 Found 4427 hits with Last Name = 'stein' and Initial = 'm'
Found 4427 hits with Last Name = 'stein' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50254445
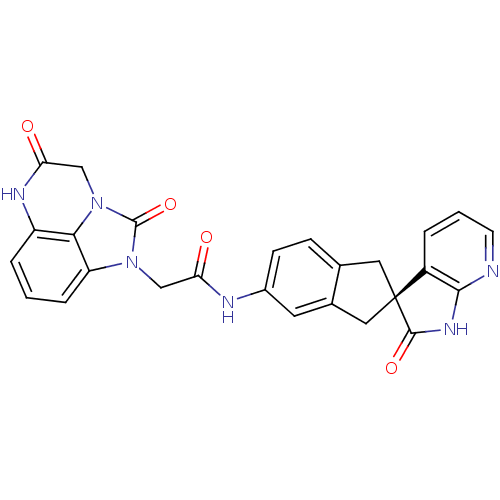
((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...)Show SMILES O=C(Cn1c2cccc3NC(=O)Cn(c23)c1=O)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C26H20N6O4/c33-20(12-31-19-5-1-4-18-22(19)32(25(31)36)13-21(34)29-18)28-16-7-6-14-10-26(11-15(14)9-16)17-3-2-8-27-23(17)30-24(26)35/h1-9H,10-13H2,(H,28,33)(H,29,34)(H,27,30,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 19: 4740-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.057
BindingDB Entry DOI: 10.7270/Q2QV3MJH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061031
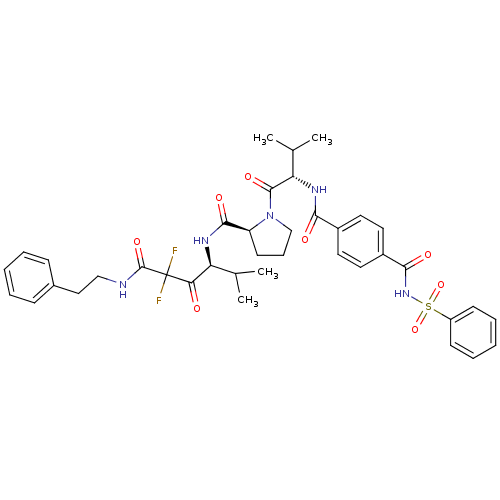
((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C39H45F2N5O8S/c1-24(2)31(33(47)39(40,41)38(52)42-22-21-26-12-7-5-8-13-26)43-36(50)30-16-11-23-46(30)37(51)32(25(3)4)44-34(48)27-17-19-28(20-18-27)35(49)45-55(53,54)29-14-9-6-10-15-29/h5-10,12-15,17-20,24-25,30-32H,11,16,21-23H2,1-4H3,(H,42,52)(H,43,50)(H,44,48)(H,45,49)/t30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50296785
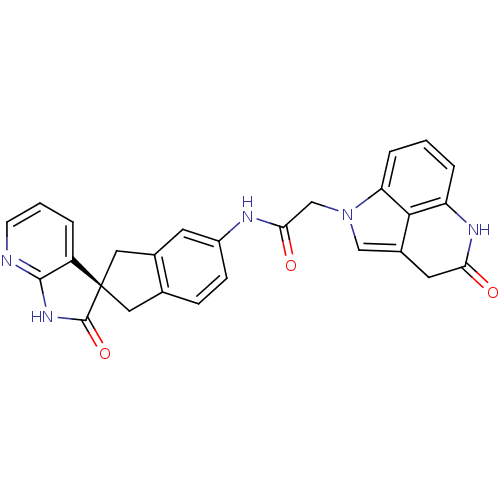
((S)-N-(2'-oxo-1,1',2',3-tetrahydrospiro[indene-2,3...)Show SMILES O=C(Cn1cc2CC(=O)Nc3cccc1c23)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C27H21N5O3/c33-22-10-17-13-32(21-5-1-4-20(30-22)24(17)21)14-23(34)29-18-7-6-15-11-27(12-16(15)9-18)19-3-2-8-28-25(19)31-26(27)35/h1-9,13H,10-12,14H2,(H,29,34)(H,30,33)(H,28,31,35)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 19: 4740-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.057
BindingDB Entry DOI: 10.7270/Q2QV3MJH |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224426
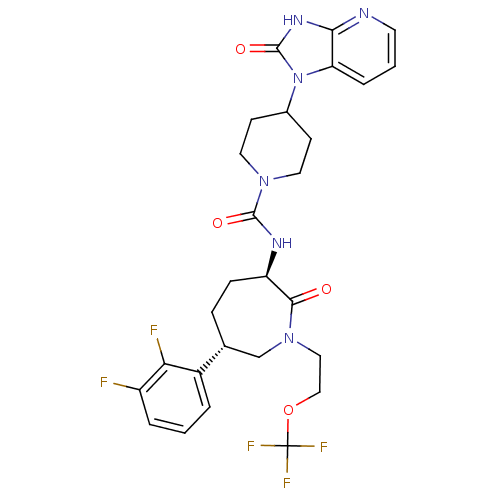
(CHEMBL238276 | N-{(3R,6S)-6-(2,3-difluorophenyl)-2...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C(=O)N(CCOC(F)(F)F)C2)c1F Show InChI InChI=1S/C27H29F5N6O4/c28-19-4-1-3-18(22(19)29)16-6-7-20(24(39)37(15-16)13-14-42-27(30,31)32)34-25(40)36-11-8-17(9-12-36)38-21-5-2-10-33-23(21)35-26(38)41/h1-5,10,16-17,20H,6-9,11-15H2,(H,34,40)(H,33,35,41)/t16-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGPR from human CL receptor expressed in HEK293 cells |
J Med Chem 50: 5564-7 (2007)
Article DOI: 10.1021/jm070668p
BindingDB Entry DOI: 10.7270/Q28C9W02 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202918
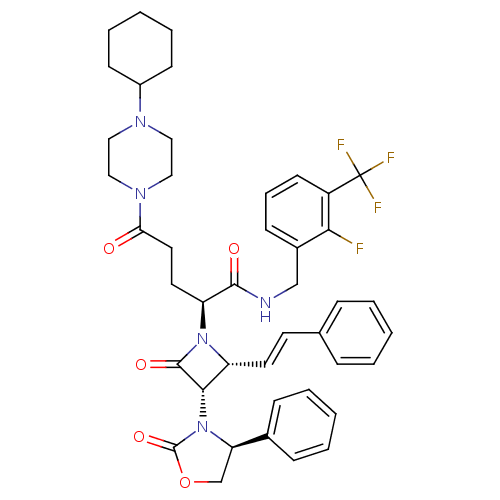
((S)-N-(2-fluoro-3-(trifluoromethyl)benzyl)-5-(4-cy...)Show SMILES Fc1c(CNC(=O)[C@H](CCC(=O)N2CCN(CC2)C2CCCCC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)cccc1C(F)(F)F Show InChI InChI=1S/C43H47F4N5O5/c44-38-31(15-10-18-33(38)43(45,46)47)27-48-40(54)35(21-22-37(53)50-25-23-49(24-26-50)32-16-8-3-9-17-32)51-34(20-19-29-11-4-1-5-12-29)39(41(51)55)52-36(28-57-42(52)56)30-13-6-2-7-14-30/h1-2,4-7,10-15,18-20,32,34-36,39H,3,8-9,16-17,21-28H2,(H,48,54)/b20-19+/t34-,35+,36-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202918

