 Found 850 hits with Last Name = 'stribling' and Initial = 's'
Found 850 hits with Last Name = 'stribling' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50174298
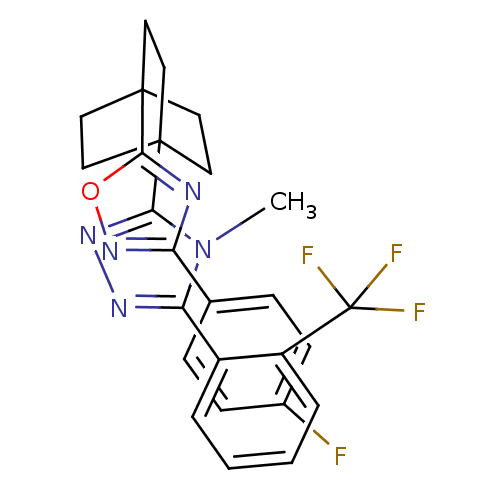
(3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50340378
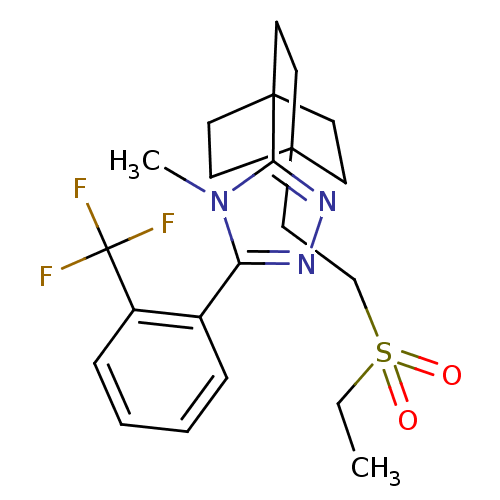
(3-(4-(2-(ethylsulfonyl)ethyl)bicyclo[2.2.2]octan-1...)Show SMILES CCS(=O)(=O)CCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C22H28F3N3O2S/c1-3-31(29,30)15-14-20-8-11-21(12-9-20,13-10-20)19-27-26-18(28(19)2)16-6-4-5-7-17(16)22(23,24)25/h4-7H,3,8-15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50435691
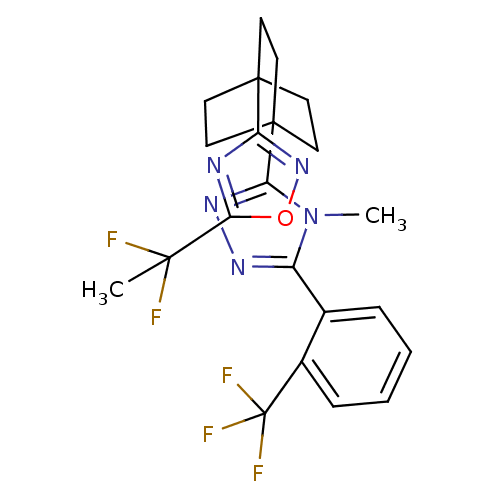
(CHEMBL2391968)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1noc(n1)C(C)(F)F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C22H22F5N5O/c1-19(23,24)18-28-16(31-33-18)20-7-10-21(11-8-20,12-9-20)17-30-29-15(32(17)2)13-5-3-4-6-14(13)22(25,26)27/h3-6H,7-12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50340386
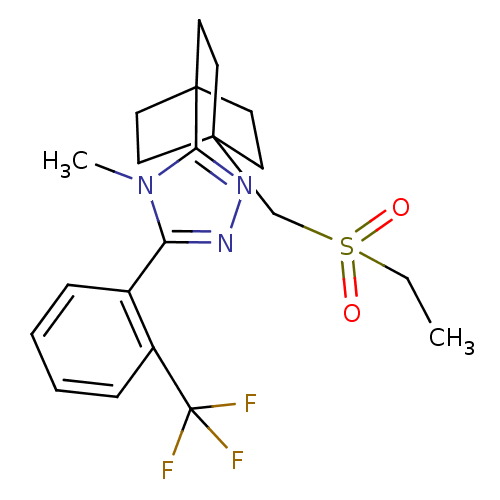
(3-(4-(ethylsulfonylmethyl)bicyclo[2.2.2]octan-1-yl...)Show SMILES CCS(=O)(=O)CC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C21H26F3N3O2S/c1-3-30(28,29)14-19-8-11-20(12-9-19,13-10-19)18-26-25-17(27(18)2)15-6-4-5-7-16(15)21(22,23)24/h4-7H,3,8-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267545

((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C1CC1 |r| Show InChI InChI=1S/C25H24F6N2O3S/c26-24(27,28)18-5-3-16(4-6-18)21-14-22(21)23(34)32-7-9-33(10-8-32)37(35,36)20-12-17(15-1-2-15)11-19(13-20)25(29,30)31/h3-6,11-13,15,21-22H,1-2,7-10,14H2/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316527
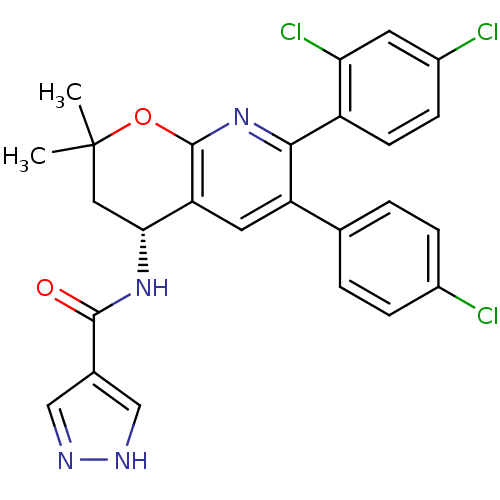
(CHEMBL1097841 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES CC1(C)C[C@@H](NC(=O)c2cn[nH]c2)c2cc(-c3ccc(Cl)cc3)c(nc2O1)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H21Cl3N4O2/c1-26(2)11-22(32-24(34)15-12-30-31-13-15)20-10-19(14-3-5-16(27)6-4-14)23(33-25(20)35-26)18-8-7-17(28)9-21(18)29/h3-10,12-13,22H,11H2,1-2H3,(H,30,31)(H,32,34)/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316529
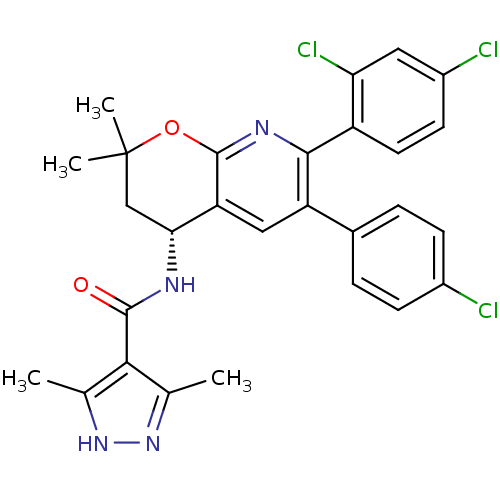
(CHEMBL1095151 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1n[nH]c(C)c1C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H25Cl3N4O2/c1-14-24(15(2)35-34-14)26(36)32-23-13-28(3,4)37-27-21(23)12-20(16-5-7-17(29)8-6-16)25(33-27)19-10-9-18(30)11-22(19)31/h5-12,23H,13H2,1-4H3,(H,32,36)(H,34,35)/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50320187
