

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297852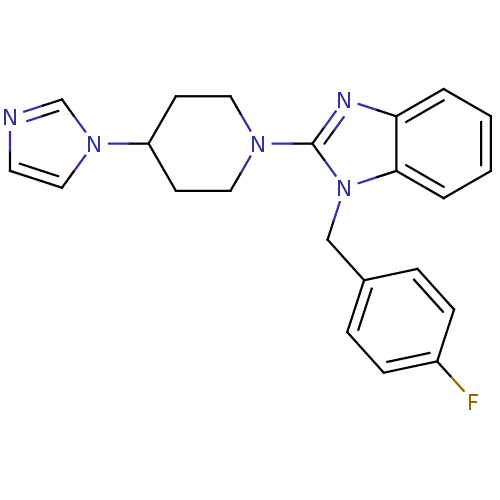 (2-(4-(1H-imidazol-1-yl)piperidin-1-yl)-1-(4-fluoro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297863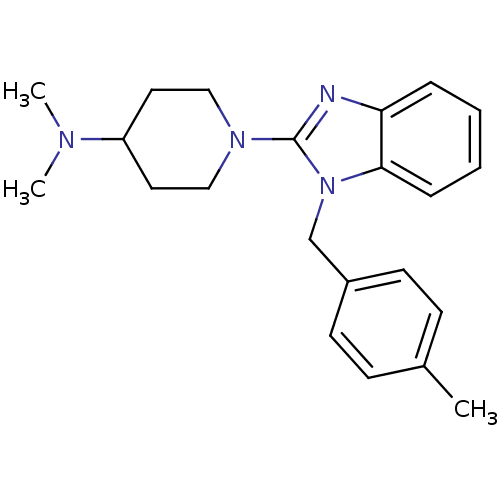 (CHEMBL563451 | N,N-dimethyl-1-(1-(4-methylbenzyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297864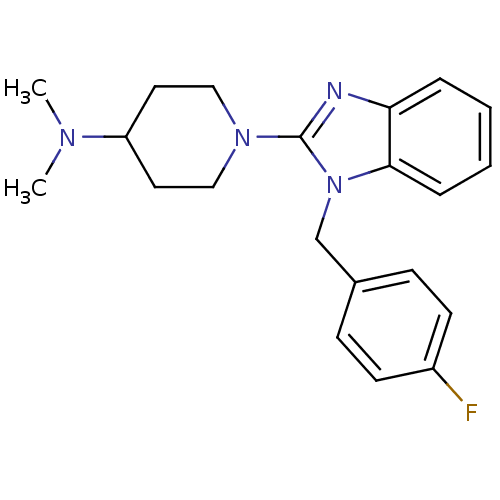 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297859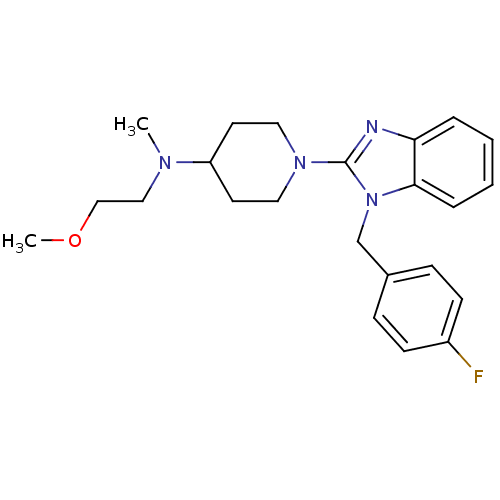 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297869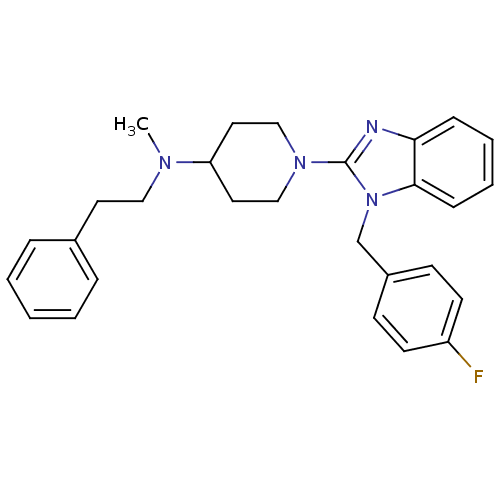 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297866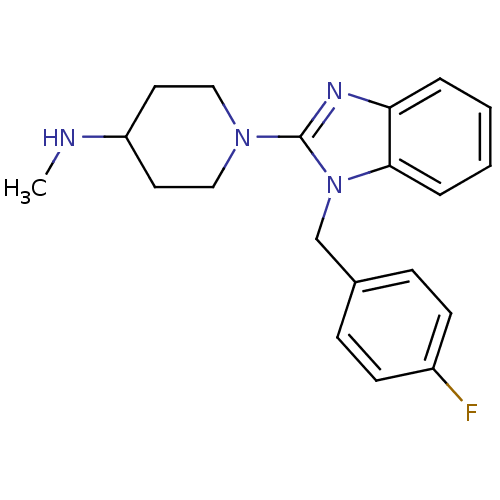 