((S)-N-(2-fluoro-3-(trifluoromethyl)benzyl)-5-(4-cy...)Show SMILES Fc1c(CNC(=O)[C@H](CCC(=O)N2CCN(CC2)C2CCCCC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)cccc1C(F)(F)F Show InChI InChI=1S/C43H47F4N5O5/c44-38-31(15-10-18-33(38)43(45,46)47)27-48-40(54)35(21-22-37(53)50-25-23-49(24-26-50)32-16-8-3-9-17-32)51-34(20-19-29-11-4-1-5-12-29)39(41(51)55)52-36(28-57-42(52)56)30-13-6-2-7-14-30/h1-2,4-7,10-15,18-20,32,34-36,39H,3,8-9,16-17,21-28H2,(H,48,54)/b20-19+/t34-,35+,36-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346209

(5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C20H24N4O2/c1-21-20(25)18-12-23-19(13-22-18)26-17-6-5-14-7-9-24(16-3-2-4-16)10-8-15(14)11-17/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50296784
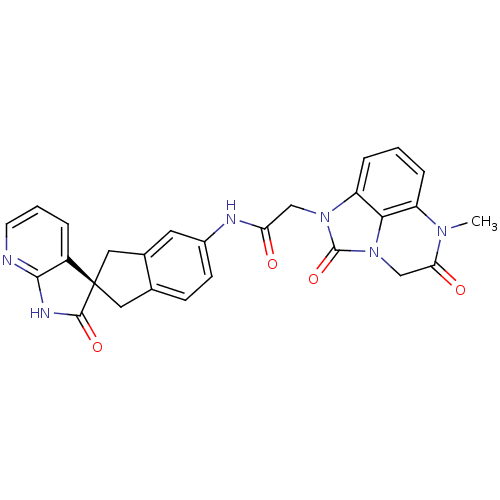
((S)-2-(6-methyl-2,5-dioxo-2,4,5,6-tetrahydro-1H-im...)Show SMILES CN1C(=O)Cn2c3c1cccc3n(CC(=O)Nc1ccc3C[C@@]4(Cc3c1)C(=O)Nc1ncccc41)c2=O |r| Show InChI InChI=1S/C27H22N6O4/c1-31-19-5-2-6-20-23(19)33(14-22(31)35)26(37)32(20)13-21(34)29-17-8-7-15-11-27(12-16(15)10-17)18-4-3-9-28-24(18)30-25(27)36/h2-10H,11-14H2,1H3,(H,29,34)(H,28,30,36)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 19: 4740-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.057
BindingDB Entry DOI: 10.7270/Q2QV3MJH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202900
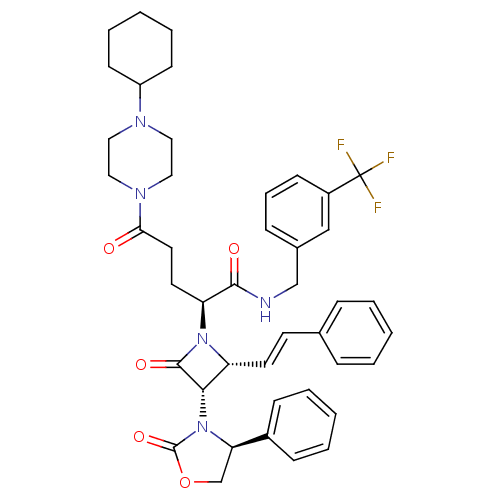
((S)-N-(3-(trifluoromethyl)benzyl)-5-(4-cyclohexylp...)Show SMILES FC(F)(F)c1cccc(CNC(=O)[C@H](CCC(=O)N2CCN(CC2)C2CCCCC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C43H48F3N5O5/c44-43(45,46)33-16-10-13-31(27-33)28-47-40(53)36(21-22-38(52)49-25-23-48(24-26-49)34-17-8-3-9-18-34)50-35(20-19-30-11-4-1-5-12-30)39(41(50)54)51-37(29-56-42(51)55)32-14-6-2-7-15-32/h1-2,4-7,10-16,19-20,27,34-37,39H,3,8-9,17-18,21-26,28-29H2,(H,47,53)/b20-19+/t35-,36+,37-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202900

((S)-N-(3-(trifluoromethyl)benzyl)-5-(4-cyclohexylp...)Show SMILES FC(F)(F)c1cccc(CNC(=O)[C@H](CCC(=O)N2CCN(CC2)C2CCCCC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C43H48F3N5O5/c44-43(45,46)33-16-10-13-31(27-33)28-47-40(53)36(21-22-38(52)49-25-23-48(24-26-49)34-17-8-3-9-18-34)50-35(20-19-30-11-4-1-5-12-30)39(41(50)54)51-37(29-56-42(51)55)32-14-6-2-7-15-32/h1-2,4-7,10-16,19-20,27,34-37,39H,3,8-9,17-18,21-26,28-29H2,(H,47,53)/b20-19+/t35-,36+,37-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50296789

(2-(2a-methyl-4-oxo-2a,3,4,5-tetrahydropyrrolo[4,3,...)Show SMILES CC12CN(CC(=O)Nc3ccc4C[C@@]5(Cc4c3)C(=O)Nc3ncccc53)c3cccc(NC(=O)C1)c23 |r| Show InChI InChI=1S/C28H25N5O3/c1-27-13-22(34)31-20-5-2-6-21(24(20)27)33(15-27)14-23(35)30-18-8-7-16-11-28(12-17(16)10-18)19-4-3-9-29-25(19)32-26(28)36/h2-10H,11-15H2,1H3,(H,30,35)(H,31,34)(H,29,32,36)/t27?,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 19: 4740-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.057
BindingDB Entry DOI: 10.7270/Q2QV3MJH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202920
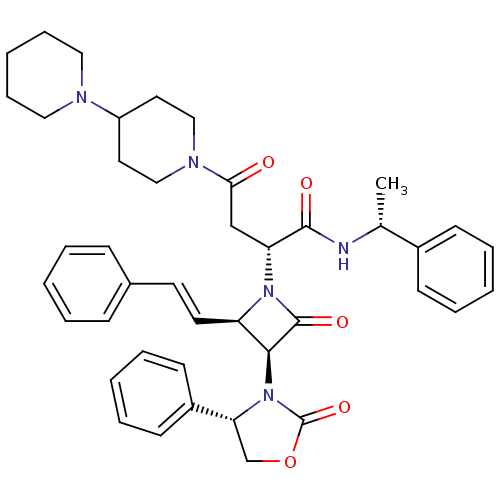
(2(R)-[[4-(piperidin-1-yl)piperidin-1-yl]carbonylme...)Show SMILES C[C@@H](NC(=O)[C@@H](CC(=O)N1CCC(CC1)N1CCCCC1)N1[C@H](\C=C\c2ccccc2)[C@H](N2[C@H](COC2=O)c2ccccc2)C1=O)c1ccccc1 Show InChI InChI=1S/C42H49N5O5/c1-30(32-16-8-3-9-17-32)43-40(49)36(28-38(48)45-26-22-34(23-27-45)44-24-12-5-13-25-44)46-35(21-20-31-14-6-2-7-15-31)39(41(46)50)47-37(29-52-42(47)51)33-18-10-4-11-19-33/h2-4,6-11,14-21,30,34-37,39H,5,12-13,22-29H2,1H3,(H,43,49)/b21-20+/t30-,35-,36-,37-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202920