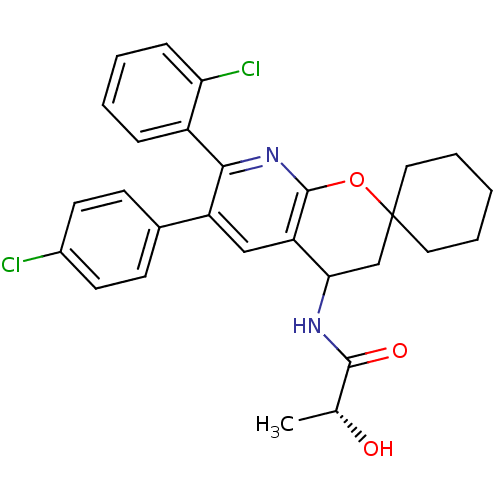
((2R)-N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3'...)Show SMILES C[C@@H](O)C(=O)NC1CC2(CCCCC2)Oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H28Cl2N2O3/c1-17(33)26(34)31-24-16-28(13-5-2-6-14-28)35-27-22(24)15-21(18-9-11-19(29)12-10-18)25(32-27)20-7-3-4-8-23(20)30/h3-4,7-12,15,17,24,33H,2,5-6,13-14,16H2,1H3,(H,31,34)/t17-,24?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267907
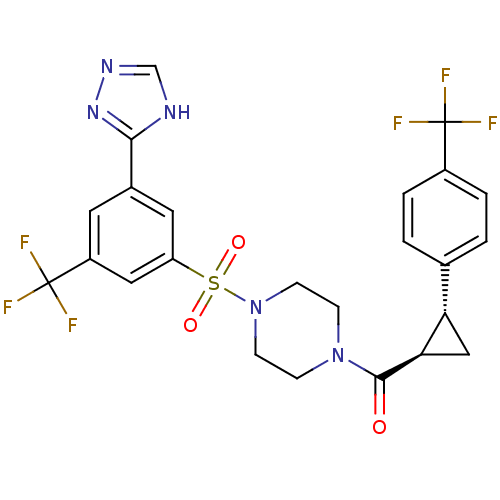
((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-c1nnc[nH]1 |r| Show InChI InChI=1S/C24H21F6N5O3S/c25-23(26,27)16-3-1-14(2-4-16)19-12-20(19)22(36)34-5-7-35(8-6-34)39(37,38)18-10-15(21-31-13-32-33-21)9-17(11-18)24(28,29)30/h1-4,9-11,13,19-20H,5-8,12H2,(H,31,32,33)/t19-,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092190
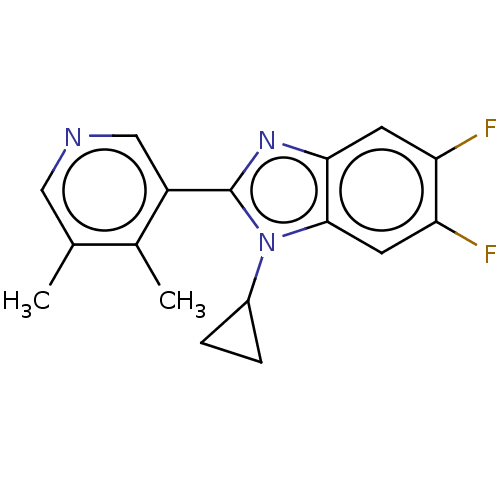
(CHEMBL3582482)Show InChI InChI=1S/C17H15F2N3/c1-9-7-20-8-12(10(9)2)17-21-15-5-13(18)14(19)6-16(15)22(17)11-3-4-11/h5-8,11H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316524
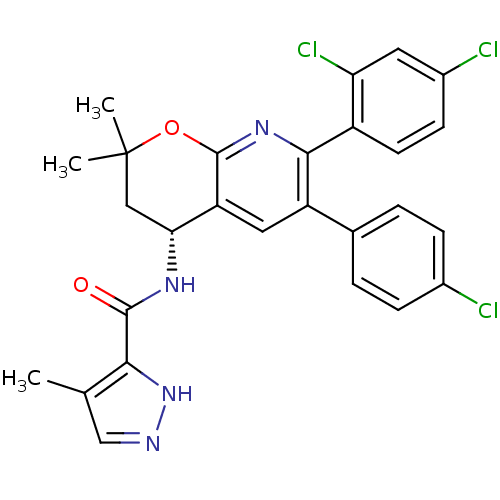
(CHEMBL1095158 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1cn[nH]c1C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H23Cl3N4O2/c1-14-13-31-34-23(14)25(35)32-22-12-27(2,3)36-26-20(22)11-19(15-4-6-16(28)7-5-15)24(33-26)18-9-8-17(29)10-21(18)30/h4-11,13,22H,12H2,1-3H3,(H,31,34)(H,32,35)/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50267545

((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C1CC1 |r| Show InChI InChI=1S/C25H24F6N2O3S/c26-24(27,28)18-5-3-16(4-6-18)21-14-22(21)23(34)32-7-9-33(10-8-32)37(35,36)20-12-17(15-1-2-15)11-19(13-20)25(29,30)31/h3-6,11-13,15,21-22H,1-2,7-10,14H2/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] SR141716 from rat recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cannabinoid CB1 receptor |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]CP-55940 binding to human recombinant CB1 receptor in CHO cells |
J Med Chem 49: 7584-7 (2006)
Article DOI: 10.1021/jm060996+
BindingDB Entry DOI: 10.7270/Q2QN67KG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50320184
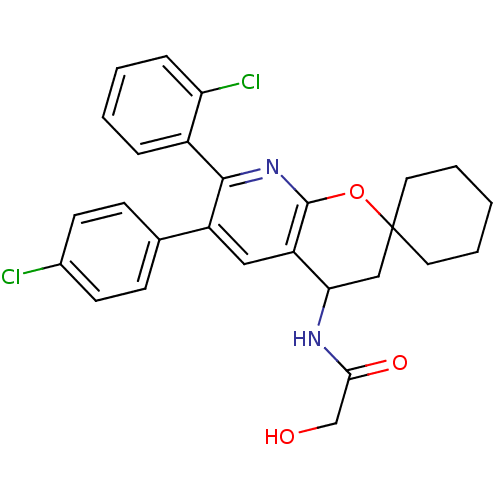
