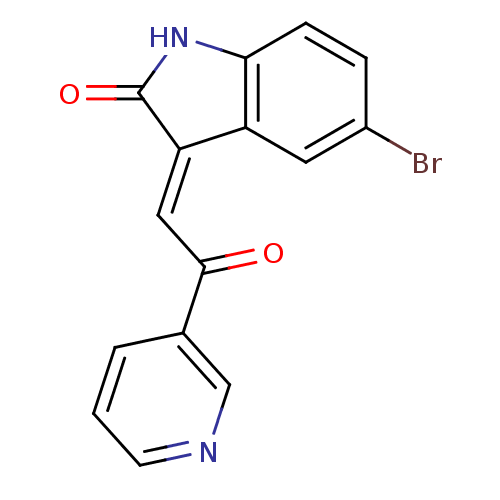
((E)-5-bromo-3-(2-oxo-2-(pyridin-3-yl)ethylidene)in...)Show InChI InChI=1S/C15H9BrN2O2/c16-10-3-4-13-11(6-10)12(15(20)18-13)7-14(19)9-2-1-5-17-8-9/h1-8H,(H,18,20)/b12-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using ZGBC as a substrate after 30 to 60 mins by fluorometric assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342562

((E)-4-chloro-3-(2-oxopropylidene)indolin-2-one | C...)Show InChI InChI=1S/C11H8ClNO2/c1-6(14)5-7-10-8(12)3-2-4-9(10)13-11(7)15/h2-5H,1H3,(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342563
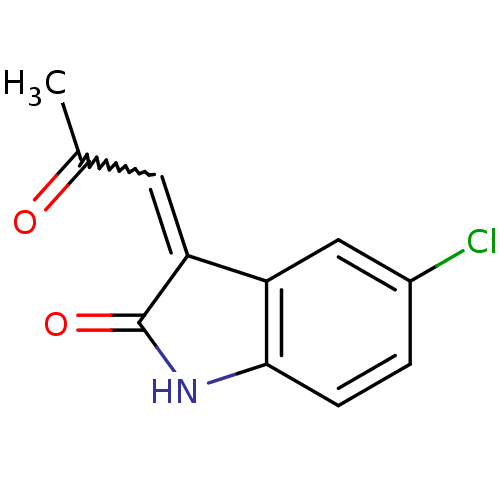
((E)-5-chloro-3-(2-oxopropylidene)indolin-2-one | C...)Show InChI InChI=1S/C11H8ClNO2/c1-6(14)4-9-8-5-7(12)2-3-10(8)13-11(9)15/h2-5H,1H3,(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342568

