 Found 394 hits with Last Name = 'staufenbiel' and Initial = 'm'
Found 394 hits with Last Name = 'staufenbiel' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin E
(Homo sapiens (Human)) | BDBM50305527
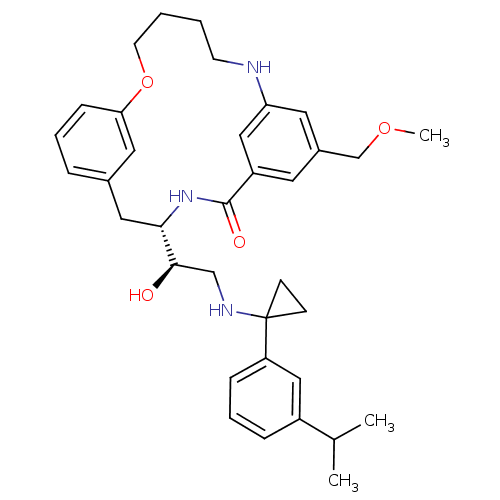
((4S)-4-[(1R)-1-hydroxy-2-({1-[3-(1-methylethyl)phe...)Show SMILES COCc1cc2NCCCCOc3cccc(C[C@H](NC(=O)c(c1)c2)[C@H](O)CNC1(CC1)c1cccc(c1)C(C)C)c3 |r| Show InChI InChI=1S/C35H45N3O4/c1-24(2)27-9-7-10-29(20-27)35(12-13-35)37-22-33(39)32-19-25-8-6-11-31(18-25)42-15-5-4-14-36-30-17-26(23-41-3)16-28(21-30)34(40)38-32/h6-11,16-18,20-21,24,32-33,36-37,39H,4-5,12-15,19,22-23H2,1-3H3,(H,38,40)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50305544
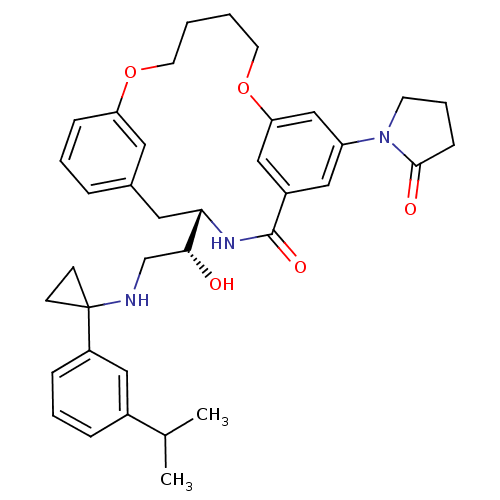
((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...)Show SMILES CC(C)c1cccc(c1)C1(CC1)NC[C@@H](O)[C@@H]1Cc2cccc(OCCCCOc3cc(cc(c3)C(=O)N1)N1CCCC1=O)c2 |r| Show InChI InChI=1S/C37H45N3O5/c1-25(2)27-9-6-10-29(20-27)37(13-14-37)38-24-34(41)33-19-26-8-5-11-31(18-26)44-16-3-4-17-45-32-22-28(36(43)39-33)21-30(23-32)40-15-7-12-35(40)42/h5-6,8-11,18,20-23,25,33-34,38,41H,3-4,7,12-17,19,24H2,1-2H3,(H,39,43)/t33-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 expressed in Escherichia coli |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50294218
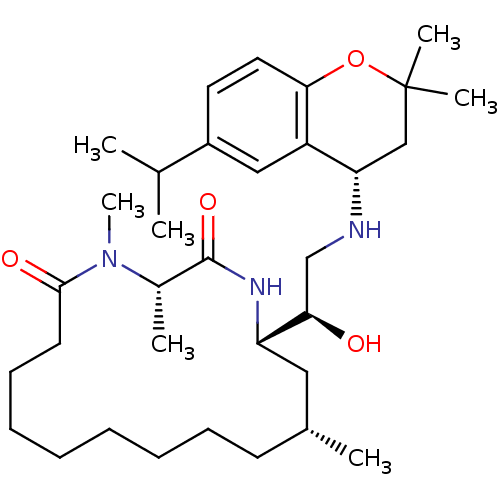
((3S,14R,16S)-16-((R)-1-hydroxy-2-((S)-6-isopropyl-...)Show SMILES CC(C)c1ccc2OC(C)(C)C[C@H](NC[C@@H](O)[C@@H]3C[C@H](C)CCCCCCCCC(=O)N(C)[C@@H](C)C(=O)N3)c2c1 |r| Show InChI InChI=1S/C33H55N3O4/c1-22(2)25-16-17-30-26(19-25)28(20-33(5,6)40-30)34-21-29(37)27-18-23(3)14-12-10-8-9-11-13-15-31(38)36(7)24(4)32(39)35-27/h16-17,19,22-24,27-29,34,37H,8-15,18,20-21H2,1-7H3,(H,35,39)/t23-,24+,27+,28+,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 19: 1366-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.055
BindingDB Entry DOI: 10.7270/Q2SB45S3 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50305542
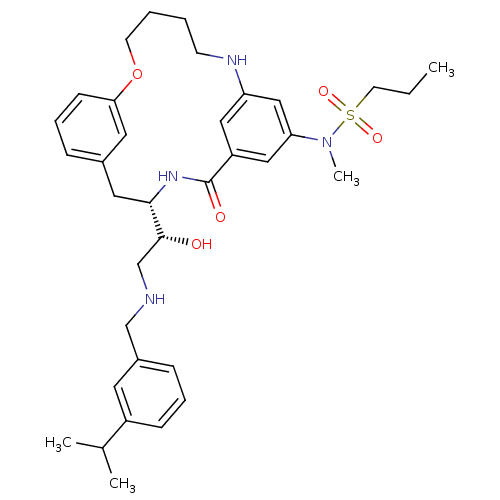
(CHEMBL595016 | Propane-1-sulfonic acid{(S)-4-[(R)-...)Show SMILES CCCS(=O)(=O)N(C)c1cc2NCCCCOc3cccc(C[C@H](NC(=O)c(c2)c1)[C@H](O)CNCc1cccc(c1)C(C)C)c3 |r| Show InChI InChI=1S/C35H48N4O5S/c1-5-16-45(42,43)39(4)31-21-29-20-30(22-31)37-14-6-7-15-44-32-13-9-10-26(18-32)19-33(38-35(29)41)34(40)24-36-23-27-11-8-12-28(17-27)25(2)3/h8-13,17-18,20-22,25,33-34,36-37,40H,5-7,14-16,19,23-24H2,1-4H3,(H,38,41)/t33-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 expressed in Escherichia coli |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50305527