(CHEMBL1086494 | rac-N-(7'-(2-chlorophenyl)-6'-(4-c...)Show SMILES OCC(=O)NC1CC2(CCCCC2)Oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H26Cl2N2O3/c28-18-10-8-17(9-11-18)20-14-21-23(30-24(33)16-32)15-27(12-4-1-5-13-27)34-26(21)31-25(20)19-6-2-3-7-22(19)29/h2-3,6-11,14,23,32H,1,4-5,12-13,15-16H2,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CB1 receptor |
Bioorg Med Chem Lett 20: 1448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.065
BindingDB Entry DOI: 10.7270/Q2028RPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092142

(CHEMBL3582477)Show SMILES CC(C)(O)c1cncc(c1)-c1nc2cc(F)c(F)cc2n1C1CC1 Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6-,8+,9-,10+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267730
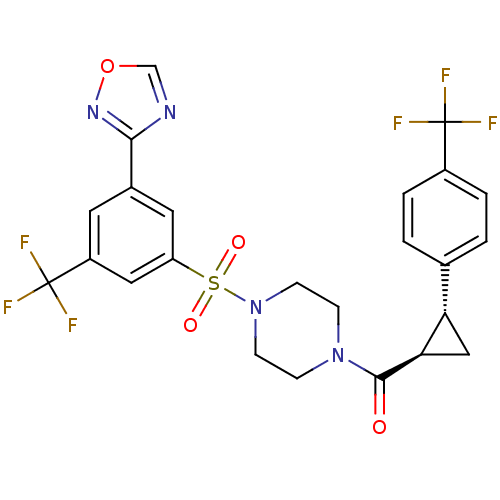
((4-(3-(1,2,4-oxadiazol-3-yl)-5-(trifluoromethyl)ph...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-c1ncon1 |r| Show InChI InChI=1S/C24H20F6N4O4S/c25-23(26,27)16-3-1-14(2-4-16)19-12-20(19)22(35)33-5-7-34(8-6-33)39(36,37)18-10-15(21-31-13-38-32-21)9-17(11-18)24(28,29)30/h1-4,9-11,13,19-20H,5-8,12H2/t19-,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50205166

(CHEMBL231636 | N-((2S,3S)-4-(4-chlorophenyl)-3-(3-...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cncc(c1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-23-13-21(15-33-16-23)27(29,30)31)24(12-18-7-9-22(28)10-8-18)20-6-4-5-19(11-20)14-32/h4-11,13,15-17,24H,12H2,1-3H3,(H,34,35)/t17-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2184-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.087
BindingDB Entry DOI: 10.7270/Q2HM584G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316528
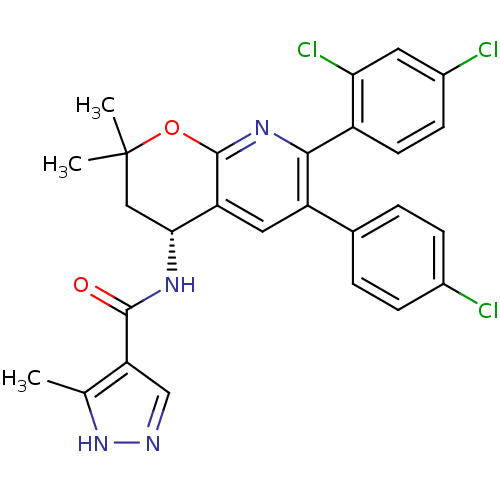
(CHEMBL1095150 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1[nH]ncc1C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H23Cl3N4O2/c1-14-21(13-31-34-14)25(35)32-23-12-27(2,3)36-26-20(23)11-19(15-4-6-16(28)7-5-15)24(33-26)18-9-8-17(29)10-22(18)30/h4-11,13,23H,12H2,1-3H3,(H,31,34)(H,32,35)/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267585
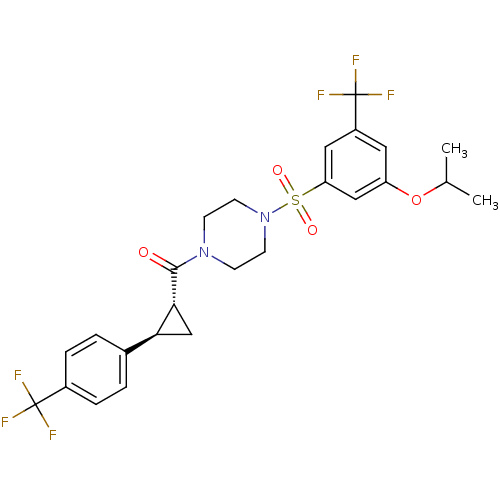
((4-(3-isopropoxy-5-(trifluoromethyl)phenylsulfonyl...)Show SMILES CC(C)Oc1cc(cc(c1)S(=O)(=O)N1CCN(CC1)C(=O)[C@@H]1C[C@H]1c1ccc(cc1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H26F6N2O4S/c1-15(2)37-19-11-18(25(29,30)31)12-20(13-19)38(35,36)33-9-7-32(8-10-33)23(34)22-14-21(22)16-3-5-17(6-4-16)24(26,27)28/h3-6,11-13,15,21-22H,7-10,14H2,1-2H3/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267775