((E)-1-benzyl-4-chloro-3-(2-oxo-2-(pyridin-3-yl)eth...)Show SMILES Clc1cccc2N(Cc3ccccc3)C(=O)\C(=C\C(=O)c3cccnc3)c12 Show InChI InChI=1S/C22H15ClN2O2/c23-18-9-4-10-19-21(18)17(12-20(26)16-8-5-11-24-13-16)22(27)25(19)14-15-6-2-1-3-7-15/h1-13H,14H2/b17-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using ZGBC as a substrate after 30 to 60 mins by fluorometric assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342565
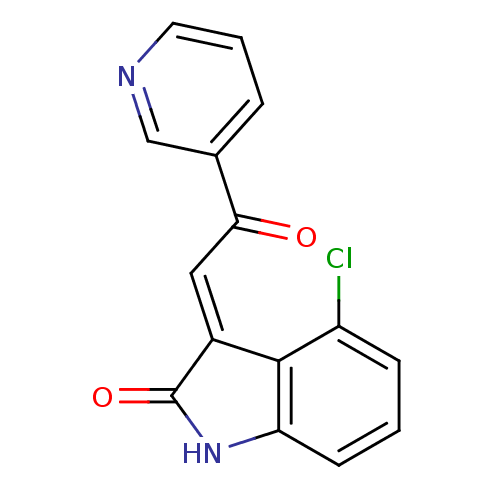
((E)-4-chloro-3-(2-oxo-2-(pyridin-3-yl)ethylidene)i...)Show InChI InChI=1S/C15H9ClN2O2/c16-11-4-1-5-12-14(11)10(15(20)18-12)7-13(19)9-3-2-6-17-8-9/h1-8H,(H,18,20)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50110259
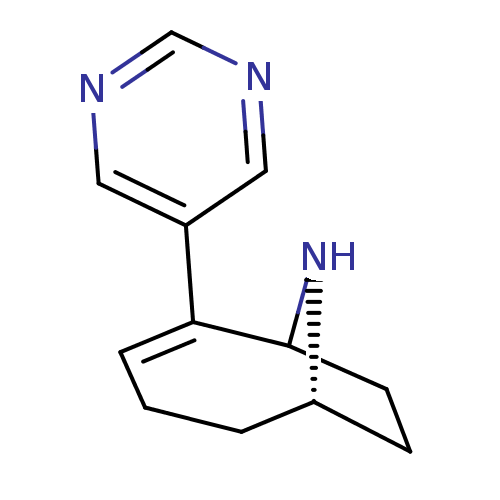
(2-Pyrimidin-5-yl-9-aza-bicyclo[4.2.1]non-2-ene | C...)Show SMILES C1CC2N[C@H]1CCC=C2c1cncnc1 |c:8,THB:9:8:3:1.0| Show InChI InChI=1S/C12H15N3/c1-2-10-4-5-12(15-10)11(3-1)9-6-13-8-14-7-9/h3,6-8,10,12,15H,1-2,4-5H2/t10-,12?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7 |
J Med Chem 45: 3235-45 (2002)
BindingDB Entry DOI: 10.7270/Q22N530X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50110258
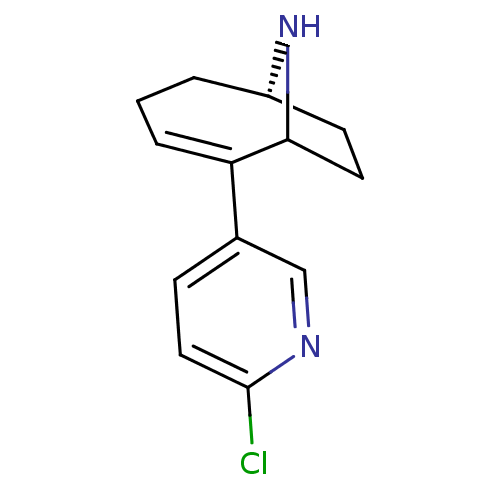
((6S)-2-(6-chloropyridin-3-yl)-9-azabicyclo[4.2.1]n...)Show SMILES Clc1ccc(cn1)C1=CCC[C@H]2CCC1N2 |t:8,THB:4:7:15:13.12| Show InChI InChI=1S/C13H15ClN2/c14-13-7-4-9(8-15-13)11-3-1-2-10-5-6-12(11)16-10/h3-4,7-8,10,12,16H,1-2,5-6H2/t10-,12?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7 |
J Med Chem 45: 3235-45 (2002)
BindingDB Entry DOI: 10.7270/Q22N530X |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342557

(5,5'-oxydiindoline-2,3-dione | CHEMBL1770389)Show InChI InChI=1S/C16H8N2O5/c19-13-9-5-7(1-3-11(9)17-15(13)21)23-8-2-4-12-10(6-8)14(20)16(22)18-12/h1-6H,(H,17,19,21)(H,18,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342571

((E)-5-bromo-3-(2-oxo-2-(pyridin-3-yl)ethylidene)in...)Show InChI InChI=1S/C15H9BrN2O2/c16-10-3-4-13-11(6-10)12(15(20)18-13)7-14(19)9-2-1-5-17-8-9/h1-8H,(H,18,20)/b12-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342566

((E)-4-chloro-3-(2-oxo-2-phenylethylidene)indolin-2...)Show InChI InChI=1S/C16H10ClNO2/c17-12-7-4-8-13-15(12)11(16(20)18-13)9-14(19)10-5-2-1-3-6-10/h1-9H,(H,18,20)/b11-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342567

((E)-4-chloro-1-methyl-3-(2-oxo-2-(pyridin-3-yl)eth...)Show InChI InChI=1S/C16H11ClN2O2/c1-19-13-6-2-5-12(17)15(13)11(16(19)21)8-14(20)10-4-3-7-18-9-10/h2-9H,1H3/b11-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342553

((E)-6-chloro-3-(2-oxo-2-(pyridin-3-yl)ethylidene)i...)Show InChI InChI=1S/C15H9ClN2O2/c16-10-3-4-11-12(15(20)18-13(11)6-10)7-14(19)9-2-1-5-17-8-9/h1-8H,(H,18,20)/b12-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342564

((E)-6-fluoro-3-(2-oxopropylidene)indolin-2-one | C...)Show InChI InChI=1S/C11H8FNO2/c1-6(14)4-9-8-3-2-7(12)5-10(8)13-11(9)15/h2-5H,1H3,(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50110263