((4S)-4-[(1R)-1-hydroxy-2-({1-[3-(1-methylethyl)phe...)Show SMILES COCc1cc2NCCCCOc3cccc(C[C@H](NC(=O)c(c1)c2)[C@H](O)CNC1(CC1)c1cccc(c1)C(C)C)c3 |r| Show InChI InChI=1S/C35H45N3O4/c1-24(2)27-9-7-10-29(20-27)35(12-13-35)37-22-33(39)32-19-25-8-6-11-31(18-25)42-15-5-4-14-36-30-17-26(23-41-3)16-28(21-30)34(40)38-32/h6-11,16-18,20-21,24,32-33,36-37,39H,4-5,12-15,19,22-23H2,1-3H3,(H,38,40)/t32-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50587074
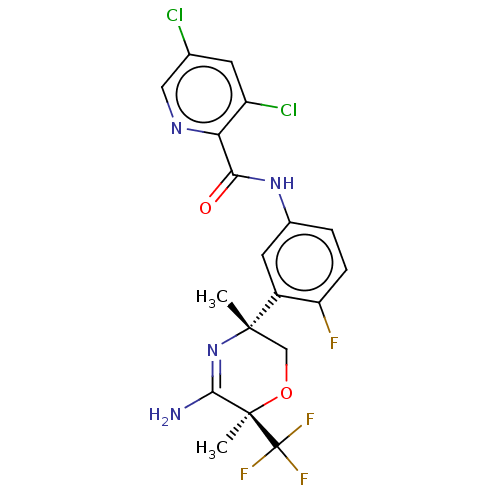
(CHEMBL5085959)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(Cl)cc2Cl)ccc1F |r,c:7| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16047

((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H64N8O14/c1-20(2)16-27(46-39(60)28(19-31(43)51)47-40(61)34(21(3)4)49-37(58)25(42)12-14-32(52)53)30(50)17-22(5)35(56)44-23(6)36(57)45-26(13-15-33(54)55)38(59)48-29(41(62)63)18-24-10-8-7-9-11-24/h7-11,20-23,25-30,34,50H,12-19,42H2,1-6H3,(H2,43,51)(H,44,56)(H,45,57)(H,46,60)(H,47,61)(H,48,59)(H,49,58)(H,52,53)(H,54,55)(H,62,63)/t22-,23+,25+,26+,27+,28+,29+,30+,34+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386481
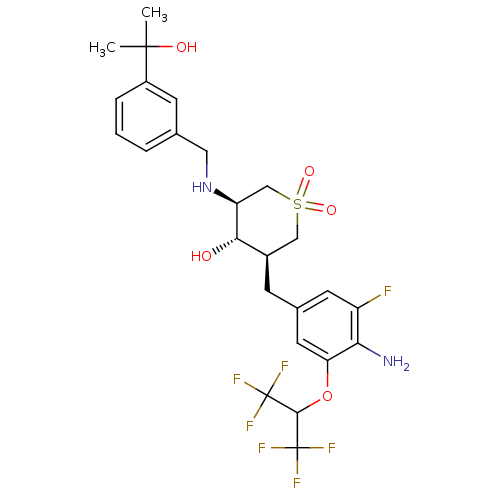
(CHEMBL2048058)Show SMILES CC(C)(O)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OC(C(F)(F)F)C(F)(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C25H29F7N2O5S/c1-23(2,36)16-5-3-4-13(7-16)10-34-18-12-40(37,38)11-15(21(18)35)6-14-8-17(26)20(33)19(9-14)39-22(24(27,28)29)25(30,31)32/h3-5,7-9,15,18,21-22,34-36H,6,10-12,33H2,1-2H3/t15-,18+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386482
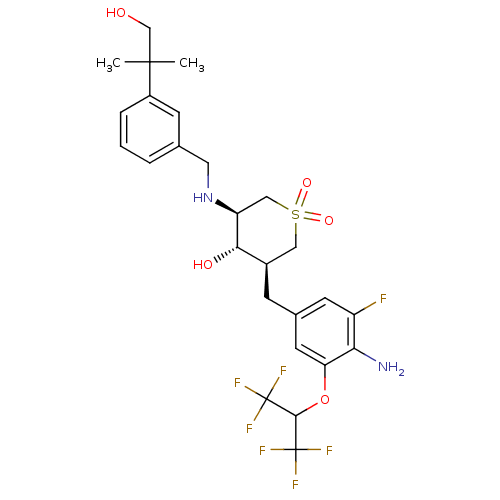
(CHEMBL2048059)Show SMILES CC(C)(CO)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OC(C(F)(F)F)C(F)(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C26H31F7N2O5S/c1-24(2,13-36)17-5-3-4-14(7-17)10-35-19-12-41(38,39)11-16(22(19)37)6-15-8-18(27)21(34)20(9-15)40-23(25(28,29)30)26(31,32)33/h3-5,7-9,16,19,22-23,35-37H,6,10-13,34H2,1-2H3/t16-,19+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386515
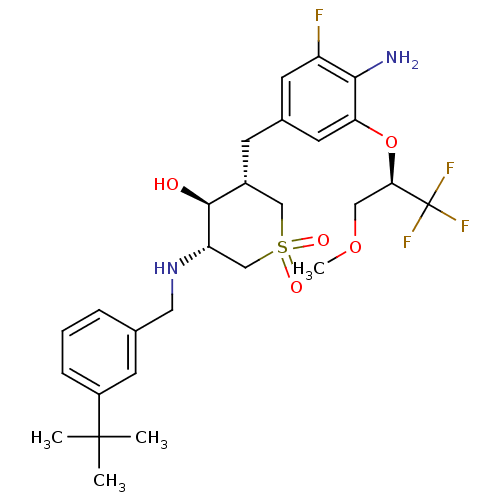
(CHEMBL2048053)Show SMILES COC[C@@H](Oc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1N)C(F)(F)F |r| Show InChI InChI=1S/C27H36F4N2O5S/c1-26(2,3)19-7-5-6-16(9-19)12-33-21-15-39(35,36)14-18(25(21)34)8-17-10-20(28)24(32)22(11-17)38-23(13-37-4)27(29,30)31/h5-7,9-11,18,21,23,25,33-34H,8,12-15,32H2,1-4H3/t18-,21+,23-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386513
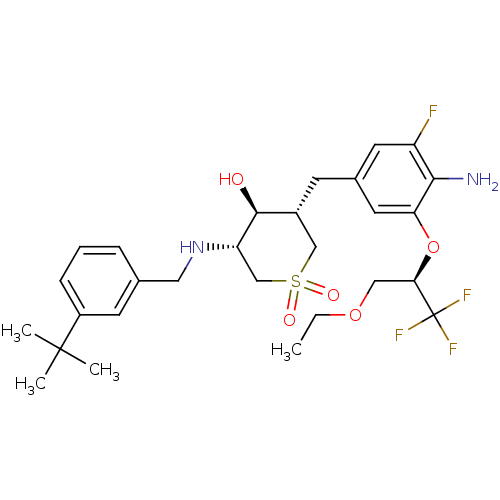
(CHEMBL2048051)Show SMILES CCOC[C@@H](Oc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1N)C(F)(F)F |r| Show InChI InChI=1S/C28H38F4N2O5S/c1-5-38-14-24(28(30,31)32)39-23-12-18(11-21(29)25(23)33)9-19-15-40(36,37)16-22(26(19)35)34-13-17-7-6-8-20(10-17)27(2,3)4/h6-8,10-12,19,22,24,26,34-35H,5,9,13-16,33H2,1-4H3/t19-,22+,24-,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386487
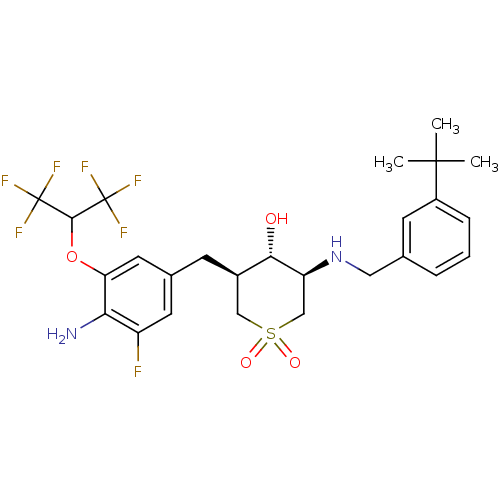
(CHEMBL2048047)Show SMILES CC(C)(C)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OC(C(F)(F)F)C(F)(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C26H31F7N2O4S/c1-24(2,3)17-6-4-5-14(8-17)11-35-19-13-40(37,38)12-16(22(19)36)7-15-9-18(27)21(34)20(10-15)39-23(25(28,29)30)26(31,32)33/h4-6,8-10,16,19,22-23,35-36H,7,11-13,34H2,1-3H3/t16-,19+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50305531
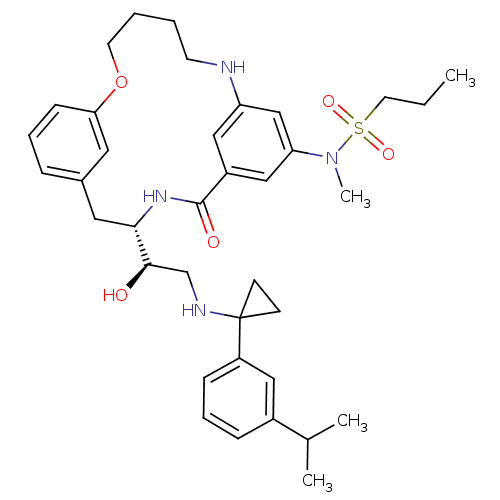
(CHEMBL595136 | Propane-1-sulfonic acid((S)-4-{(R)-...)Show SMILES CCCS(=O)(=O)N(C)c1cc2NCCCCOc3cccc(C[C@H](NC(=O)c(c2)c1)[C@H](O)CNC1(CC1)c1cccc(c1)C(C)C)c3 |r| Show InChI InChI=1S/C37H50N4O5S/c1-5-18-47(44,45)41(4)32-23-29-22-31(24-32)38-16-6-7-17-46-33-13-8-10-27(19-33)20-34(40-36(29)43)35(42)25-39-37(14-15-37)30-12-9-11-28(21-30)26(2)3/h8-13,19,21-24,26,34-35,38-39,42H,5-7,14-18,20,25H2,1-4H3,(H,40,43)/t34-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 expressed in Escherichia coli |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50294218