(2(R)-[[4-(piperidin-1-yl)piperidin-1-yl]carbonylme...)Show SMILES C[C@@H](NC(=O)[C@@H](CC(=O)N1CCC(CC1)N1CCCCC1)N1[C@H](\C=C\c2ccccc2)[C@H](N2[C@H](COC2=O)c2ccccc2)C1=O)c1ccccc1 Show InChI InChI=1S/C42H49N5O5/c1-30(32-16-8-3-9-17-32)43-40(49)36(28-38(48)45-26-22-34(23-27-45)44-24-12-5-13-25-44)46-35(21-20-31-14-6-2-7-15-31)39(41(46)50)47-37(29-52-42(47)51)33-18-10-4-11-19-33/h2-4,6-11,14-21,30,34-37,39H,5,12-13,22-29H2,1H3,(H,43,49)/b21-20+/t30-,35-,36-,37-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224419
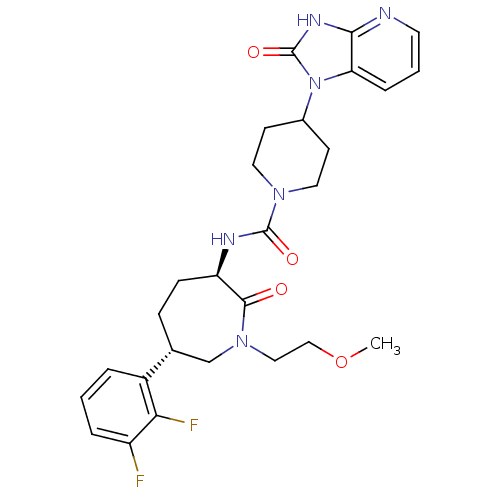
(CHEMBL237662 | N-[(3R,6S)-6-(2,3-difluorophenyl)-1...)Show SMILES COCCN1C[C@@H](CC[C@@H](NC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)C1=O)c1cccc(F)c1F Show InChI InChI=1S/C27H32F2N6O4/c1-39-15-14-34-16-17(19-4-2-5-20(28)23(19)29)7-8-21(25(34)36)31-26(37)33-12-9-18(10-13-33)35-22-6-3-11-30-24(22)32-27(35)38/h2-6,11,17-18,21H,7-10,12-16H2,1H3,(H,31,37)(H,30,32,38)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGPR from human CL receptor expressed in HEK293 cells |
J Med Chem 50: 5564-7 (2007)
Article DOI: 10.1021/jm070668p
BindingDB Entry DOI: 10.7270/Q28C9W02 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418481
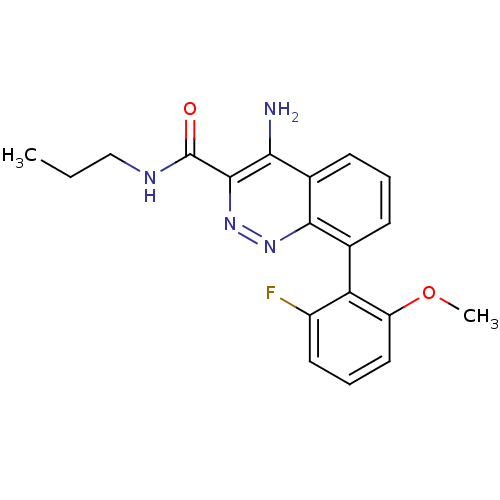
(CHEMBL1783282)Show SMILES CCCNC(=O)c1nnc2c(cccc2c1N)-c1c(F)cccc1OC |(3.52,-21.84,;2.19,-22.61,;.85,-21.84,;-.48,-22.61,;-1.81,-21.84,;-1.81,-20.3,;-3.15,-22.61,;-3.16,-24.17,;-4.5,-24.95,;-5.84,-24.18,;-7.18,-24.95,;-8.51,-24.18,;-8.51,-22.63,;-7.18,-21.86,;-5.85,-22.63,;-4.51,-21.84,;-4.52,-20.3,;-7.18,-26.49,;-5.85,-27.25,;-4.52,-26.47,;-5.85,-28.78,;-7.19,-29.56,;-8.52,-28.78,;-8.52,-27.25,;-9.85,-26.47,;-9.84,-24.93,)| Show InChI InChI=1S/C19H19FN4O2/c1-3-10-22-19(25)18-16(21)12-7-4-6-11(17(12)23-24-18)15-13(20)8-5-9-14(15)26-2/h4-9H,3,10H2,1-2H3,(H2,21,23)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
J Biol Chem 266: 6365-9 (1991)
BindingDB Entry DOI: 10.7270/Q2X928SG |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50296787
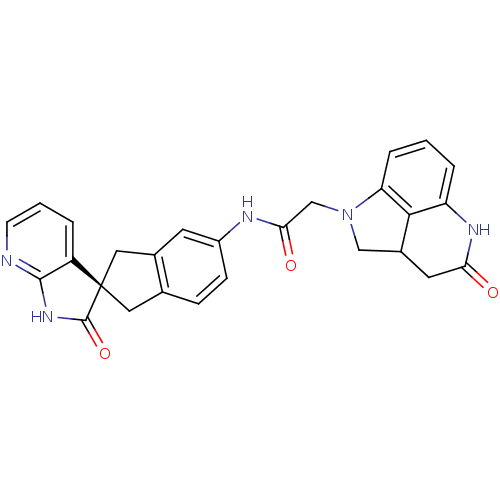
(CHEMBL564088 | N-((S)-2'-oxo-1,1',2',3-tetrahydros...)Show SMILES O=C(CN1CC2CC(=O)Nc3cccc1c23)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C27H23N5O3/c33-22-10-17-13-32(21-5-1-4-20(30-22)24(17)21)14-23(34)29-18-7-6-15-11-27(12-16(15)9-18)19-3-2-8-28-25(19)31-26(27)35/h1-9,17H,10-14H2,(H,29,34)(H,30,33)(H,28,31,35)/t17?,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 19: 4740-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.057
BindingDB Entry DOI: 10.7270/Q2QV3MJH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061047
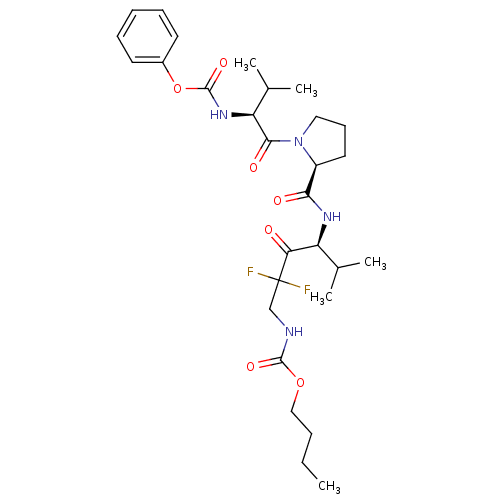
(((S)-2,2-Difluoro-5-methyl-4-{[(S)-1-((S)-3-methyl...)Show SMILES CCCCOC(=O)NCC(F)(F)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)Oc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C29H42F2N4O7/c1-6-7-16-41-27(39)32-17-29(30,31)24(36)22(18(2)3)33-25(37)21-14-11-15-35(21)26(38)23(19(4)5)34-28(40)42-20-12-9-8-10-13-20/h8-10,12-13,18-19,21-23H,6-7,11,14-17H2,1-5H3,(H,32,39)(H,33,37)(H,34,40)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061043