(2-Pyrazin-2-yl-9-aza-bicyclo[4.2.1]non-2-ene | CHE...)Show SMILES C1CC2N[C@H]1CCC=C2c1cnccn1 |c:8,THB:9:8:3:1.0| Show InChI InChI=1S/C12H15N3/c1-2-9-4-5-11(15-9)10(3-1)12-8-13-6-7-14-12/h3,6-9,11,15H,1-2,4-5H2/t9-,11?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7 |
J Med Chem 45: 3235-45 (2002)
BindingDB Entry DOI: 10.7270/Q22N530X |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342560
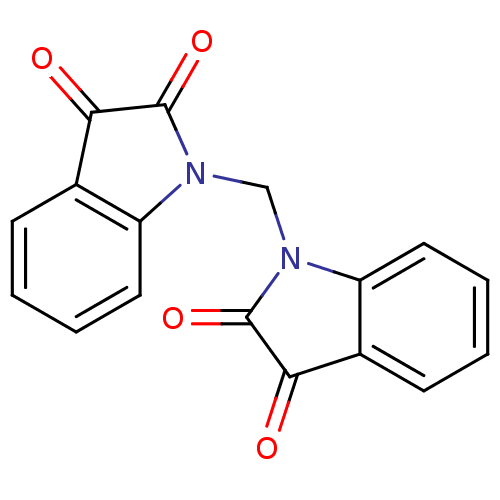
(1,1'-methylenediindoline-2,3-dione | CHEMBL1770393)Show InChI InChI=1S/C17H10N2O4/c20-14-10-5-1-3-7-12(10)18(16(14)22)9-19-13-8-4-2-6-11(13)15(21)17(19)23/h1-8H,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50132009
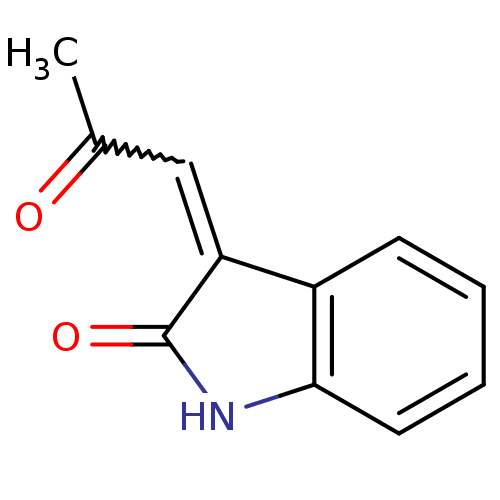
(3-(2-Oxo-propylidene)-1,3-dihydro-indol-2-one | CH...)Show InChI InChI=1S/C11H9NO2/c1-7(13)6-9-8-4-2-3-5-10(8)12-11(9)14/h2-6H,1H3,(H,12,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342559
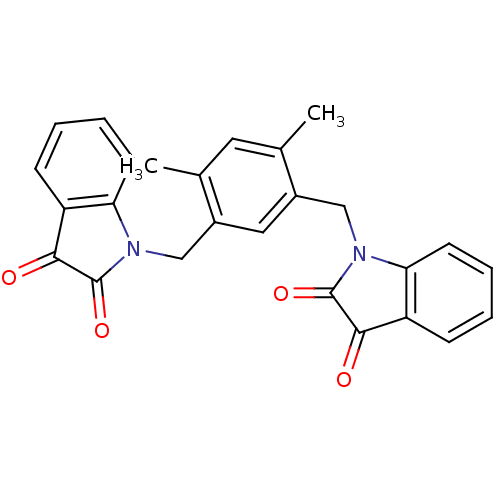
(1,1'-(4,6-dimethyl-1,3-phenylene)bis(methylene)dii...)Show SMILES Cc1cc(C)c(CN2C(=O)C(=O)c3ccccc23)cc1CN1C(=O)C(=O)c2ccccc12 Show InChI InChI=1S/C26H20N2O4/c1-15-11-16(2)18(14-28-22-10-6-4-8-20(22)24(30)26(28)32)12-17(15)13-27-21-9-5-3-7-19(21)23(29)25(27)31/h3-12H,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342569
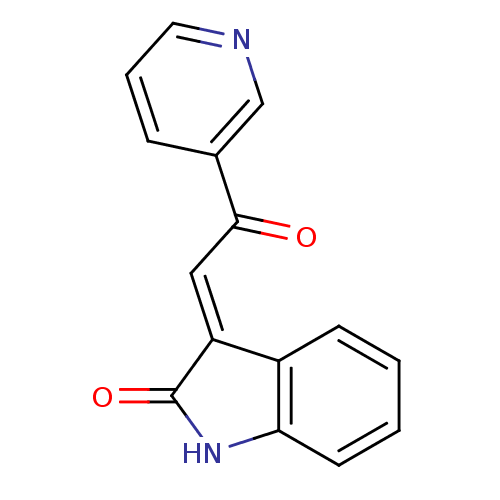
((E)-3-(2-oxo-2-(pyridin-3-yl)ethylidene)indolin-2-...)Show InChI InChI=1S/C15H10N2O2/c18-14(10-4-3-7-16-9-10)8-12-11-5-1-2-6-13(11)17-15(12)19/h1-9H,(H,17,19)/b12-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342558