((3S,14R,16S)-16-((R)-1-hydroxy-2-((S)-6-isopropyl-...)Show SMILES CC(C)c1ccc2OC(C)(C)C[C@H](NC[C@@H](O)[C@@H]3C[C@H](C)CCCCCCCCC(=O)N(C)[C@@H](C)C(=O)N3)c2c1 |r| Show InChI InChI=1S/C33H55N3O4/c1-22(2)25-16-17-30-26(19-25)28(20-33(5,6)40-30)34-21-29(37)27-18-23(3)14-12-10-8-9-11-13-15-31(38)36(7)24(4)32(39)35-27/h16-17,19,22-24,27-29,34,37H,8-15,18,20-21H2,1-7H3,(H,35,39)/t23-,24+,27+,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 19: 1366-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.055
BindingDB Entry DOI: 10.7270/Q2SB45S3 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM134401
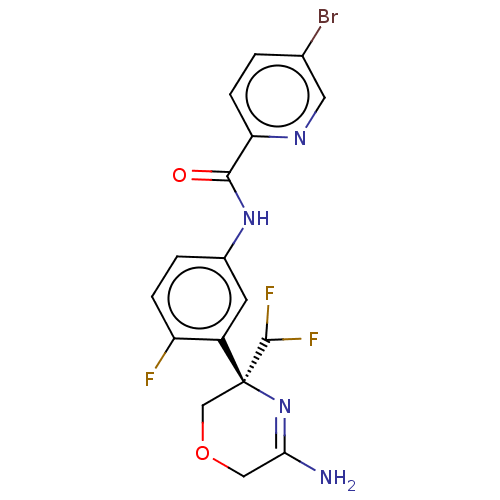
(US8846658, 118)Show SMILES NC1=N[C@](COC1)(C(F)F)c1cc(NC(=O)c2ccc(Br)cn2)ccc1F |r,t:1| Show InChI InChI=1S/C17H14BrF3N4O2/c18-9-1-4-13(23-6-9)15(26)24-10-2-3-12(19)11(5-10)17(16(20)21)8-27-7-14(22)25-17/h1-6,16H,7-8H2,(H2,22,25)(H,24,26)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... |
Bioorg Med Chem Lett 28: 2195-2200 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.003
BindingDB Entry DOI: 10.7270/Q26T0Q5P |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386512