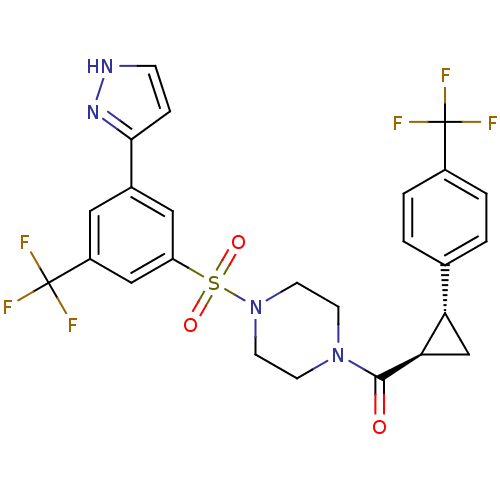
((4-(3-(1H-pyrazol-5-yl)-5-(trifluoromethyl)phenyls...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-c1cc[nH]n1 |r| Show InChI InChI=1S/C25H22F6N4O3S/c26-24(27,28)17-3-1-15(2-4-17)20-14-21(20)23(36)34-7-9-35(10-8-34)39(37,38)19-12-16(22-5-6-32-33-22)11-18(13-19)25(29,30)31/h1-6,11-13,20-21H,7-10,14H2,(H,32,33)/t20-,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50314119
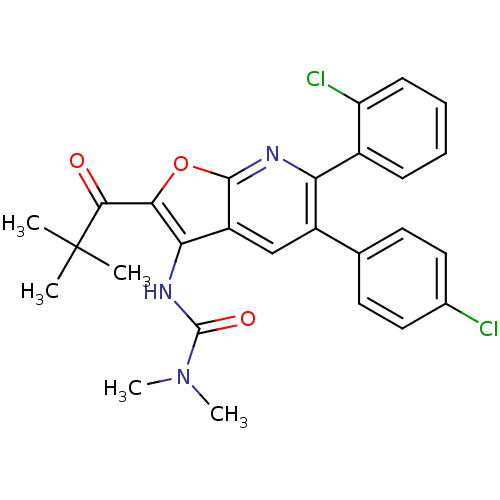
(3-(6-(2-chlorophenyl)-5-(4-chlorophenyl)-2-pivaloy...)Show SMILES CN(C)C(=O)Nc1c(oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1)C(=O)C(C)(C)C Show InChI InChI=1S/C27H25Cl2N3O3/c1-27(2,3)24(33)23-22(31-26(34)32(4)5)19-14-18(15-10-12-16(28)13-11-15)21(30-25(19)35-23)17-8-6-7-9-20(17)29/h6-14H,1-5H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CB1 receptor |
Bioorg Med Chem Lett 20: 1448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.065
BindingDB Entry DOI: 10.7270/Q2028RPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092182
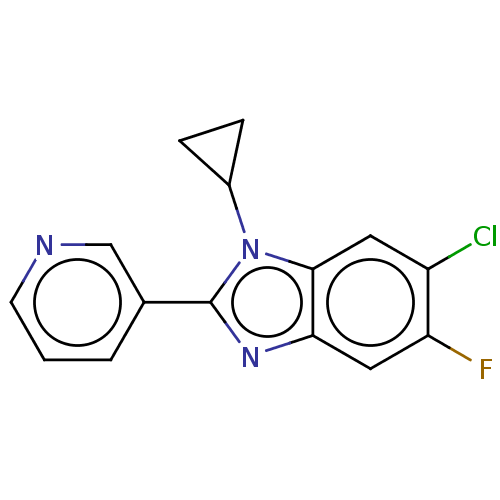
(CHEMBL3582470)Show InChI InChI=1S/C15H11ClFN3/c16-11-6-14-13(7-12(11)17)19-15(20(14)10-3-4-10)9-2-1-5-18-8-9/h1-2,5-8,10H,3-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50320186
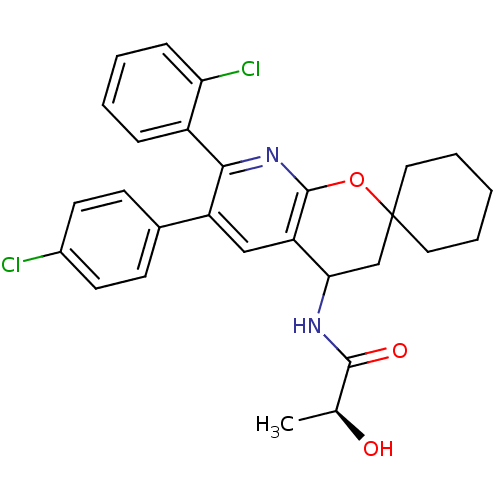
((2S)-N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3'...)Show SMILES C[C@H](O)C(=O)NC1CC2(CCCCC2)Oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H28Cl2N2O3/c1-17(33)26(34)31-24-16-28(13-5-2-6-14-28)35-27-22(24)15-21(18-9-11-19(29)12-10-18)25(32-27)20-7-3-4-8-23(20)30/h3-4,7-12,15,17,24,33H,2,5-6,13-14,16H2,1H3,(H,31,34)/t17-,24?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50314122
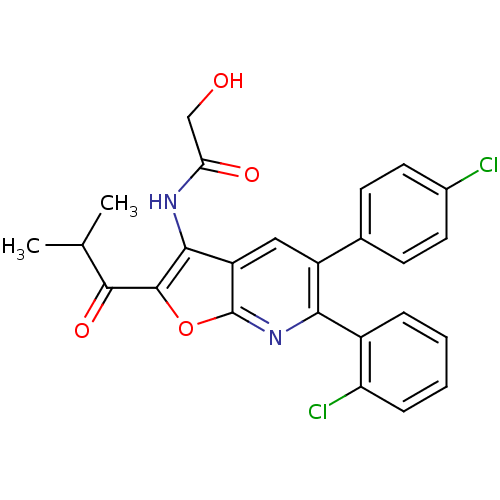
(CHEMBL1091832 | N-(6-(2-chlorophenyl)-5-(4-chlorop...)Show SMILES CC(C)C(=O)c1oc2nc(-c3ccccc3Cl)c(cc2c1NC(=O)CO)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H20Cl2N2O4/c1-13(2)23(32)24-22(28-20(31)12-30)18-11-17(14-7-9-15(26)10-8-14)21(29-25(18)33-24)16-5-3-4-6-19(16)27/h3-11,13,30H,12H2,1-2H3,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CB1 receptor |
Bioorg Med Chem Lett 20: 1448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.065