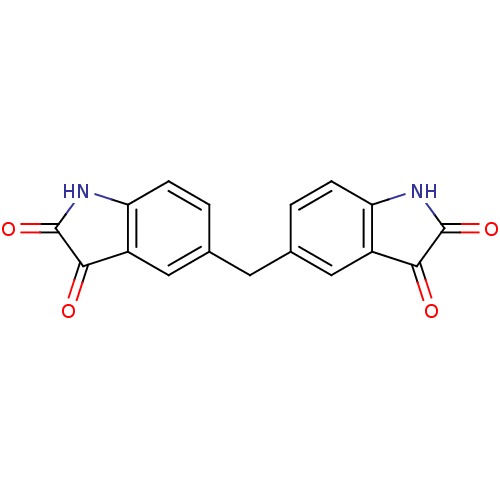
(5,5'-methylenediindoline-2,3-dione | CHEMBL1770390)Show InChI InChI=1S/C17H10N2O4/c20-14-10-6-8(1-3-12(10)18-16(14)22)5-9-2-4-13-11(7-9)15(21)17(23)19-13/h1-4,6-7H,5H2,(H,18,20,22)(H,19,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50342561
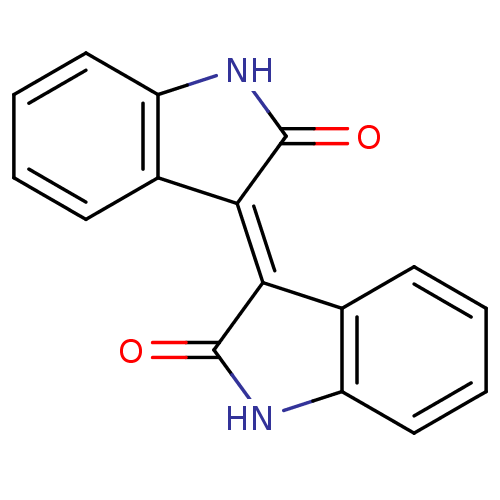
((2-oxoindolin-3-ylidene)indolin-2-one | (E)-[3,3'-...)Show InChI InChI=1S/C16H10N2O2/c19-15-13(9-5-1-3-7-11(9)17-15)14-10-6-2-4-8-12(10)18-16(14)20/h1-8H,(H,17,19)(H,18,20)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assay |
Bioorg Med Chem Lett 21: 2692-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.037
BindingDB Entry DOI: 10.7270/Q2CN7471 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50115624
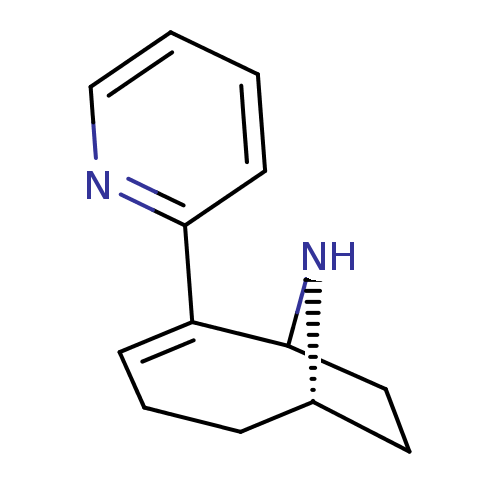
(2-Pyridin-2-yl-9-aza-bicyclo[4.2.1]non-2-ene | CHE...)Show SMILES C1CC2N[C@H]1CCC=C2c1ccccn1 |c:8,THB:9:8:3:1.0| Show InChI InChI=1S/C13H16N2/c1-2-9-14-12(6-1)11-5-3-4-10-7-8-13(11)15-10/h1-2,5-6,9-10,13,15H,3-4,7-8H2/t10-,13?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7 |
J Med Chem 45: 3235-45 (2002)
BindingDB Entry DOI: 10.7270/Q22N530X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50115623
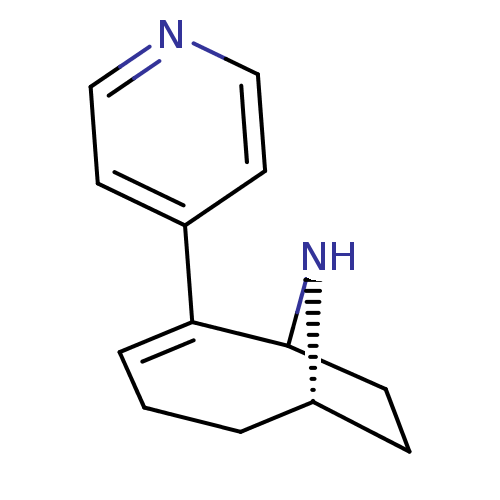
(2-Pyridin-4-yl-9-aza-bicyclo[4.2.1]non-2-ene | CHE...)Show SMILES C1CC2N[C@H]1CCC=C2c1ccncc1 |c:8,THB:9:8:3:1.0| Show InChI InChI=1S/C13H16N2/c1-2-11-4-5-13(15-11)12(3-1)10-6-8-14-9-7-10/h3,6-9,11,13,15H,1-2,4-5H2/t11-,13?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7 |
J Med Chem 45: 3235-45 (2002)