(CHEMBL2048050)Show SMILES C[C@@H](Oc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1N)C(F)(F)F |r| Show InChI InChI=1S/C26H34F4N2O4S/c1-15(26(28,29)30)36-22-11-17(10-20(27)23(22)31)8-18-13-37(34,35)14-21(24(18)33)32-12-16-6-5-7-19(9-16)25(2,3)4/h5-7,9-11,15,18,21,24,32-33H,8,12-14,31H2,1-4H3/t15-,18-,21+,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50012653

(CHEMBL3261067)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C21H19F4N5O2/c1-11-6-12(8-26)9-28-16(11)17(31)29-13-4-5-15(22)14(7-13)19(2)10-32-20(3,18(27)30-19)21(23,24)25/h4-7,9H,10H2,1-3H3,(H2,27,30)(H,29,31)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type human APP expressed in CHO cells assessed as reduction in amyloid beta 40 secretion incubated for 24 hrs by immunoassay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50012653

(CHEMBL3261067)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C21H19F4N5O2/c1-11-6-12(8-26)9-28-16(11)17(31)29-13-4-5-15(22)14(7-13)19(2)10-32-20(3,18(27)30-19)21(23,24)25/h4-7,9H,10H2,1-3H3,(H2,27,30)(H,29,31)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type human APP expressed in CHO cells assessed as reduction in amyloid beta 42 secretion incubated for 24 hrs by immunoassay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50012653