BindingDB Entry DOI: 10.7270/Q2028RPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092185
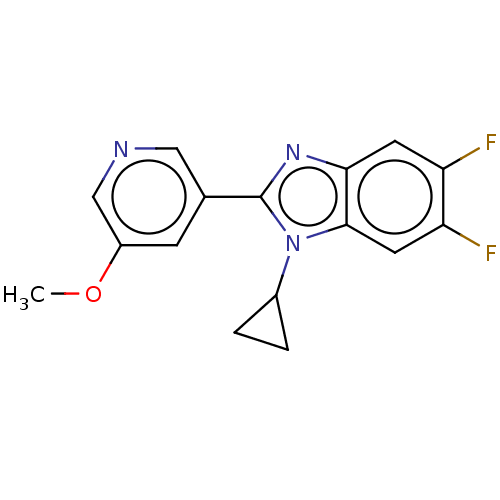
(CHEMBL3582474)Show InChI InChI=1S/C16H13F2N3O/c1-22-11-4-9(7-19-8-11)16-20-14-5-12(17)13(18)6-15(14)21(16)10-2-3-10/h4-8,10H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316525
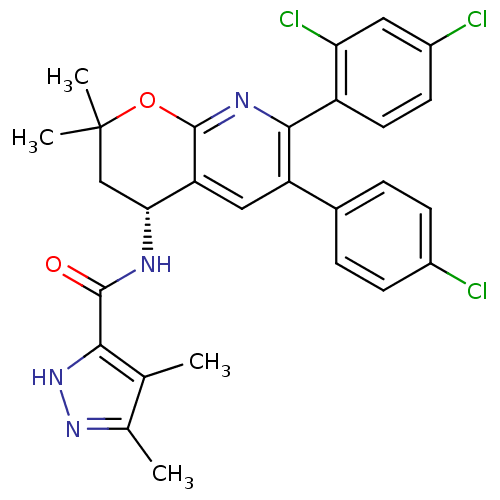
(CHEMBL1095476 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1n[nH]c(C(=O)N[C@@H]2CC(C)(C)Oc3nc(-c4ccc(Cl)cc4Cl)c(cc23)-c2ccc(Cl)cc2)c1C |r| Show InChI InChI=1S/C28H25Cl3N4O2/c1-14-15(2)34-35-24(14)26(36)32-23-13-28(3,4)37-27-21(23)12-20(16-5-7-17(29)8-6-16)25(33-27)19-10-9-18(30)11-22(19)31/h5-12,23H,13H2,1-4H3,(H,32,36)(H,34,35)/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50193700
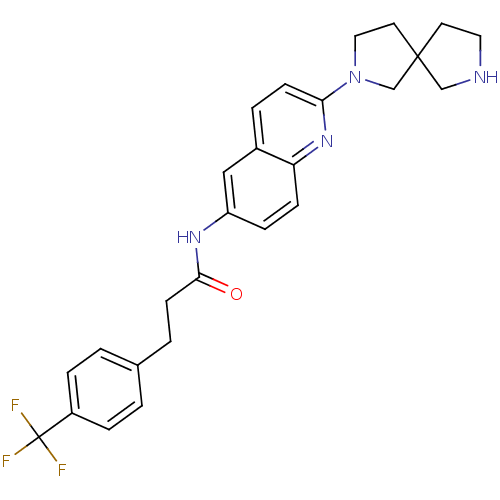
(CHEMBL217502 | N-(2-(2,7-diazaspiro[4.4]nonan-2-yl...)Show SMILES FC(F)(F)c1ccc(CCC(=O)Nc2ccc3nc(ccc3c2)N2CCC3(CCNC3)C2)cc1 Show InChI InChI=1S/C26H27F3N4O/c27-26(28,29)20-5-1-18(2-6-20)3-10-24(34)31-21-7-8-22-19(15-21)4-9-23(32-22)33-14-12-25(17-33)11-13-30-16-25/h1-2,4-9,15,30H,3,10-14,16-17H2,(H,31,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of 125I-[Phe13,Tyr19]-MCH from human MCH-R1 |
Bioorg Med Chem Lett 16: 5270-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.006
BindingDB Entry DOI: 10.7270/Q2ZC82HP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200833
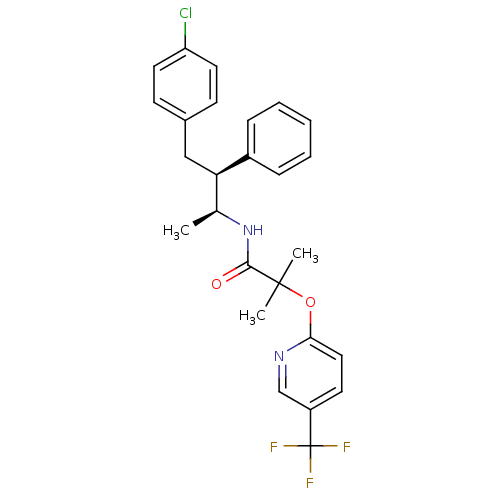
(CHEMBL373626 | N-((2S,3S)-4-(4-chlorophenyl)-3-phe...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C26H26ClF3N2O2/c1-17(22(19-7-5-4-6-8-19)15-18-9-12-21(27)13-10-18)32-24(33)25(2,3)34-23-14-11-20(16-31-23)26(28,29)30/h4-14,16-17,22H,15H2,1-3H3,(H,32,33)/t17-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]CP-55940 binding to human recombinant CB1 receptor in CHO cells |
J Med Chem 49: 7584-7 (2006)
Article DOI: 10.1021/jm060996+
BindingDB Entry DOI: 10.7270/Q2QN67KG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267487
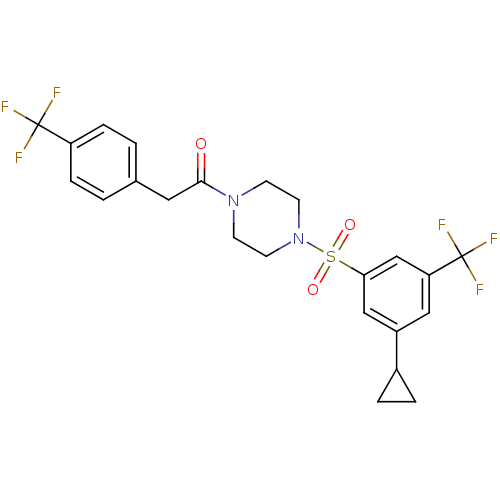
(1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...)Show SMILES FC(F)(F)c1ccc(CC(=O)N2CCN(CC2)S(=O)(=O)c2cc(cc(c2)C(F)(F)F)C2CC2)cc1 Show InChI InChI=1S/C23H22F6N2O3S/c24-22(25,26)18-5-1-15(2-6-18)11-21(32)30-7-9-31(10-8-30)35(33,34)20-13-17(16-3-4-16)12-19(14-20)23(27,28)29/h1-2,5-6,12-14,16H,3-4,7-11H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267584