BindingDB Entry DOI: 10.7270/Q22N530X |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM381823
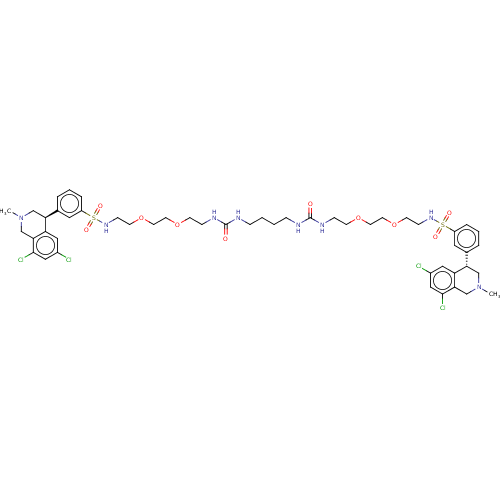
(CVD-0019453 | US10272079, Compound 002 | US1027207...)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)NCCCCNC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| Show InChI InChI=1S/C50H66Cl4N8O10S2/c1-61-31-43(41-27-37(51)29-47(53)45(41)33-61)35-7-5-9-39(25-35)73(65,66)59-15-19-71-23-21-69-17-13-57-49(63)55-11-3-4-12-56-50(64)58-14-18-70-22-24-72-20-16-60-74(67,68)40-10-6-8-36(26-40)44-32-62(2)34-46-42(44)28-38(52)30-48(46)54/h5-10,25-30,43-44,59-60H,3-4,11-24,31-34H2,1-2H3,(H2,55,57,63)(H2,56,58,64)/t43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601173
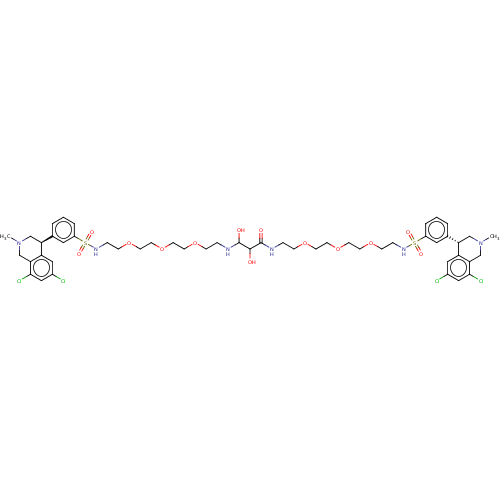
(CHEMBL5176968)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(O)C(O)C(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM381826
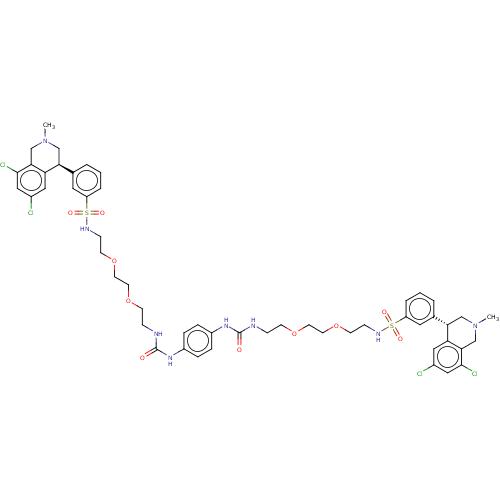
(US10272079, Compound 003)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)Nc2ccc(NC(=O)NCCOCCOCCNS(=O)(=O)c3cccc(c3)[C@@H]3CN(C)Cc4c(Cl)cc(Cl)cc34)cc2)c2cc(Cl)cc(Cl)c2C1 |r| Show InChI InChI=1S/C52H62Cl4N8O10S2/c1-63-31-45(43-27-37(53)29-49(55)47(43)33-63)35-5-3-7-41(25-35)75(67,68)59-15-19-73-23-21-71-17-13-57-51(65)61-39-9-11-40(12-10-39)62-52(66)58-14-18-72-22-24-74-20-16-60-76(69,70)42-8-4-6-36(26-42)46-32-64(2)34-48-44(46)28-38(54)30-50(48)56/h3-12,25-30,45-46,59-60H,13-24,31-34H2,1-2H3,(H2,57,61,65)(H2,58,62,66)/t45-,46-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601180