(CHEMBL3261067)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C21H19F4N5O2/c1-11-6-12(8-26)9-28-16(11)17(31)29-13-4-5-15(22)14(7-13)19(2)10-32-20(3,18(27)30-19)21(23,24)25/h4-7,9H,10H2,1-3H3,(H2,27,30)(H,29,31)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM134348
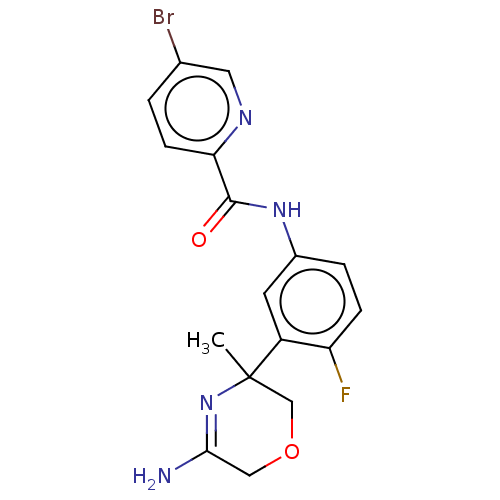
(US8846658, 60)Show SMILES CC1(COCC(N)=N1)c1cc(NC(=O)c2ccc(Br)cn2)ccc1F |c:6| Show InChI InChI=1S/C17H16BrFN4O2/c1-17(9-25-8-15(20)23-17)12-6-11(3-4-13(12)19)22-16(24)14-5-2-10(18)7-21-14/h2-7H,8-9H2,1H3,(H2,20,23)(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50273411
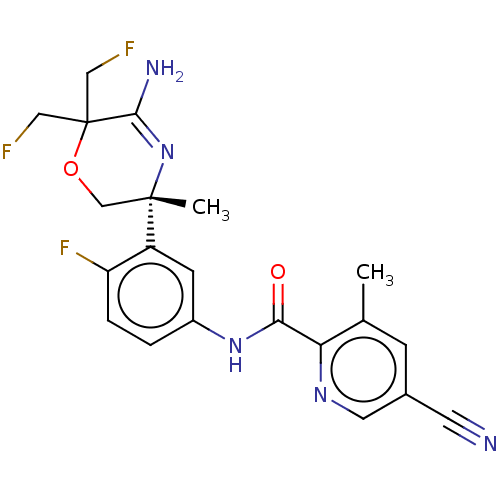
(CHEMBL4127572)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)COC(CF)(CF)C(N)=N1)C#N |r,c:29| Show InChI InChI=1S/C21H20F3N5O2/c1-12-5-13(7-25)8-27-17(12)18(30)28-14-3-4-16(24)15(6-14)20(2)11-31-21(9-22,10-23)19(26)29-20/h3-6,8H,9-11H2,1-2H3,(H2,26,29)(H,28,30)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... |
Bioorg Med Chem Lett 28: 2195-2200 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.003
BindingDB Entry DOI: 10.7270/Q26T0Q5P |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM134359
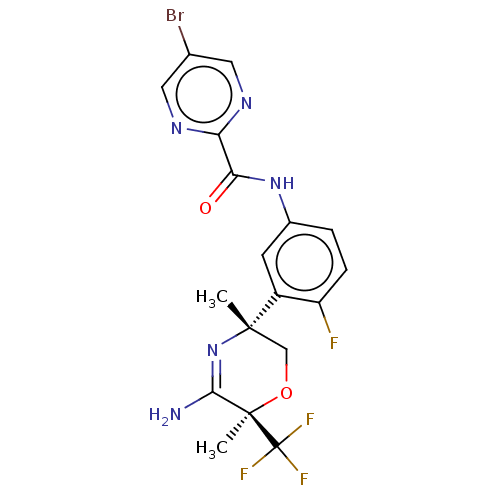
(US8846658, 71)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(Br)cn2)ccc1F |r,c:7| Show InChI InChI=1S/C18H16BrF4N5O2/c1-16(8-30-17(2,15(24)28-16)18(21,22)23)11-5-10(3-4-12(11)20)27-14(29)13-25-6-9(19)7-26-13/h3-7H,8H2,1-2H3,(H2,24,28)(H,27,29)/t16-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM134356
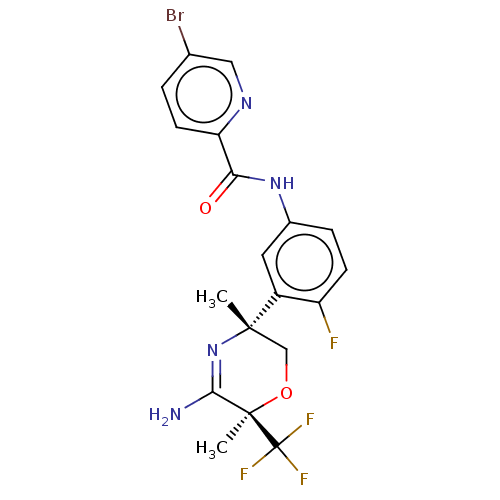
(US8846658, 68)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ccc(Br)cn2)ccc1F |r,c:7| Show InChI InChI=1S/C19H17BrF4N4O2/c1-17(9-30-18(2,16(25)28-17)19(22,23)24)12-7-11(4-5-13(12)21)27-15(29)14-6-3-10(20)8-26-14/h3-8H,9H2,1-2H3,(H2,25,28)(H,27,29)/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50305536
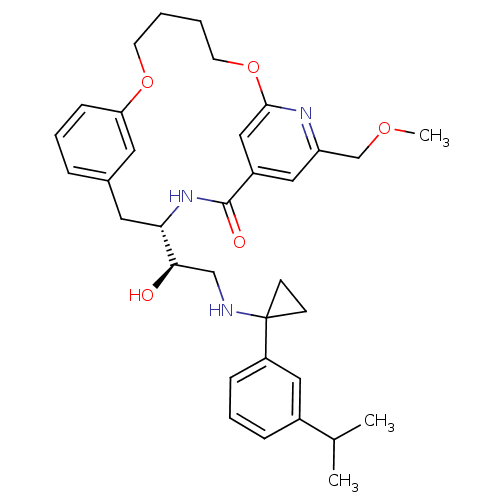
((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...)Show SMILES COCc1cc2cc(OCCCCOc3cccc(C[C@H](NC2=O)[C@H](O)CNC2(CC2)c2cccc(c2)C(C)C)c3)n1 |r| Show InChI InChI=1S/C34H43N3O5/c1-23(2)25-9-7-10-27(18-25)34(12-13-34)35-21-31(38)30-17-24-8-6-11-29(16-24)41-14-4-5-15-42-32-20-26(33(39)37-30)19-28(36-32)22-40-3/h6-11,16,18-20,23,30-31,35,38H,4-5,12-15,17,21-22H2,1-3H3,(H,37,39)/t30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50587069
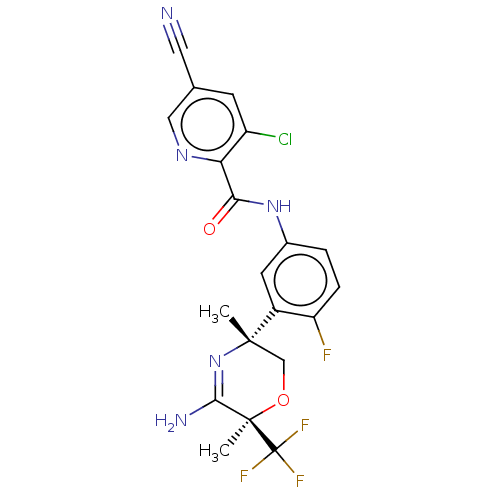
(CHEMBL5073160)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(cc2Cl)C#N)ccc1F |r,c:7| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50587070
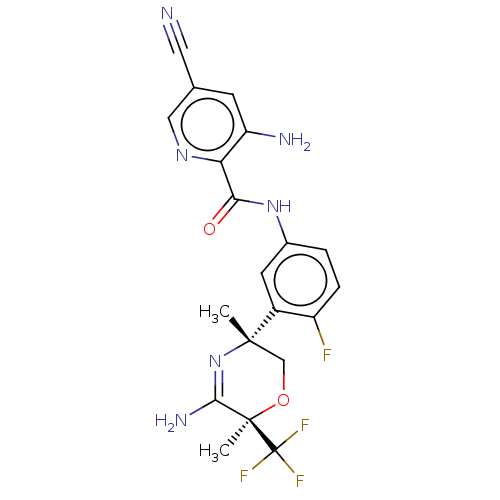
(CHEMBL5090563)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(cc2N)C#N)ccc1F |r,c:7| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50305537
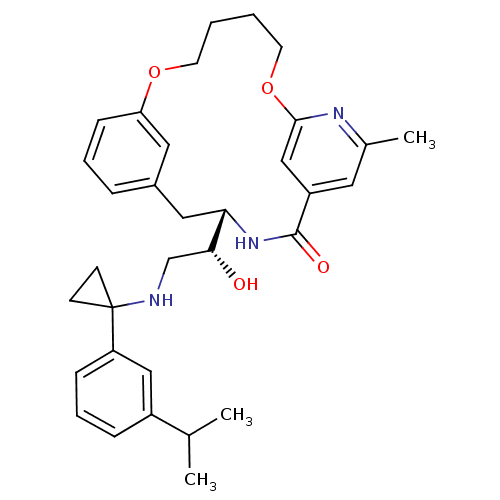
((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...)Show SMILES CC(C)c1cccc(c1)C1(CC1)NC[C@@H](O)[C@@H]1Cc2cccc(OCCCCOc3cc(cc(C)n3)C(=O)N1)c2 |r| Show InChI InChI=1S/C33H41N3O4/c1-22(2)25-9-7-10-27(19-25)33(12-13-33)34-21-30(37)29-18-24-8-6-11-28(17-24)39-14-4-5-15-40-31-20-26(32(38)36-29)16-23(3)35-31/h6-11,16-17,19-20,22,29-30,34,37H,4-5,12-15,18,21H2,1-3H3,(H,36,38)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50273406
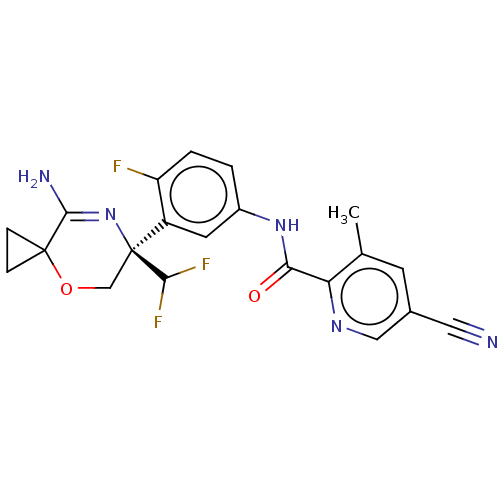
(CHEMBL4128406)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(c1)[C@@]1(COC2(CC2)C(N)=N1)C(F)F)C#N |r,c:27| Show InChI InChI=1S/C21H18F3N5O2/c1-11-6-12(8-25)9-27-16(11)17(30)28-13-2-3-15(22)14(7-13)21(18(23)24)10-31-20(4-5-20)19(26)29-21/h2-3,6-7,9,18H,4-5,10H2,1H3,(H2,26,29)(H,28,30)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... |
Bioorg Med Chem Lett 28: 2195-2200 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.003
BindingDB Entry DOI: 10.7270/Q26T0Q5P |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386516
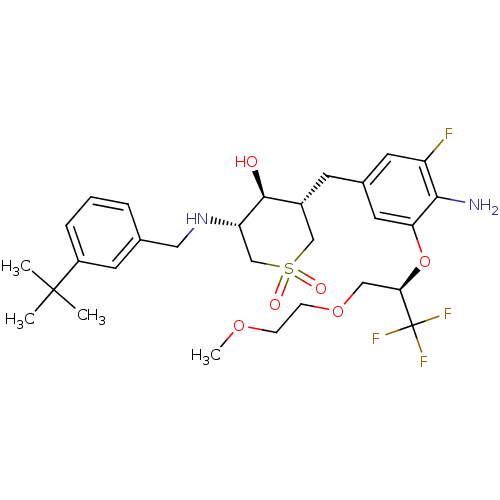
(CHEMBL2048054)Show SMILES COCCOC[C@@H](Oc1cc(C[C@@H]2CS(=O)(=O)C[C@H](NCc3cccc(c3)C(C)(C)C)[C@H]2O)cc(F)c1N)C(F)(F)F |r| Show InChI InChI=1S/C29H40F4N2O6S/c1-28(2,3)21-7-5-6-18(11-21)14-35-23-17-42(37,38)16-20(27(23)36)10-19-12-22(30)26(34)24(13-19)41-25(29(31,32)33)15-40-9-8-39-4/h5-7,11-13,20,23,25,27,35-36H,8-10,14-17,34H2,1-4H3/t20-,23+,25-,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50012653