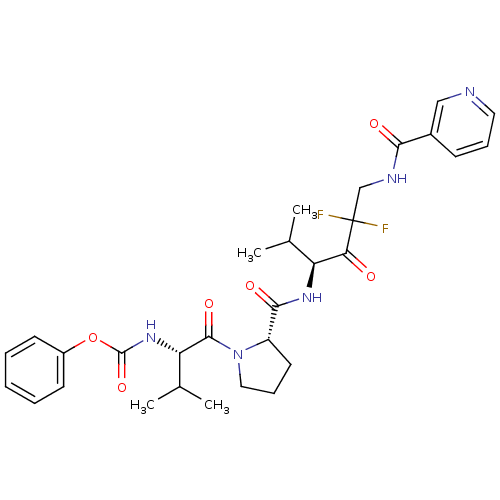
(CHEMBL106592 | [(S)-1-((S)-2-{(S)-3,3-Difluoro-1-i...)Show SMILES CC(C)[C@H](NC(=O)Oc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNC(=O)c1cccnc1 Show InChI InChI=1S/C30H37F2N5O6/c1-18(2)23(25(38)30(31,32)17-34-26(39)20-10-8-14-33-16-20)35-27(40)22-13-9-15-37(22)28(41)24(19(3)4)36-29(42)43-21-11-6-5-7-12-21/h5-8,10-12,14,16,18-19,22-24H,9,13,15,17H2,1-4H3,(H,34,39)(H,35,40)(H,36,42)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061028
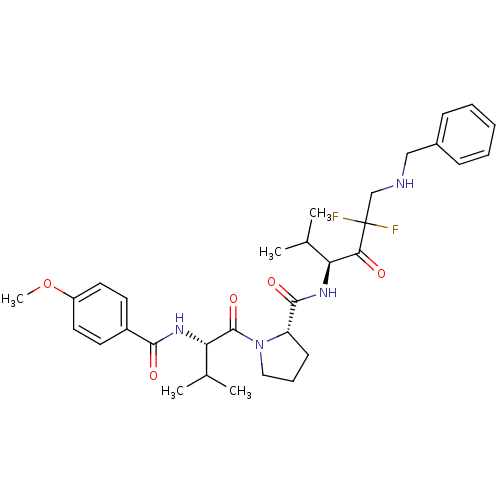
((S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3-methyl-but...)Show SMILES COc1ccc(cc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNCc1ccccc1 Show InChI InChI=1S/C32H42F2N4O5/c1-20(2)26(28(39)32(33,34)19-35-18-22-10-7-6-8-11-22)36-30(41)25-12-9-17-38(25)31(42)27(21(3)4)37-29(40)23-13-15-24(43-5)16-14-23/h6-8,10-11,13-16,20-21,25-27,35H,9,12,17-19H2,1-5H3,(H,36,41)(H,37,40)/t25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50296787

(CHEMBL564088 | N-((S)-2'-oxo-1,1',2',3-tetrahydros...)Show SMILES O=C(CN1CC2CC(=O)Nc3cccc1c23)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C27H23N5O3/c33-22-10-17-13-32(21-5-1-4-20(30-22)24(17)21)14-23(34)29-18-7-6-15-11-27(12-16(15)9-18)19-3-2-8-28-25(19)31-26(27)35/h1-9,17H,10-14H2,(H,29,34)(H,30,33)(H,28,31,35)/t17?,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 19: 4740-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.057
BindingDB Entry DOI: 10.7270/Q2QV3MJH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
J Biol Chem 266: 6365-9 (1991)
BindingDB Entry DOI: 10.7270/Q2X928SG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061037
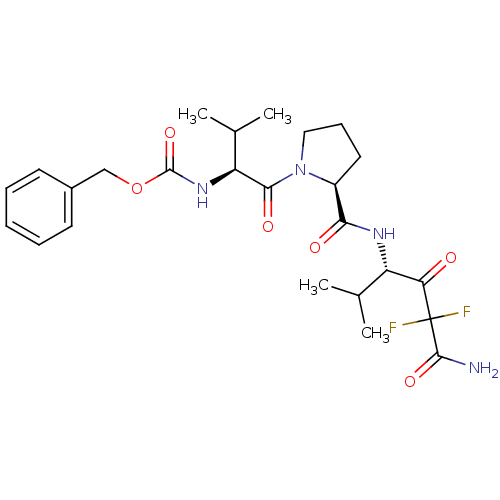
(CHEMBL302961 | {(S)-1-[(S)-2-((S)-3-Carbamoyl-3,3-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(N)=O Show InChI InChI=1S/C25H34F2N4O6/c1-14(2)18(20(32)25(26,27)23(28)35)29-21(33)17-11-8-12-31(17)22(34)19(15(3)4)30-24(36)37-13-16-9-6-5-7-10-16/h5-7,9-10,14-15,17-19H,8,11-13H2,1-4H3,(H2,28,35)(H,29,33)(H,30,36)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418483
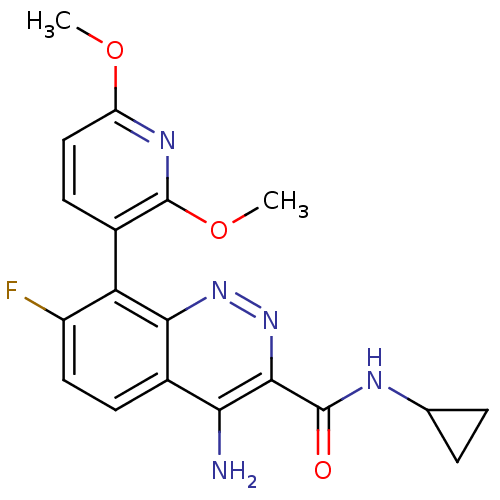
(CHEMBL1783284)Show SMILES COc1ccc(c(OC)n1)-c1c(F)ccc2c(N)c(nnc12)C(=O)NC1CC1 |(-7.37,-43.23,;-6.03,-42.46,;-6.03,-40.92,;-4.69,-40.15,;-4.69,-38.61,;-6.02,-37.85,;-7.36,-38.61,;-8.69,-37.83,;-10.03,-38.6,;-7.36,-40.15,;-6.02,-36.31,;-7.35,-35.54,;-8.69,-36.31,;-7.35,-34,;-6.02,-33.23,;-4.69,-33.99,;-3.35,-33.2,;-3.36,-31.66,;-1.99,-33.98,;-2,-35.54,;-3.34,-36.31,;-4.68,-35.54,;-.65,-33.21,;-.65,-31.67,;.68,-33.98,;2.01,-33.21,;3.55,-33.2,;2.78,-31.87,)| Show InChI InChI=1S/C19H18FN5O3/c1-27-13-8-6-10(19(23-13)28-2)14-12(20)7-5-11-15(21)17(25-24-16(11)14)18(26)22-9-3-4-9/h5-9H,3-4H2,1-2H3,(H2,21,24)(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202882
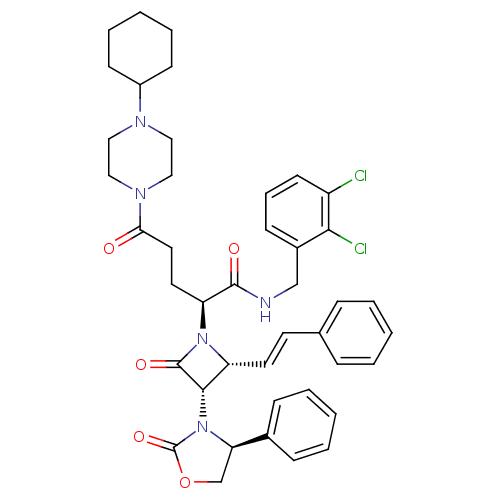