(CHEMBL5170002)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)CCC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601178
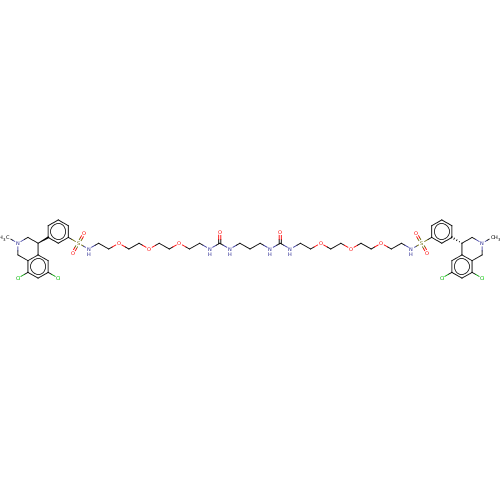
(CHEMBL5202545)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(=O)NCCCNC(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM381826

(US10272079, Compound 003)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)Nc2ccc(NC(=O)NCCOCCOCCNS(=O)(=O)c3cccc(c3)[C@@H]3CN(C)Cc4c(Cl)cc(Cl)cc34)cc2)c2cc(Cl)cc(Cl)c2C1 |r| Show InChI InChI=1S/C52H62Cl4N8O10S2/c1-63-31-45(43-27-37(53)29-49(55)47(43)33-63)35-5-3-7-41(25-35)75(67,68)59-15-19-73-23-21-71-17-13-57-51(65)61-39-9-11-40(12-10-39)62-52(66)58-14-18-72-22-24-74-20-16-60-76(69,70)42-8-4-6-36(26-42)46-32-64(2)34-48-44(46)28-38(54)30-50(48)56/h3-12,25-30,45-46,59-60H,13-24,31-34H2,1-2H3,(H2,57,61,65)(H2,58,62,66)/t45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM426615
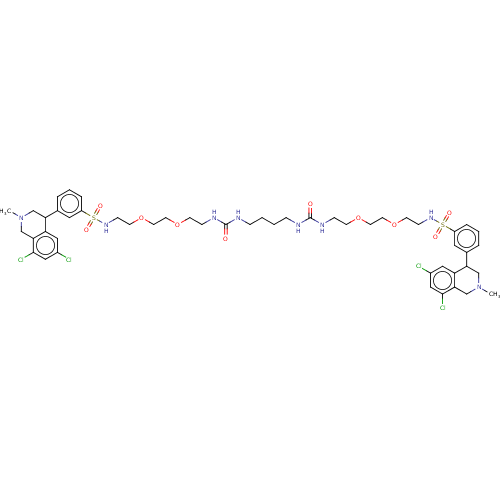
(N,N′-(10,17-dioxo-3,6,21,24-tetraoxa-9,11,16...)Show SMILES CN1CC(c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)NCCCCNC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)C2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 Show InChI InChI=1S/C50H66Cl4N8O10S2/c1-61-31-43(41-27-37(51)29-47(53)45(41)33-61)35-7-5-9-39(25-35)73(65,66)59-15-19-71-23-21-69-17-13-57-49(63)55-11-3-4-12-56-50(64)58-14-18-70-22-24-72-20-16-60-74(67,68)40-10-6-8-36(26-40)44-32-62(2)34-46-42(44)28-38(52)30-48(46)54/h5-10,25-30,43-44,59-60H,3-4,11-24,31-34H2,1-2H3,(H2,55,57,63)(H2,56,58,64) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601172