(CHEMBL3261067)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C21H19F4N5O2/c1-11-6-12(8-26)9-28-16(11)17(31)29-13-4-5-15(22)14(7-13)19(2)10-32-20(3,18(27)30-19)21(23,24)25/h4-7,9H,10H2,1-3H3,(H2,27,30)(H,29,31)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type human APP expressed in CHO cells assessed as reduction in soluble APP beta level incubated for 24 hrs by immunoassay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM134358
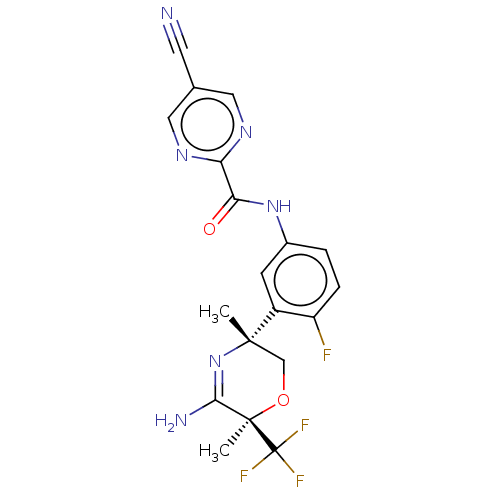
(US8846658, 70)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(cn2)C#N)ccc1F |r,c:7| Show InChI InChI=1S/C19H16F4N6O2/c1-17(9-31-18(2,16(25)29-17)19(21,22)23)12-5-11(3-4-13(12)20)28-15(30)14-26-7-10(6-24)8-27-14/h3-5,7-8H,9H2,1-2H3,(H2,25,29)(H,28,30)/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50587067
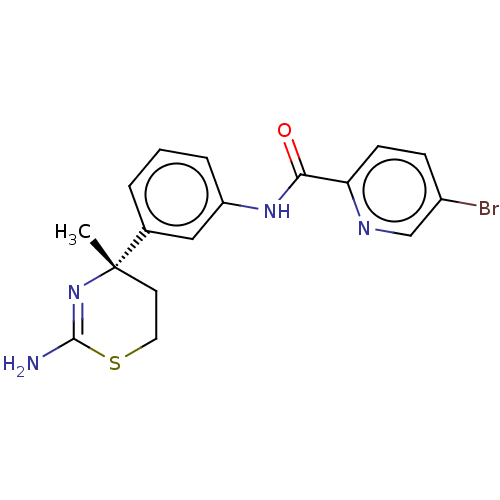
(CHEMBL5093426)Show SMILES C[C@]1(CCSC(N)=N1)c1cccc(NC(=O)c2ccc(Br)cn2)c1 |r,c:6| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APP751 (unknown origin) expressed in CHO cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50273421
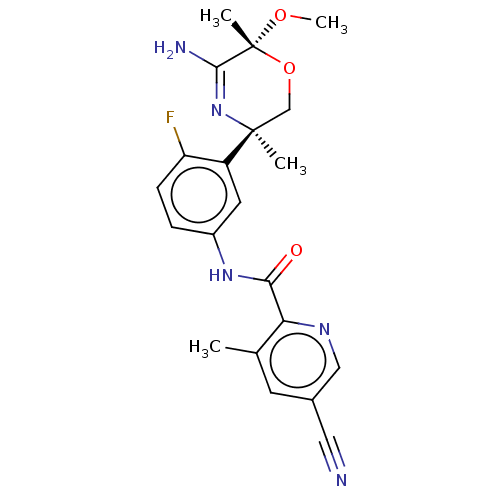
(CHEMBL4126351)Show SMILES CO[C@]1(C)OC[C@](C)(N=C1N)c1cc(NC(=O)c2ncc(cc2C)C#N)ccc1F |r,c:8| Show InChI InChI=1S/C21H22FN5O3/c1-12-7-13(9-23)10-25-17(12)18(28)26-14-5-6-16(22)15(8-14)20(2)11-30-21(3,29-4)19(24)27-20/h5-8,10H,11H2,1-4H3,(H2,24,27)(H,26,28)/t20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... |
Bioorg Med Chem Lett 28: 2195-2200 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.003
BindingDB Entry DOI: 10.7270/Q26T0Q5P |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM134322
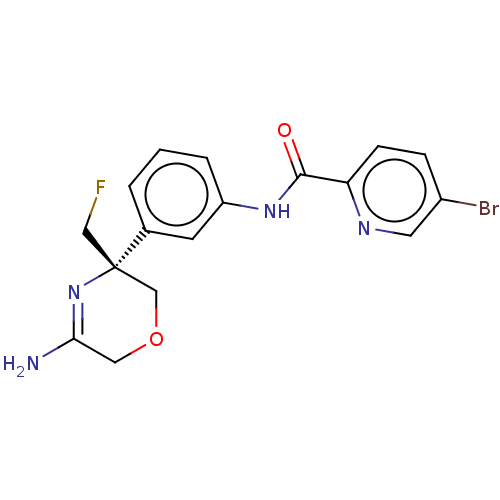
(US8846658, 33)Show SMILES NC1=N[C@@](CF)(COC1)c1cccc(NC(=O)c2ccc(Br)cn2)c1 |r,t:1| Show InChI InChI=1S/C17H16BrFN4O2/c18-12-4-5-14(21-7-12)16(24)22-13-3-1-2-11(6-13)17(9-19)10-25-8-15(20)23-17/h1-7H,8-10H2,(H2,20,23)(H,22,24)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... |
Bioorg Med Chem Lett 28: 2195-2200 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.003
BindingDB Entry DOI: 10.7270/Q26T0Q5P |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50587071
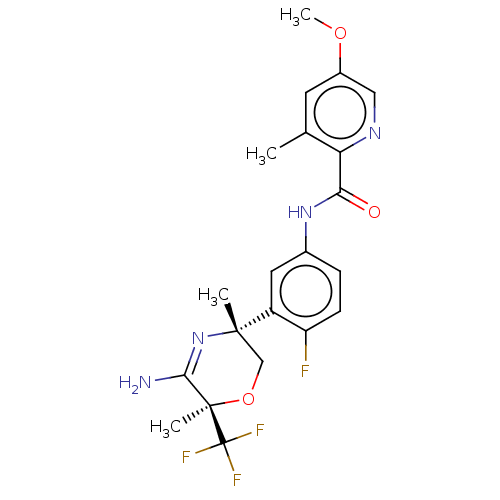
(CHEMBL5075177)Show SMILES COc1cnc(C(=O)Nc2ccc(F)c(c2)[C@]2(C)CO[C@](C)(C(N)=N2)C(F)(F)F)c(C)c1 |r,c:24| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50587069