((S)-N-(2,3-dichlorobenzyl)-5-(4-cyclohexylpiperazi...)Show SMILES Clc1cccc(CNC(=O)[C@H](CCC(=O)N2CCN(CC2)C2CCCCC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)c1Cl Show InChI InChI=1S/C42H47Cl2N5O5/c43-33-18-10-15-31(38(33)44)27-45-40(51)35(21-22-37(50)47-25-23-46(24-26-47)32-16-8-3-9-17-32)48-34(20-19-29-11-4-1-5-12-29)39(41(48)52)49-36(28-54-42(49)53)30-13-6-2-7-14-30/h1-2,4-7,10-15,18-20,32,34-36,39H,3,8-9,16-17,21-28H2,(H,45,51)/b20-19+/t34-,35+,36-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202882

((S)-N-(2,3-dichlorobenzyl)-5-(4-cyclohexylpiperazi...)Show SMILES Clc1cccc(CNC(=O)[C@H](CCC(=O)N2CCN(CC2)C2CCCCC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)c1Cl Show InChI InChI=1S/C42H47Cl2N5O5/c43-33-18-10-15-31(38(33)44)27-45-40(51)35(21-22-37(50)47-25-23-46(24-26-47)32-16-8-3-9-17-32)48-34(20-19-29-11-4-1-5-12-29)39(41(48)52)49-36(28-54-42(49)53)30-13-6-2-7-14-30/h1-2,4-7,10-15,18-20,32,34-36,39H,3,8-9,16-17,21-28H2,(H,45,51)/b20-19+/t34-,35+,36-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418482
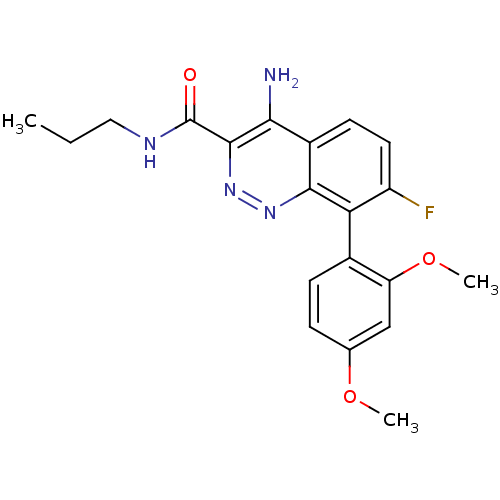
(CHEMBL1783283)Show SMILES CCCNC(=O)c1nnc2c(c(F)ccc2c1N)-c1ccc(OC)cc1OC |(30.61,-19.8,;29.28,-20.57,;27.94,-19.8,;26.61,-20.57,;25.28,-19.8,;25.28,-18.26,;23.94,-20.57,;23.93,-22.13,;22.59,-22.9,;21.25,-22.14,;19.91,-22.9,;18.58,-22.13,;17.24,-22.9,;18.58,-20.59,;19.91,-19.82,;21.24,-20.58,;22.58,-19.79,;22.57,-18.25,;19.91,-24.44,;21.24,-25.2,;21.24,-26.74,;19.9,-27.51,;19.9,-29.05,;18.57,-29.82,;18.57,-26.74,;18.57,-25.2,;17.24,-24.42,;15.9,-25.19,)| Show InChI InChI=1S/C20H21FN4O3/c1-4-9-23-20(26)19-17(22)13-7-8-14(21)16(18(13)24-25-19)12-6-5-11(27-2)10-15(12)28-3/h5-8,10H,4,9H2,1-3H3,(H2,22,24)(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202902
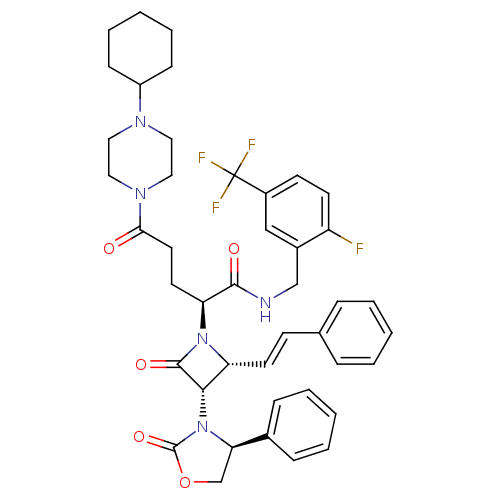
((S)-N-(2-fluoro-5-(trifluoromethyl)benzyl)-5-(4-cy...)Show SMILES Fc1ccc(cc1CNC(=O)[C@H](CCC(=O)N1CCN(CC1)C1CCCCC1)N1[C@H](\C=C\c2ccccc2)[C@H](N2[C@H](COC2=O)c2ccccc2)C1=O)C(F)(F)F Show InChI InChI=1S/C43H47F4N5O5/c44-34-18-17-32(43(45,46)47)26-31(34)27-48-40(54)36(20-21-38(53)50-24-22-49(23-25-50)33-14-8-3-9-15-33)51-35(19-16-29-10-4-1-5-11-29)39(41(51)55)52-37(28-57-42(52)56)30-12-6-2-7-13-30/h1-2,4-7,10-13,16-19,26,33,35-37,39H,3,8-9,14-15,20-25,27-28H2,(H,48,54)/b19-16+/t35-,36+,37-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202902

((S)-N-(2-fluoro-5-(trifluoromethyl)benzyl)-5-(4-cy...)Show SMILES Fc1ccc(cc1CNC(=O)[C@H](CCC(=O)N1CCN(CC1)C1CCCCC1)N1[C@H](\C=C\c2ccccc2)[C@H](N2[C@H](COC2=O)c2ccccc2)C1=O)C(F)(F)F Show InChI InChI=1S/C43H47F4N5O5/c44-34-18-17-32(43(45,46)47)26-31(34)27-48-40(54)36(20-21-38(53)50-24-22-49(23-25-50)33-14-8-3-9-15-33)51-35(19-16-29-10-4-1-5-11-29)39(41(51)55)52-37(28-57-42(52)56)30-12-6-2-7-13-30/h1-2,4-7,10-13,16-19,26,33,35-37,39H,3,8-9,14-15,20-25,27-28H2,(H,48,54)/b19-16+/t35-,36+,37-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50263407
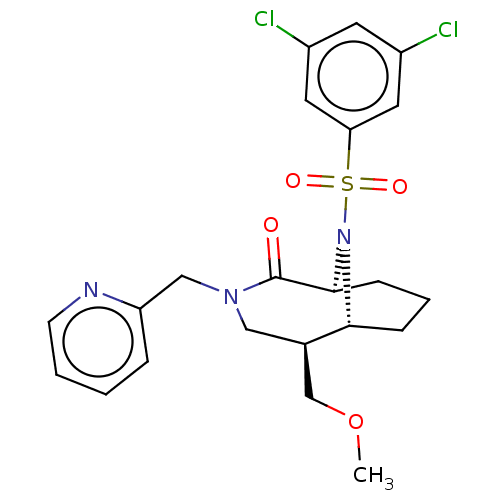
(CHEMBL4090599)Show SMILES [H][C@]12CCC[C@]([H])(N1S(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N(Cc1ccccn1)C[C@@H]2COC |r,TLB:31:30:7:2.3.4,20:19:7:2.3.4,THB:22:21:7:2.3.4| Show InChI InChI=1S/C22H25Cl2N3O4S/c1-31-14-15-12-26(13-18-5-2-3-8-25-18)22(28)21-7-4-6-20(15)27(21)32(29,30)19-10-16(23)9-17(24)11-19/h2-3,5,8-11,15,20-21H,4,6-7,12-14H2,1H3/t15-,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany.