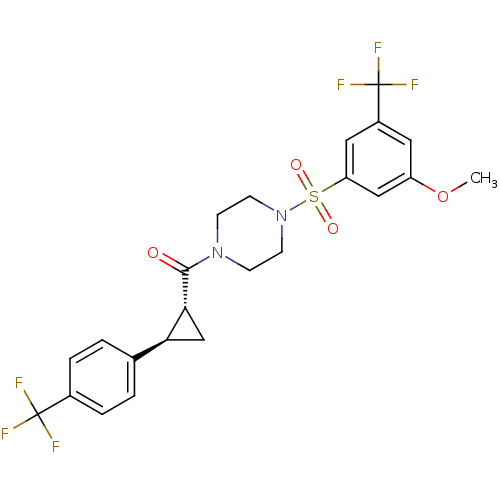
((4-(3-methoxy-5-(trifluoromethyl)phenylsulfonyl)pi...)Show SMILES COc1cc(cc(c1)S(=O)(=O)N1CCN(CC1)C(=O)[C@@H]1C[C@H]1c1ccc(cc1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H22F6N2O4S/c1-35-17-10-16(23(27,28)29)11-18(12-17)36(33,34)31-8-6-30(7-9-31)21(32)20-13-19(20)14-2-4-15(5-3-14)22(24,25)26/h2-5,10-12,19-20H,6-9,13H2,1H3/t19-,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267545

((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C1CC1 |r| Show InChI InChI=1S/C25H24F6N2O3S/c26-24(27,28)18-5-3-16(4-6-18)21-14-22(21)23(34)32-7-9-33(10-8-32)37(35,36)20-12-17(15-1-2-15)11-19(13-20)25(29,30)31/h3-6,11-13,15,21-22H,1-2,7-10,14H2/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092137
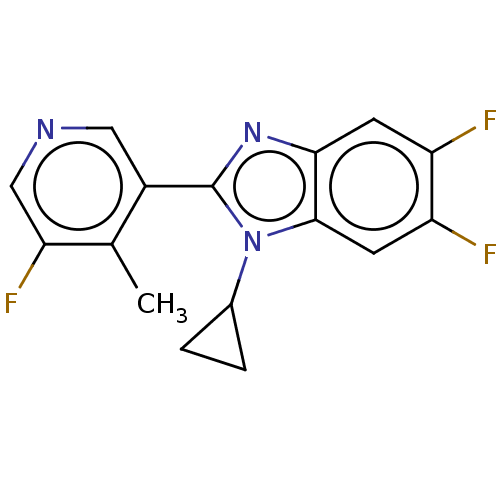
(CHEMBL3582481)Show InChI InChI=1S/C16H12F3N3/c1-8-10(6-20-7-13(8)19)16-21-14-4-11(17)12(18)5-15(14)22(16)9-2-3-9/h4-7,9H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50314118
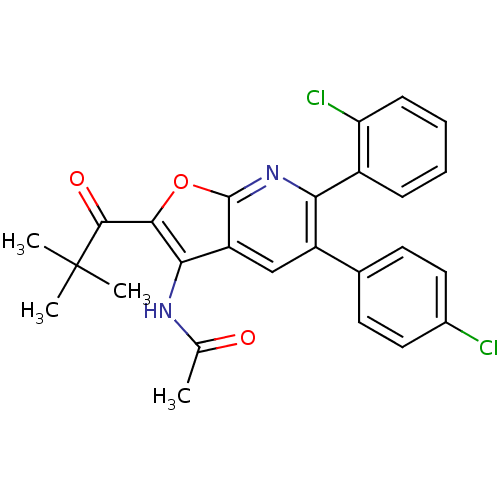
(CHEMBL1090402 | N-(6-(2-chlorophenyl)-5-(4-chlorop...)Show SMILES CC(=O)Nc1c(oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1)C(=O)C(C)(C)C Show InChI InChI=1S/C26H22Cl2N2O3/c1-14(31)29-22-19-13-18(15-9-11-16(27)12-10-15)21(17-7-5-6-8-20(17)28)30-25(19)33-23(22)24(32)26(2,3)4/h5-13H,1-4H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CB1 receptor |
Bioorg Med Chem Lett 20: 1448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.065
BindingDB Entry DOI: 10.7270/Q2028RPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092183
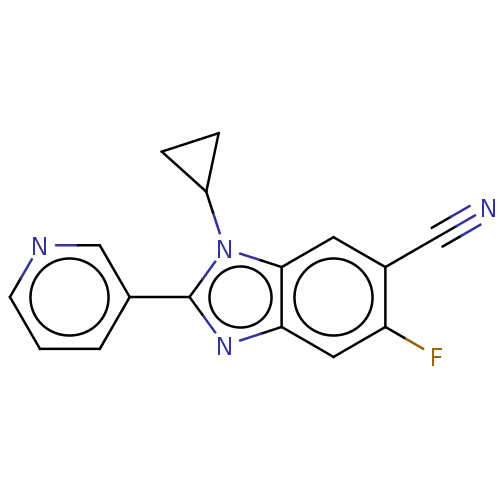
(CHEMBL3582471)Show InChI InChI=1S/C16H11FN4/c17-13-7-14-15(6-11(13)8-18)21(12-3-4-12)16(20-14)10-2-1-5-19-9-10/h1-2,5-7,9,12H,3-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092191
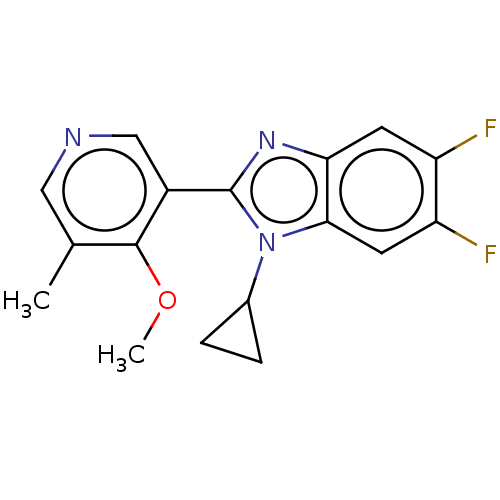
(CHEMBL3582483)Show InChI InChI=1S/C17H15F2N3O/c1-9-7-20-8-11(16(9)23-2)17-21-14-5-12(18)13(19)6-15(14)22(17)10-3-4-10/h5-8,10H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267688
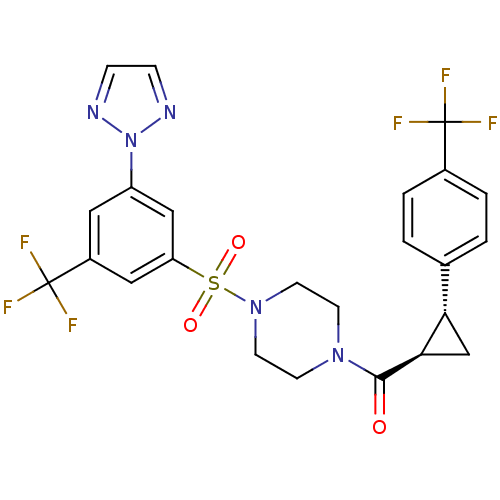
((4-(3-(2H-1,2,3-triazol-2-yl)-5-(trifluoromethyl)p...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-n1nccn1 |r| Show InChI InChI=1S/C24H21F6N5O3S/c25-23(26,27)16-3-1-15(2-4-16)20-14-21(20)22(36)33-7-9-34(10-8-33)39(37,38)19-12-17(24(28,29)30)11-18(13-19)35-31-5-6-32-35/h1-6,11-13,20-21H,7-10,14H2/t20-,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316520