(CHEMBL5073160)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(cc2Cl)C#N)ccc1F |r,c:7| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50587070

(CHEMBL5090563)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(cc2N)C#N)ccc1F |r,c:7| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM134359

(US8846658, 71)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(Br)cn2)ccc1F |r,c:7| Show InChI InChI=1S/C18H16BrF4N5O2/c1-16(8-30-17(2,15(24)28-16)18(21,22)23)11-5-10(3-4-12(11)20)27-14(29)13-25-6-9(19)7-26-13/h3-7H,8H2,1-2H3,(H2,24,28)(H,27,29)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50305536

((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...)Show SMILES COCc1cc2cc(OCCCCOc3cccc(C[C@H](NC2=O)[C@H](O)CNC2(CC2)c2cccc(c2)C(C)C)c3)n1 |r| Show InChI InChI=1S/C34H43N3O5/c1-23(2)25-9-7-10-27(18-25)34(12-13-34)35-21-31(38)30-17-24-8-6-11-29(16-24)41-14-4-5-15-42-32-20-26(33(39)37-30)19-28(36-32)22-40-3/h6-11,16,18-20,23,30-31,35,38H,4-5,12-15,17,21-22H2,1-3H3,(H,37,39)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM134365
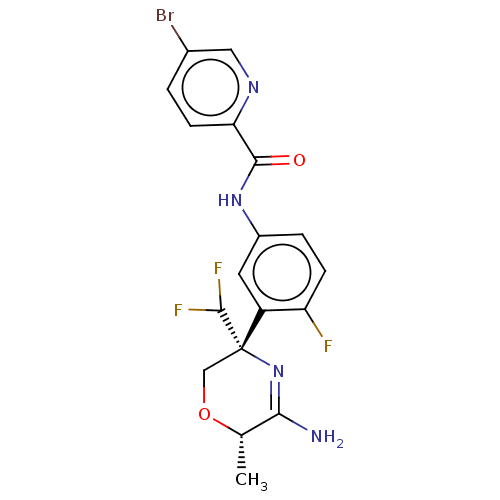
(US8846658, 78)Show SMILES C[C@@H]1OC[C@@](N=C1N)(C(F)F)c1cc(NC(=O)c2ccc(Br)cn2)ccc1F |r,c:5| Show InChI InChI=1S/C18H16BrF3N4O2/c1-9-15(23)26-18(8-28-9,17(21)22)12-6-11(3-4-13(12)20)25-16(27)14-5-2-10(19)7-24-14/h2-7,9,17H,8H2,1H3,(H2,23,26)(H,25,27)/t9-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... |
Bioorg Med Chem Lett 28: 2195-2200 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.003
BindingDB Entry DOI: 10.7270/Q26T0Q5P |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50012653

(CHEMBL3261067)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C21H19F4N5O2/c1-11-6-12(8-26)9-28-16(11)17(31)29-13-4-5-15(22)14(7-13)19(2)10-32-20(3,18(27)30-19)21(23,24)25/h4-7,9H,10H2,1-3H3,(H2,27,30)(H,29,31)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE1 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50587070

(CHEMBL5090563)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(cc2N)C#N)ccc1F |r,c:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE1 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50012653