(CHEMBL5183104)Show SMILES CN1CC(c2ccc(cc2)S(=O)(=O)NCCOCCOCCNC(O)C(O)C(=O)NCCOCCOCCNS(=O)(=O)c2ccc(cc2)C2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM50601178

(CHEMBL5202545)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(=O)NCCCNC(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601181
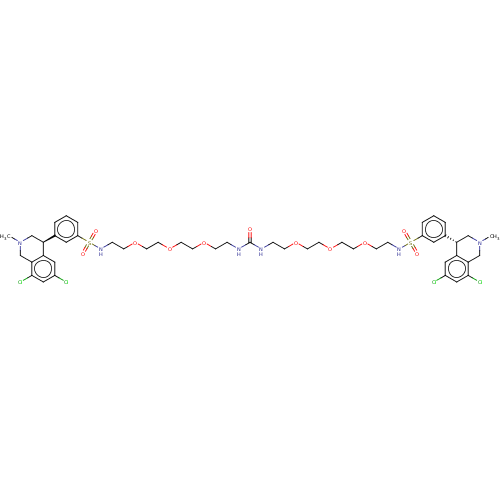
(CHEMBL5170188)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601179
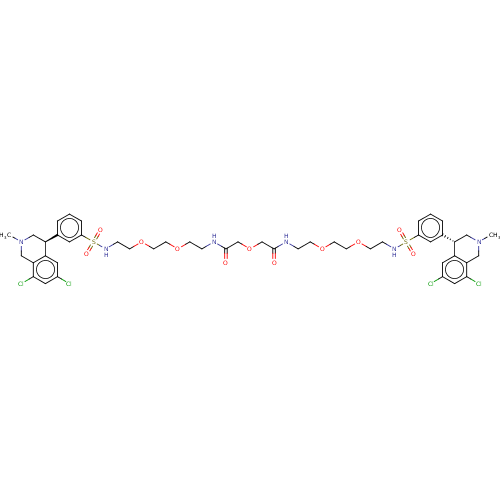
(CHEMBL5202068)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)COCC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM426616

(N,N′-(2,2′-(2,2′-(2,2′-(1,...)Show SMILES CN1CC(c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)Nc2ccc(NC(=O)NCCOCCOCCNS(=O)(=O)c3cccc(c3)C3CN(C)Cc4c(Cl)cc(Cl)cc34)cc2)c2cc(Cl)cc(Cl)c2C1 Show InChI InChI=1S/C52H62Cl4N8O10S2/c1-63-31-45(43-27-37(53)29-49(55)47(43)33-63)35-5-3-7-41(25-35)75(67,68)59-15-19-73-23-21-71-17-13-57-51(65)61-39-9-11-40(12-10-39)62-52(66)58-14-18-72-22-24-74-20-16-60-76(69,70)42-8-4-6-36(26-42)46-32-64(2)34-48-44(46)28-38(54)30-50(48)56/h3-12,25-30,45-46,59-60H,13-24,31-34H2,1-2H3,(H2,57,61,65)(H2,58,62,66) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM50601179

(CHEMBL5202068)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)COCC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM50601180

(CHEMBL5170002)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)CCC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM426507
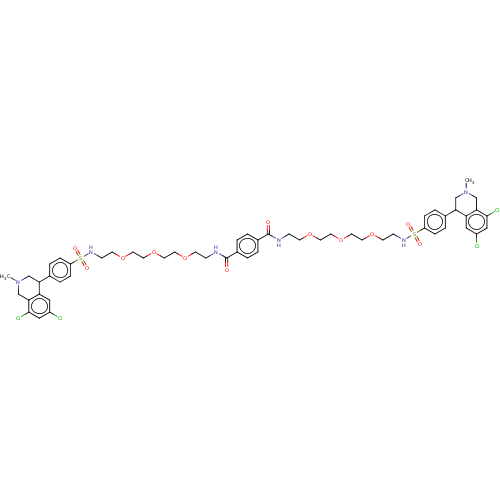
(N1,N4-bis(2-(2-(2-(2-(4-(6,8-dichloro-2-methyl-1,2...)Show SMILES CN1CC(c2ccc(cc2)S(=O)(=O)NCCOCCOCCOCCNC(=O)c2ccc(cc2)C(=O)NCCOCCOCCOCCNS(=O)(=O)c2ccc(cc2)C2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 Show InChI InChI=1S/C56H68Cl4N6O12S2/c1-65-35-49(47-31-43(57)33-53(59)51(47)37-65)39-7-11-45(12-8-39)79(69,70)63-17-21-75-25-29-77-27-23-73-19-15-61-55(67)41-3-5-42(6-4-41)56(68)62-16-20-74-24-28-78-30-26-76-22-18-64-80(71,72)46-13-9-40(10-14-46)50-36-66(2)38-52-48(50)32-44(58)34-54(52)60/h3-14,31-34,49-50,63-64H,15-30,35-38H2,1-2H3,(H,61,67)(H,62,68) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM50601181