Curated by ChEMBL
| Assay Description
Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... |
J Med Chem 61: 3660-3673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00137
BindingDB Entry DOI: 10.7270/Q2833VGW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202873
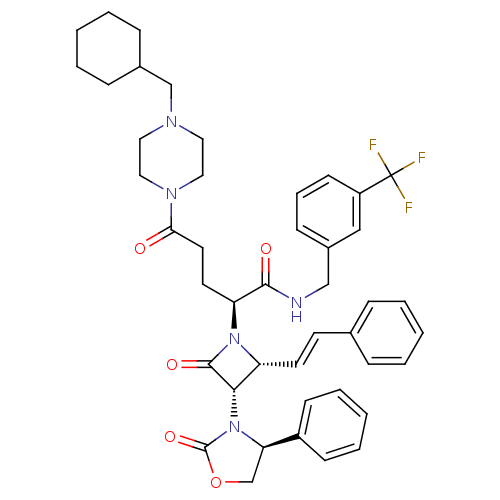
((S)-N-(3-(trifluoromethyl)benzyl)-5-(4-(cyclohexyl...)Show SMILES FC(F)(F)c1cccc(CNC(=O)[C@H](CCC(=O)N2CCN(CC3CCCCC3)CC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C44H50F3N5O5/c45-44(46,47)35-18-10-15-33(27-35)28-48-41(54)37(21-22-39(53)50-25-23-49(24-26-50)29-32-13-6-2-7-14-32)51-36(20-19-31-11-4-1-5-12-31)40(42(51)55)52-38(30-57-43(52)56)34-16-8-3-9-17-34/h1,3-5,8-12,15-20,27,32,36-38,40H,2,6-7,13-14,21-26,28-30H2,(H,48,54)/b20-19+/t36-,37+,38-,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343682

(((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-ylox...)Show SMILES [#6]-[#6]-[#6](-[#6]-[#6])-[#8]-[#6@@H]-1-[#6]-[#6](=[#6]-[#6@H](\[#7]=[#6](/[#7])-[#7])-[#6@H]-1-[#7]-[#6](-[#6])=O)-[#6](-[#8])=O |r,c:8| Show InChI InChI=1S/C15H26N4O4/c1-4-10(5-2)23-12-7-9(14(21)22)6-11(19-15(16)17)13(12)18-8(3)20/h6,10-13H,4-5,7H2,1-3H3,(H,18,20)(H,21,22)(H4,16,17,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202873

((S)-N-(3-(trifluoromethyl)benzyl)-5-(4-(cyclohexyl...)Show SMILES FC(F)(F)c1cccc(CNC(=O)[C@H](CCC(=O)N2CCN(CC3CCCCC3)CC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C44H50F3N5O5/c45-44(46,47)35-18-10-15-33(27-35)28-48-41(54)37(21-22-39(53)50-25-23-49(24-26-50)29-32-13-6-2-7-14-32)51-36(20-19-31-11-4-1-5-12-31)40(42(51)55)52-38(30-57-43(52)56)34-16-8-3-9-17-34/h1,3-5,8-12,15-20,27,32,36-38,40H,2,6-7,13-14,21-26,28-30H2,(H,48,54)/b20-19+/t36-,37+,38-,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228078
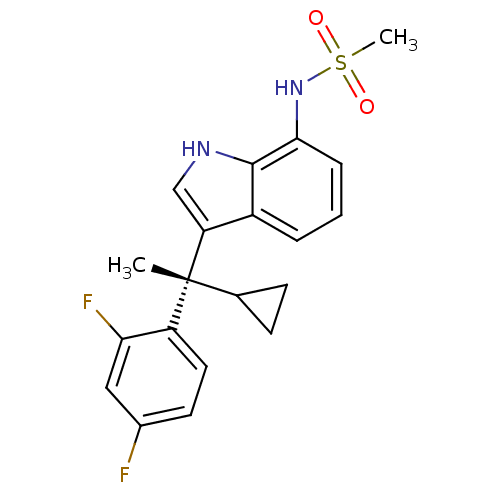
((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.494 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224413
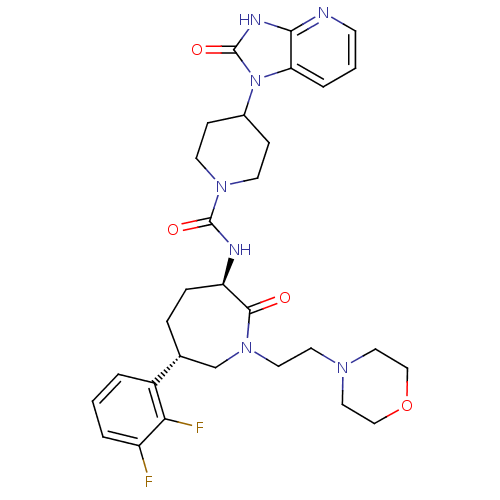
(CHEMBL392634 | N-[(3R,6S)-6-(2,3-difluorophenyl)-1...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C(=O)N(CCN3CCOCC3)C2)c1F Show InChI InChI=1S/C30H37F2N7O4/c31-23-4-1-3-22(26(23)32)20-6-7-24(28(40)38(19-20)14-13-36-15-17-43-18-16-36)34-29(41)37-11-8-21(9-12-37)39-25-5-2-10-33-27(25)35-30(39)42/h1-5,10,20-21,24H,6-9,11-19H2,(H,34,41)(H,33,35,42)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGPR from human CL receptor expressed in HEK293 cells |
J Med Chem 50: 5564-7 (2007)
Article DOI: 10.1021/jm070668p
BindingDB Entry DOI: 10.7270/Q28C9W02 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224430
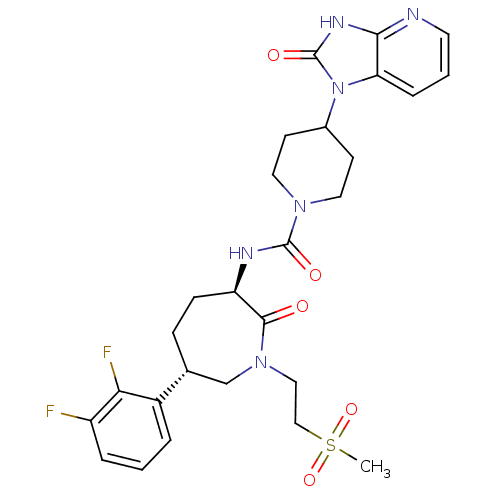
(CHEMBL392636 | N-{(3R,6S)-6-(2,3-difluorophenyl)-1...)Show SMILES CS(=O)(=O)CCN1C[C@@H](CC[C@@H](NC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)C1=O)c1cccc(F)c1F Show InChI InChI=1S/C27H32F2N6O5S/c1-41(39,40)15-14-34-16-17(19-4-2-5-20(28)23(19)29)7-8-21(25(34)36)31-26(37)33-12-9-18(10-13-33)35-22-6-3-11-30-24(22)32-27(35)38/h2-6,11,17-18,21H,7-10,12-16H2,1H3,(H,31,37)(H,30,32,38)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGPR from human CL receptor expressed in HEK293 cells |