(CHEMBL1095159 | N-[6-(4-Chlorophenyl)-7-(2,4-dichl...)Show SMILES CC1(C)CC(NC(=O)C(F)(F)CO)c2cc(-c3ccc(Cl)cc3)c(nc2O1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H21Cl3F2N2O3/c1-24(2)11-20(31-23(34)25(29,30)12-33)18-10-17(13-3-5-14(26)6-4-13)21(32-22(18)35-24)16-8-7-15(27)9-19(16)28/h3-10,20,33H,11-12H2,1-2H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316532

(CHEMBL1095554 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES CC1(C)C[C@@H](NC(=O)c2ccc3[nH]ncc3c2)c2cc(-c3ccc(Cl)cc3)c(nc2O1)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C30H23Cl3N4O2/c1-30(2)14-26(35-28(38)17-5-10-25-18(11-17)15-34-37-25)23-13-22(16-3-6-19(31)7-4-16)27(36-29(23)39-30)21-9-8-20(32)12-24(21)33/h3-13,15,26H,14H2,1-2H3,(H,34,37)(H,35,38)/t26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316531
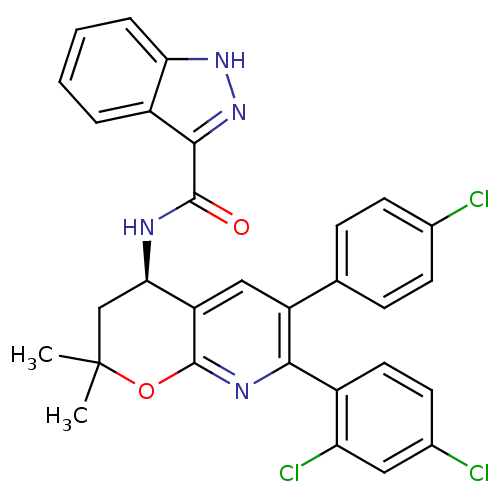
(CHEMBL1095553 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES CC1(C)C[C@@H](NC(=O)c2n[nH]c3ccccc23)c2cc(-c3ccc(Cl)cc3)c(nc2O1)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C30H23Cl3N4O2/c1-30(2)15-25(34-28(38)27-20-5-3-4-6-24(20)36-37-27)22-14-21(16-7-9-17(31)10-8-16)26(35-29(22)39-30)19-12-11-18(32)13-23(19)33/h3-14,25H,15H2,1-2H3,(H,34,38)(H,36,37)/t25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316518
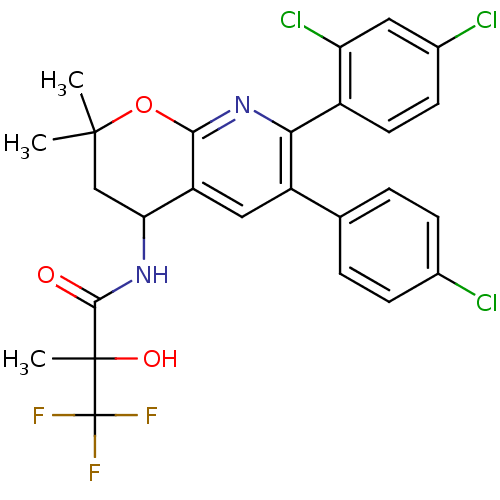
(CHEMBL1097455 | N-[6-(4-Chlorophenyl)-7-(2,4-dichl...)Show SMILES CC(O)(C(=O)NC1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C26H22Cl3F3N2O3/c1-24(2)12-20(33-23(35)25(3,36)26(30,31)32)18-11-17(13-4-6-14(27)7-5-13)21(34-22(18)37-24)16-9-8-15(28)10-19(16)29/h4-11,20,36H,12H2,1-3H3,(H,33,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267687
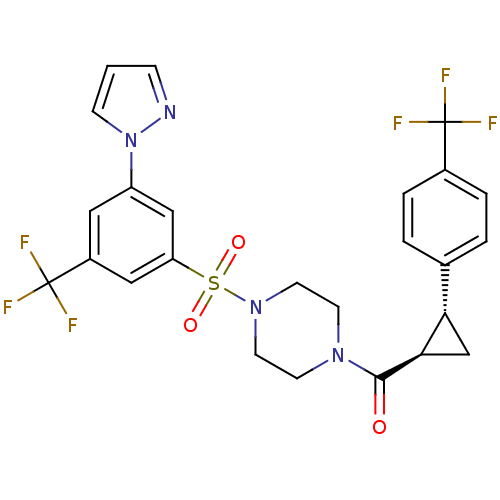
((4-(3-(1H-pyrazol-1-yl)-5-(trifluoromethyl)phenyls...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-n1cccn1 |r| Show InChI InChI=1S/C25H22F6N4O3S/c26-24(27,28)17-4-2-16(3-5-17)21-15-22(21)23(36)33-8-10-34(11-9-33)39(37,38)20-13-18(25(29,30)31)12-19(14-20)35-7-1-6-32-35/h1-7,12-14,21-22H,8-11,15H2/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50320185
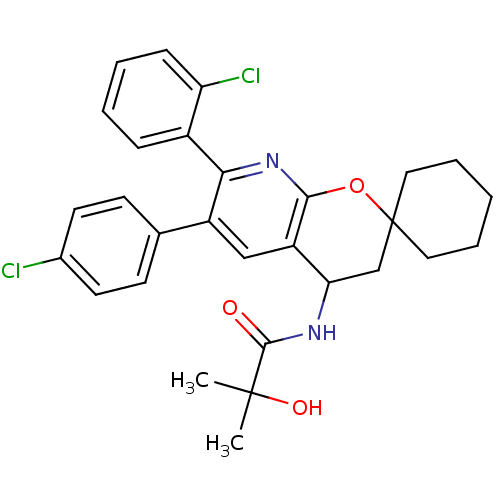