(CHEMBL5170188)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50543044
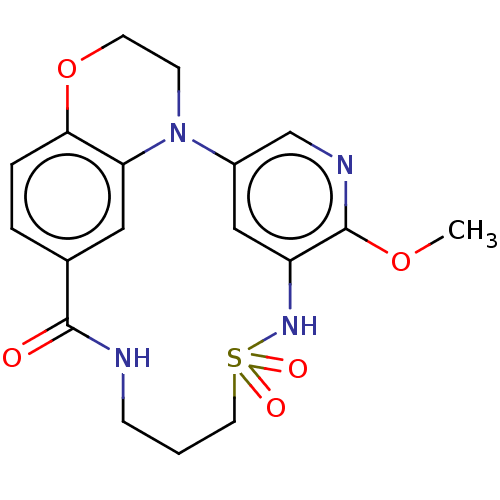
(CHEMBL4636926)Show SMILES COc1ncc2cc1NS(=O)(=O)CCCNC(=O)c1ccc3OCCN2c3c1 Show InChI InChI=1S/C18H20N4O5S/c1-26-18-14-10-13(11-20-18)22-6-7-27-16-4-3-12(9-15(16)22)17(23)19-5-2-8-28(24,25)21-14/h3-4,9-11,21H,2,5-8H2,1H3,(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
ACS Med Chem Lett 11: 1386-1391 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00061
BindingDB Entry DOI: 10.7270/Q2988BKZ |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM381823

(CVD-0019453 | US10272079, Compound 002 | US1027207...)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)NCCCCNC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| Show InChI InChI=1S/C50H66Cl4N8O10S2/c1-61-31-43(41-27-37(51)29-47(53)45(41)33-61)35-7-5-9-39(25-35)73(65,66)59-15-19-71-23-21-69-17-13-57-49(63)55-11-3-4-12-56-50(64)58-14-18-70-22-24-72-20-16-60-74(67,68)40-10-6-8-36(26-40)44-32-62(2)34-46-42(44)28-38(52)30-48(46)54/h5-10,25-30,43-44,59-60H,3-4,11-24,31-34H2,1-2H3,(H2,55,57,63)(H2,56,58,64)/t43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50543037
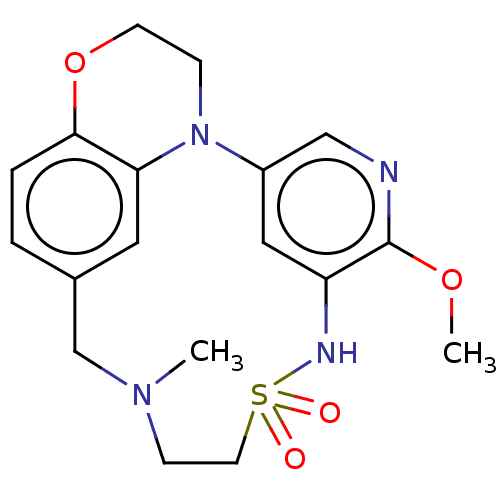
(CHEMBL4638471)Show SMILES COc1ncc2cc1NS(=O)(=O)CCN(C)Cc1ccc3OCCN2c3c1 Show InChI InChI=1S/C18H22N4O4S/c1-21-6-8-27(23,24)20-15-10-14(11-19-18(15)25-2)22-5-7-26-17-4-3-13(12-21)9-16(17)22/h3-4,9-11,20H,5-8,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
ACS Med Chem Lett 11: 1386-1391 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00061
BindingDB Entry DOI: 10.7270/Q2988BKZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM426610
(N1,N4-bis(2-(2-(2-(2-(3-(6,8-dichloro-2-methyl-1,2...)Show SMILES CN1CC(c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(=O)c2ccc(cc2)C(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)C2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 Show InChI InChI=1S/C56H68Cl4N6O12S2/c1-65-35-49(47-31-43(57)33-53(59)51(47)37-65)41-5-3-7-45(29-41)79(69,70)63-15-19-75-23-27-77-25-21-73-17-13-61-55(67)39-9-11-40(12-10-39)56(68)62-14-18-74-22-26-78-28-24-76-20-16-64-80(71,72)46-8-4-6-42(30-46)50-36-66(2)38-52-48(50)32-44(58)34-54(52)60/h3-12,29-34,49-50,63-64H,13-28,35-38H2,1-2H3,(H,61,67)(H,62,68) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM381824
(US10272079, Compound 001)Show SMILES CN1C[C@@H](c2cccc(NC(=O)[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO)c2)c2cc(Cl)cc(Cl)c2C1 |r| Show InChI InChI=1S/C22H26Cl2N2O6/c1-26-8-15(14-6-12(23)7-17(24)16(14)9-26)11-3-2-4-13(5-11)25-22(32)21(31)20(30)19(29)18(28)10-27/h2-7,15,18-21,27-31H,8-10H2,1H3,(H,25,32)/t15-,18+,19+,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM50601167
(CHEMBL5200502)Show SMILES CN1CC(c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(O)C(O)C(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)C2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data