(CHEMBL3261067)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F)C#N |r,c:26| Show InChI InChI=1S/C21H19F4N5O2/c1-11-6-12(8-26)9-28-16(11)17(31)29-13-4-5-15(22)14(7-13)19(2)10-32-20(3,18(27)30-19)21(23,24)25/h4-7,9H,10H2,1-3H3,(H2,27,30)(H,29,31)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50587072
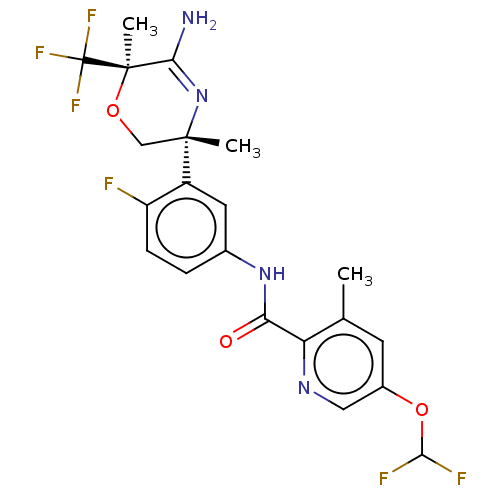
(CHEMBL5086270)Show SMILES Cc1cc(OC(F)F)cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)CO[C@](C)(C(N)=N1)C(F)(F)F |r,c:30| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM134360
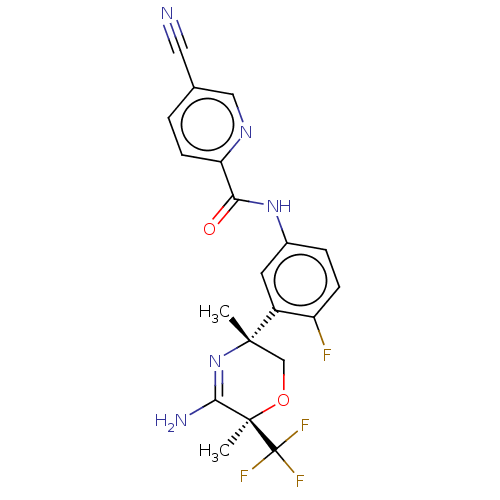
(US8846658, 73)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,c:7| Show InChI InChI=1S/C20H17F4N5O2/c1-18(10-31-19(2,17(26)29-18)20(22,23)24)13-7-12(4-5-14(13)21)28-16(30)15-6-3-11(8-25)9-27-15/h3-7,9H,10H2,1-2H3,(H2,26,29)(H,28,30)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE1 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386483
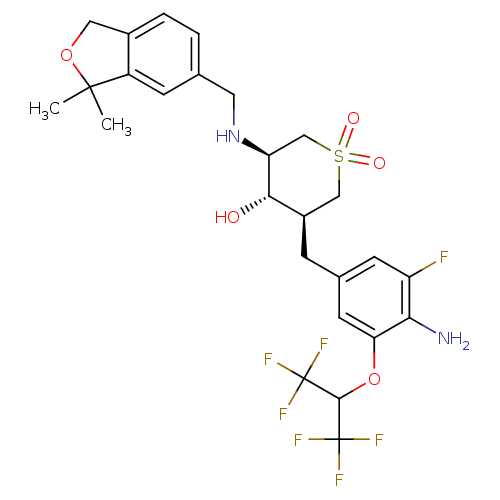
(CHEMBL2048060)Show SMILES CC1(C)OCc2ccc(CN[C@H]3CS(=O)(=O)C[C@@H](Cc4cc(F)c(N)c(OC(C(F)(F)F)C(F)(F)F)c4)[C@@H]3O)cc12 |r| Show InChI InChI=1S/C26H29F7N2O5S/c1-24(2)17-6-13(3-4-15(17)10-39-24)9-35-19-12-41(37,38)11-16(22(19)36)5-14-7-18(27)21(34)20(8-14)40-23(25(28,29)30)26(31,32)33/h3-4,6-8,16,19,22-23,35-36H,5,9-12,34H2,1-2H3/t16-,19+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386508
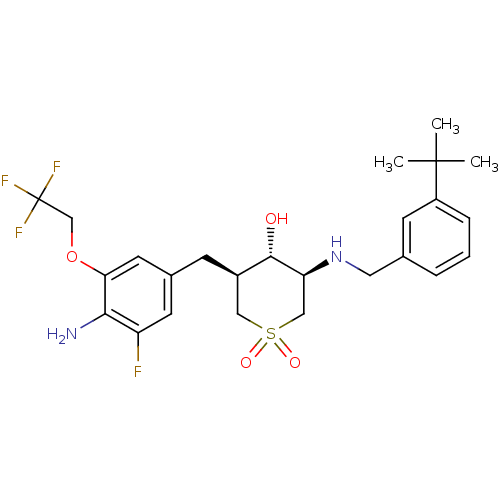
(CHEMBL2048045)Show SMILES CC(C)(C)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OCC(F)(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C25H32F4N2O4S/c1-24(2,3)18-6-4-5-15(8-18)11-31-20-13-36(33,34)12-17(23(20)32)7-16-9-19(26)22(30)21(10-16)35-14-25(27,28)29/h4-6,8-10,17,20,23,31-32H,7,11-14,30H2,1-3H3/t17-,20+,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50386507
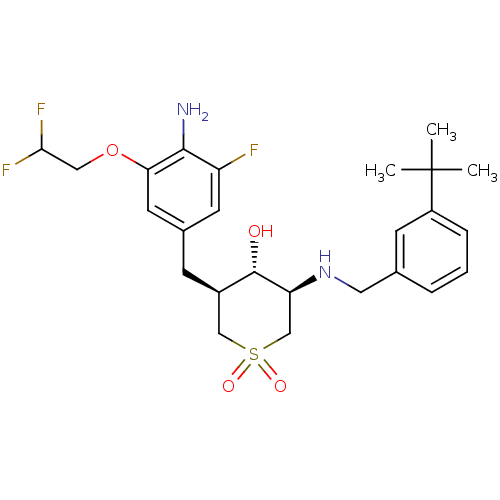
(CHEMBL2048044)Show SMILES CC(C)(C)c1cccc(CN[C@H]2CS(=O)(=O)C[C@@H](Cc3cc(F)c(N)c(OCC(F)F)c3)[C@@H]2O)c1 |r| Show InChI InChI=1S/C25H33F3N2O4S/c1-25(2,3)18-6-4-5-15(8-18)11-30-20-14-35(32,33)13-17(24(20)31)7-16-9-19(26)23(29)21(10-16)34-12-22(27)28/h4-6,8-10,17,20,22,24,30-31H,7,11-14,29H2,1-3H3/t17-,20+,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 ectodomain after 1 hr by fluorescence analysis |
J Med Chem 55: 3364-86 (2012)
Article DOI: 10.1021/jm300069y
BindingDB Entry DOI: 10.7270/Q2P55PJW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50587069

(CHEMBL5073160)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ncc(cc2Cl)C#N)ccc1F |r,c:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE1 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM134360

(US8846658, 73)Show SMILES C[C@]1(CO[C@](C)(C(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,c:7| Show InChI InChI=1S/C20H17F4N5O2/c1-18(10-31-19(2,17(26)29-18)20(22,23)24)13-7-12(4-5-14(13)21)28-16(30)15-6-3-11(8-25)9-27-15/h3-7,9H,10H2,1-2H3,(H2,26,29)(H,28,30)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant BACE2 catalytic domain using FRET substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02143
BindingDB Entry DOI: 10.7270/Q20P13X7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data