(CHEMBL1085566 | N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOR...)Show SMILES CC(C)(O)C(=O)NC1CC2(CCCCC2)Oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C29H30Cl2N2O3/c1-28(2,35)27(34)32-24-17-29(14-6-3-7-15-29)36-26-22(24)16-21(18-10-12-19(30)13-11-18)25(33-26)20-8-4-5-9-23(20)31/h4-5,8-13,16,24,35H,3,6-7,14-15,17H2,1-2H3,(H,32,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50316513
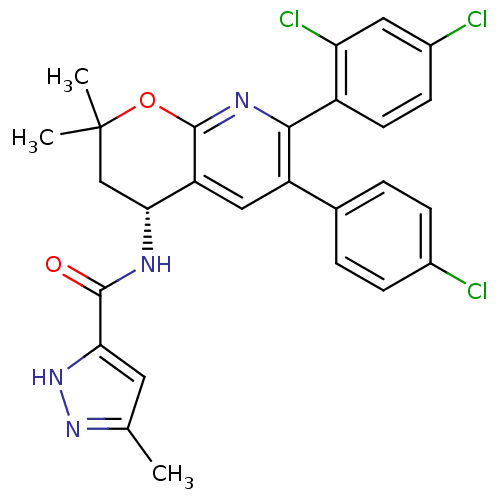
(CHEMBL1096182 | N-((4R)-6-(4-chlorophenyl)-7-(2,4-...)Show SMILES Cc1cc([nH]n1)C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H23Cl3N4O2/c1-14-10-22(34-33-14)25(35)31-23-13-27(2,3)36-26-20(23)12-19(15-4-6-16(28)7-5-15)24(32-26)18-9-8-17(29)11-21(18)30/h4-12,23H,13H2,1-3H3,(H,31,35)(H,33,34)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat cannabinoid CB1 receptor |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316519
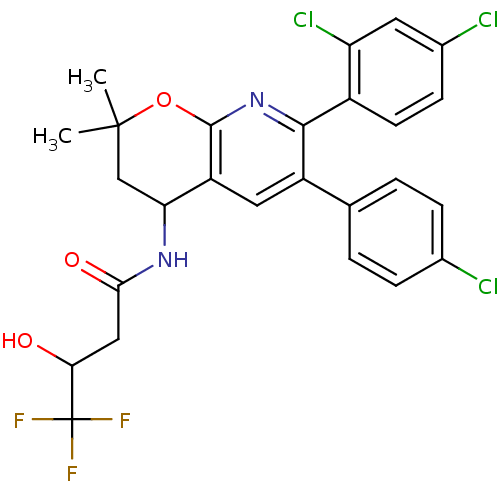
(CHEMBL1097456 | N-[6-(4-CHLOROPHENYL)-7-(2,4-DICHL...)Show SMILES CC1(C)CC(NC(=O)CC(O)C(F)(F)F)c2cc(-c3ccc(Cl)cc3)c(nc2O1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H22Cl3F3N2O3/c1-25(2)12-20(33-22(36)11-21(35)26(30,31)32)18-10-17(13-3-5-14(27)6-4-13)23(34-24(18)37-25)16-8-7-15(28)9-19(16)29/h3-10,20-21,35H,11-12H2,1-2H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092186
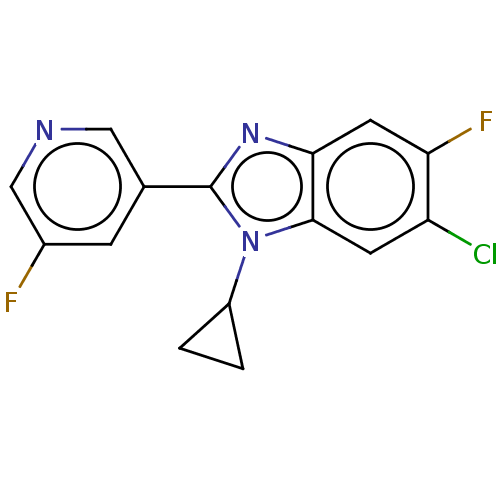
(CHEMBL3582475)Show InChI InChI=1S/C15H10ClF2N3/c16-11-4-14-13(5-12(11)18)20-15(21(14)10-1-2-10)8-3-9(17)7-19-6-8/h3-7,10H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50204183
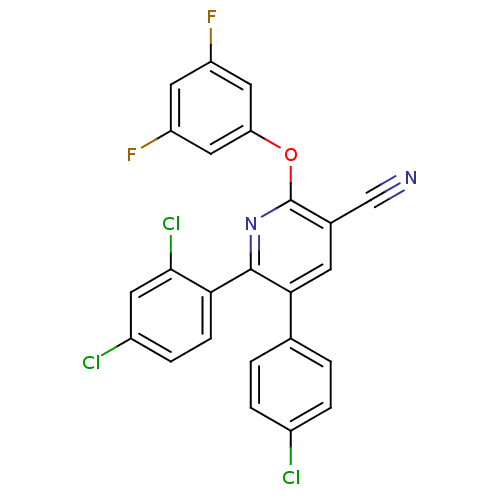
(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)-2-(3,5-d...)Show SMILES Fc1cc(F)cc(Oc2nc(-c3ccc(Cl)cc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C24H11Cl3F2N2O/c25-15-3-1-13(2-4-15)21-7-14(12-30)24(32-19-10-17(28)9-18(29)11-19)31-23(21)20-6-5-16(26)8-22(20)27/h1-11H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at CB1 receptor |
Bioorg Med Chem Lett 17: 2031-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.005
BindingDB Entry DOI: 10.7270/Q27944BJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316513

(CHEMBL1096182 | N-((4R)-6-(4-chlorophenyl)-7-(2,4-...)Show SMILES Cc1cc([nH]n1)C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H23Cl3N4O2/c1-14-10-22(34-33-14)25(35)31-23-13-27(2,3)36-26-20(23)12-19(15-4-6-16(28)7-5-15)24(32-26)18-9-8-17(29)11-21(18)30/h4-12,23H,13H2,1-3H3,(H,31,35)(H,33,34)/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data