J Med Chem 50: 5564-7 (2007)
Article DOI: 10.1021/jm070668p
BindingDB Entry DOI: 10.7270/Q28C9W02 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202916
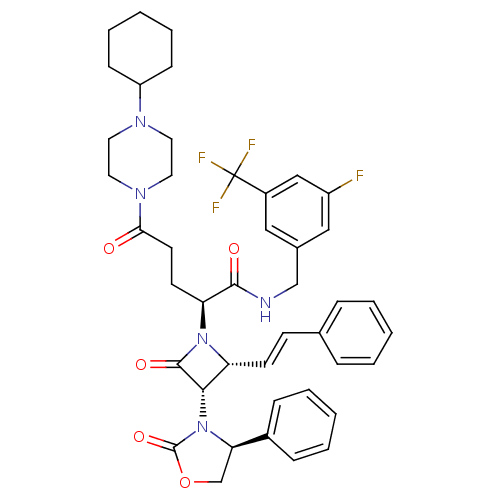
((S)-N-(3-fluoro-5-(trifluoromethyl)benzyl)-5-(4-cy...)Show SMILES Fc1cc(CNC(=O)[C@H](CCC(=O)N2CCN(CC2)C2CCCCC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)cc(c1)C(F)(F)F Show InChI InChI=1S/C43H47F4N5O5/c44-33-25-30(24-32(26-33)43(45,46)47)27-48-40(54)36(18-19-38(53)50-22-20-49(21-23-50)34-14-8-3-9-15-34)51-35(17-16-29-10-4-1-5-11-29)39(41(51)55)52-37(28-57-42(52)56)31-12-6-2-7-13-31/h1-2,4-7,10-13,16-17,24-26,34-37,39H,3,8-9,14-15,18-23,27-28H2,(H,48,54)/b17-16+/t35-,36+,37-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50202916

((S)-N-(3-fluoro-5-(trifluoromethyl)benzyl)-5-(4-cy...)Show SMILES Fc1cc(CNC(=O)[C@H](CCC(=O)N2CCN(CC2)C2CCCCC2)N2[C@H](\C=C\c3ccccc3)[C@H](N3[C@H](COC3=O)c3ccccc3)C2=O)cc(c1)C(F)(F)F Show InChI InChI=1S/C43H47F4N5O5/c44-33-25-30(24-32(26-33)43(45,46)47)27-48-40(54)36(18-19-38(53)50-22-20-49(21-23-50)34-14-8-3-9-15-34)51-35(17-16-29-10-4-1-5-11-29)39(41(51)55)52-37(28-57-42(52)56)31-12-6-2-7-13-31/h1-2,4-7,10-13,16-17,24-26,34-37,39H,3,8-9,14-15,18-23,27-28H2,(H,48,54)/b17-16+/t35-,36+,37-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells |
Bioorg Med Chem 15: 2054-80 (2007)
Article DOI: 10.1016/j.bmc.2006.12.031
BindingDB Entry DOI: 10.7270/Q2GX4B6M |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
J Biol Chem 266: 6365-9 (1991)
BindingDB Entry DOI: 10.7270/Q2X928SG |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418488

(CHEMBL1783285)Show SMILES COc1cc(c(OC)nn1)-c1c(F)ccc2c(N)c(nnc12)C(=O)NC1CC1 |(23.34,-39.57,;22.01,-40.34,;20.67,-39.57,;20.67,-38.03,;19.34,-37.28,;18,-38.03,;16.67,-37.26,;15.34,-38.02,;18,-39.57,;19.33,-40.35,;19.34,-35.74,;18.01,-34.97,;16.67,-35.73,;18.01,-33.42,;19.34,-32.65,;20.67,-33.41,;22.01,-32.63,;22,-31.09,;23.38,-33.4,;23.37,-34.96,;22.02,-35.74,;20.68,-34.97,;24.71,-32.63,;24.71,-31.09,;26.04,-33.4,;27.38,-32.63,;28.91,-32.63,;28.14,-31.29,)| Show InChI InChI=1S/C18H17FN6O3/c1-27-12-7-10(18(28-2)25-22-12)13-11(19)6-5-9-14(20)16(24-23-15(9)13)17(26)21-8-3-4-8/h5-8H,3-4H2,1-2H3,(H2,20,23)(H,21,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50031210
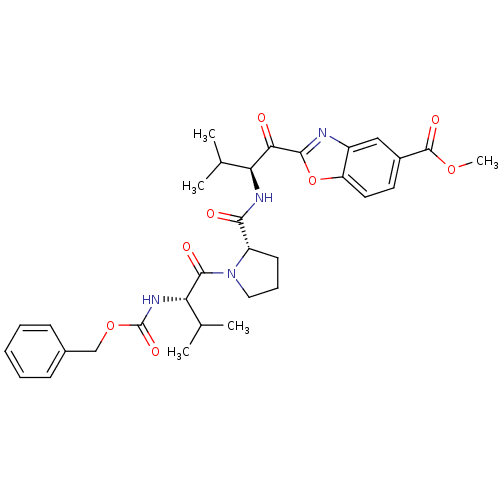
(2-((S)-2-{[(S)-1-((S)-2-Benzyloxycarbonylamino-3-m...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C32H38N4O8/c1-18(2)25(27(37)29-33-22-16-21(31(40)42-5)13-14-24(22)44-29)34-28(38)23-12-9-15-36(23)30(39)26(19(3)4)35-32(41)43-17-20-10-7-6-8-11-20/h6-8,10-11,13-14,16,18-19,23,25-26H,9,12,15,17H2,1-5H3,(H,34,38)(H,35,41)/t23-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50362873
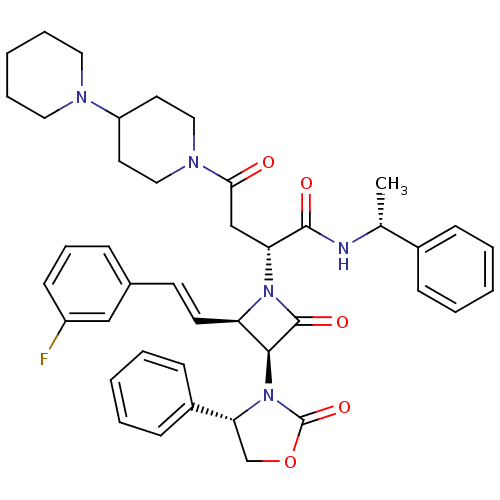
(CHEMBL1940587)Show SMILES C[C@@H](NC(=O)[C@@H](CC(=O)N1CCC(CC1)N1CCCCC1)N1[C@H](\C=C\c2cccc(F)c2)[C@H](N2[C@H](COC2=O)c2ccccc2)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C42H48FN5O5/c1-29(31-13-5-2-6-14-31)44-40(50)36(27-38(49)46-24-20-34(21-25-46)45-22-9-4-10-23-45)47-35(19-18-30-12-11-17-33(43)26-30)39(41(47)51)48-37(28-53-42(48)52)32-15-7-3-8-16-32/h2-3,5-8,11-19,26,29,34-37,39H,4,9-10,20-25,27-28H2,1H3,(H,44,50)/b19-18+/t29-,35-,36-,37-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Displacement of [3H]arginine-vasopressin from human Vasopressin V1a receptor after 30 mins by liquid scintillation counter |
Bioorg Med Chem 20: 1337-45 (2012)
Article DOI: 10.1016/j.bmc.2011.12.013
BindingDB Entry DOI: 10.7270/Q2KD1ZCX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50362871
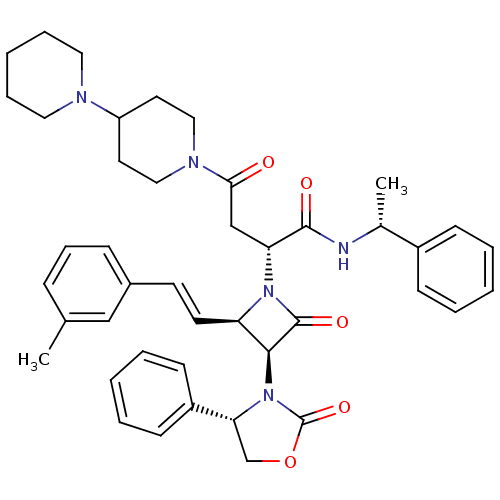
(CHEMBL1940588)Show SMILES C[C@@H](NC(=O)[C@@H](CC(=O)N1CCC(CC1)N1CCCCC1)N1[C@H](\C=C\c2cccc(C)c2)[C@H](N2[C@H](COC2=O)c2ccccc2)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C43H51N5O5/c1-30-13-12-14-32(27-30)19-20-36-40(48-38(29-53-43(48)52)34-17-8-4-9-18-34)42(51)47(36)37(41(50)44-31(2)33-15-6-3-7-16-33)28-39(49)46-25-21-35(22-26-46)45-23-10-5-11-24-45/h3-4,6-9,12-20,27,31,35-38,40H,5,10-11,21-26,28-29H2,1-2H3,(H,44,50)/b20-19+/t31-,36-,37-,38-,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University
Curated by ChEMBL
| Assay Description
Displacement of [3H]arginine-vasopressin from human Vasopressin V1a receptor after 30 mins by liquid scintillation counter |
Bioorg Med Chem 20: 1337-45 (2012)
Article DOI: 10.1016/j.bmc.2011.12.013
BindingDB Entry DOI: 10.7270/Q2KD1ZCX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human histamine H3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116462
BindingDB Entry DOI: 10.7270/Q2DR30DF |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061044
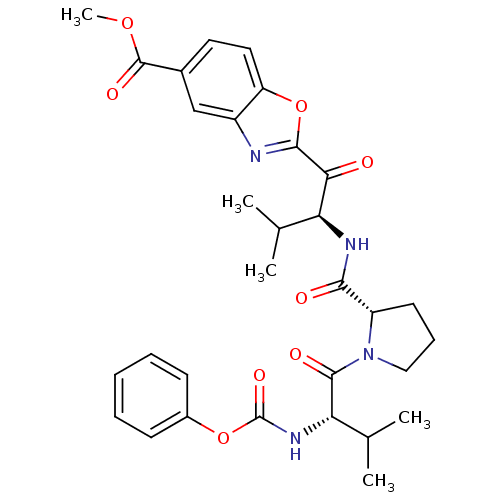
(2-((S)-3-Methyl-2-{[(S)-1-((S)-3-methyl-2-phenoxyc...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)Oc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C31H36N4O8/c1-17(2)24(26(36)28-32-21-16-19(30(39)41-5)13-14-23(21)43-28)33-27(37)22-12-9-15-35(22)29(38)25(18(3)4)34-31(40)42-20-10-7-6-8-11-20/h6-8,10-11,13-14,16-18,22,24-25H,9,12,15H2,1-5H3,(H,33,37)(H,34,40)/t22-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061035
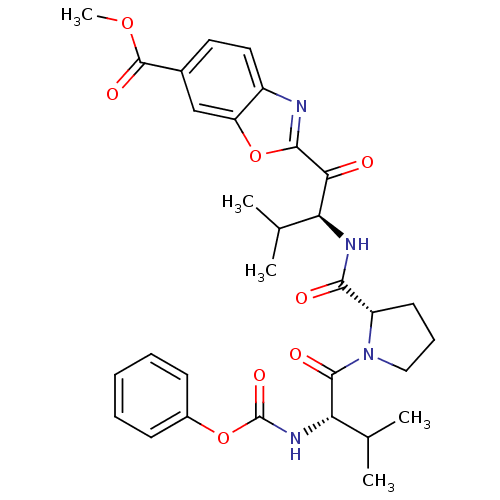
(2-((S)-3-Methyl-2-{[(S)-1-((S)-3-methyl-2-phenoxyc...)Show SMILES COC(=O)c1ccc2nc(oc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)Oc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C31H36N4O8/c1-17(2)24(26(36)28-32-21-14-13-19(30(39)41-5)16-23(21)43-28)33-27(37)22-12-9-15-35(22)29(38)25(18(3)4)34-31(40)42-20-10-7-6-8-11-20/h6-8,10-11,13-14,16-18,22,24-25H,9,12,15H2,1-5H3,(H,33,37)(H,34,40)/t22-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061049
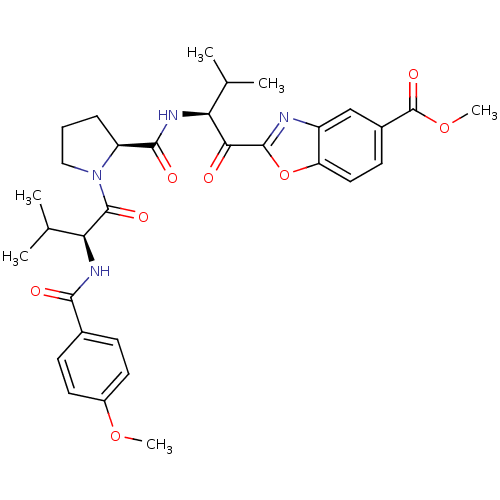
(2-[(S)-2-({(S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)c1ccc(OC)cc1)C(C)C)C(C)C Show InChI InChI=1S/C32H38N4O8/c1-17(2)25(27(37)30-33-22-16-20(32(41)43-6)11-14-24(22)44-30)34-29(39)23-8-7-15-36(23)31(40)26(18(3)4)35-28(38)19-9-12-21(42-5)13-10-19/h9-14,16-18,23,25-26H,7-8,15H2,1-6H3,(H,34,39)(H,35,38)/t23-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data