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297862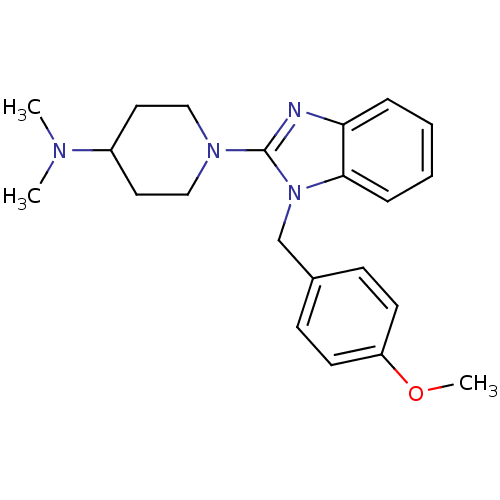 (1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22877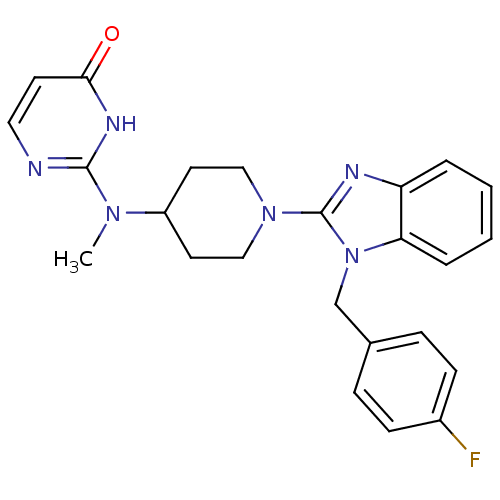 (2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297867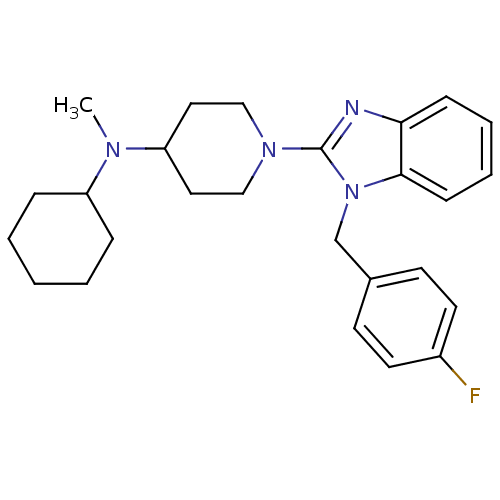 (CHEMBL558933 | N-cyclohexyl-1-(1-(4-fluorobenzyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297855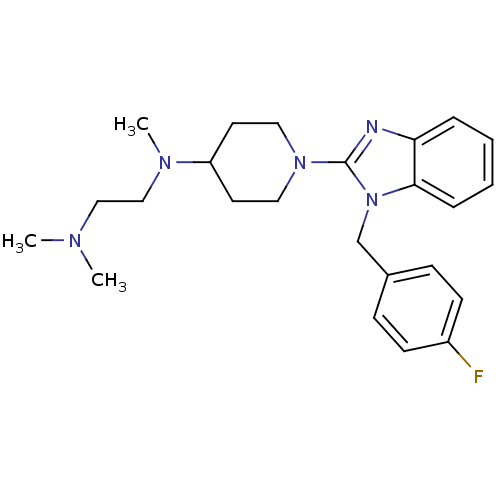 (CHEMBL559061 | N1-(1-(1-(4-fluorobenzyl)-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297858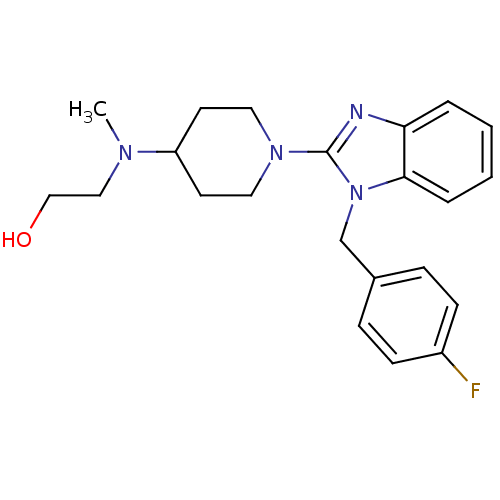 (2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297870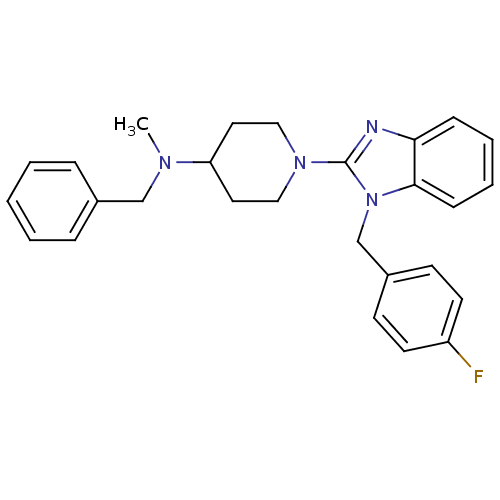 (CHEMBL551888 | N-benzyl-1-(1-(4-fluorobenzyl)-1H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297861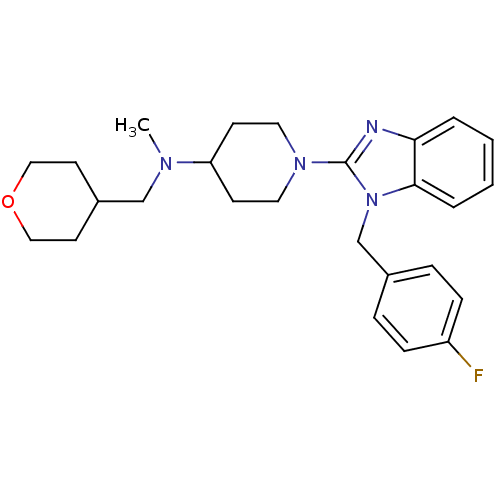 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297857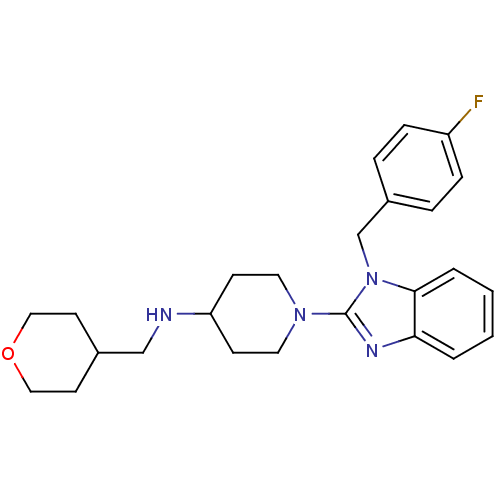 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297854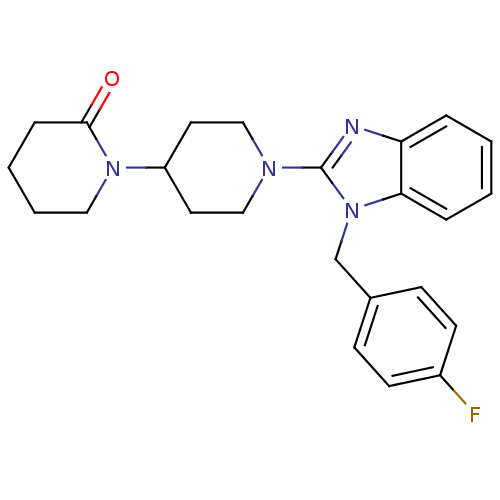 (1'-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297850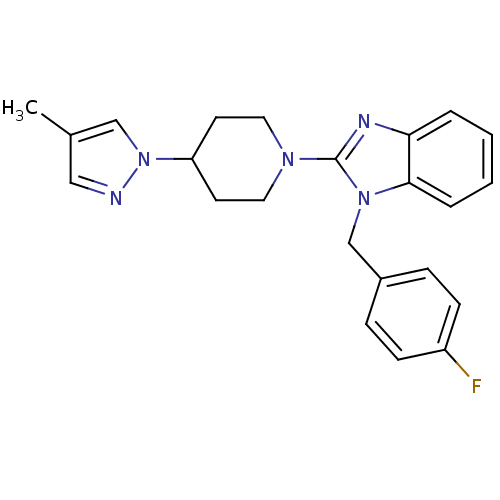 (1-(4-fluorobenzyl)-2-(4-(4-methyl-1H-pyrazol-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297849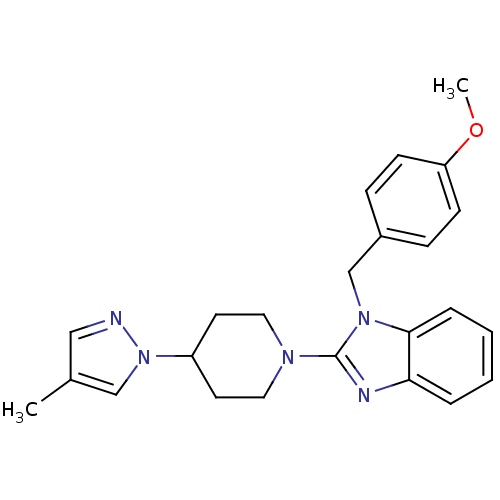 (1-(4-methoxybenzyl)-2-(4-(4-methyl-1H-pyrazol-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297860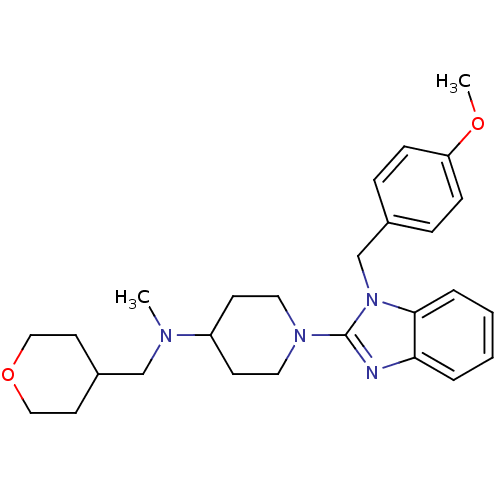 (1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297856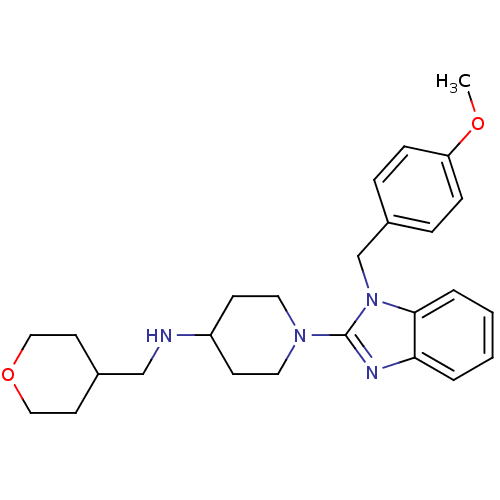 (1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297861 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297851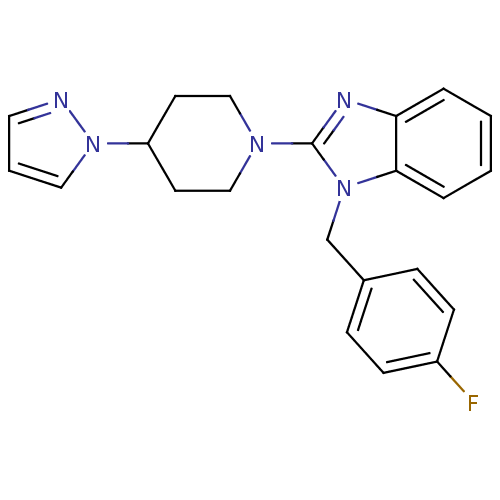 (2-(4-(1H-pyrazol-1-yl)piperidin-1-yl)-1-(4-fluorob...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897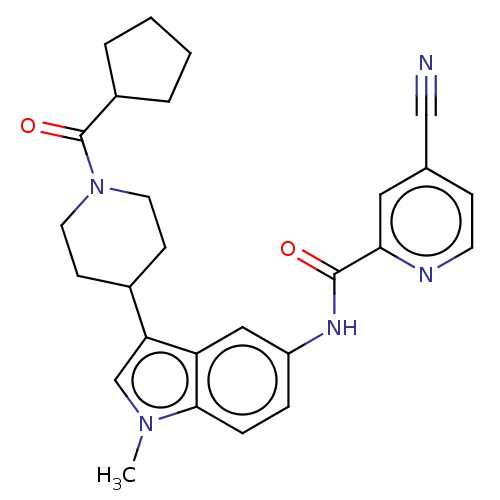 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891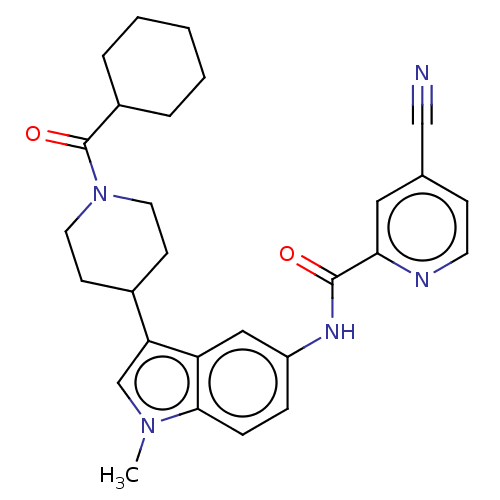 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249567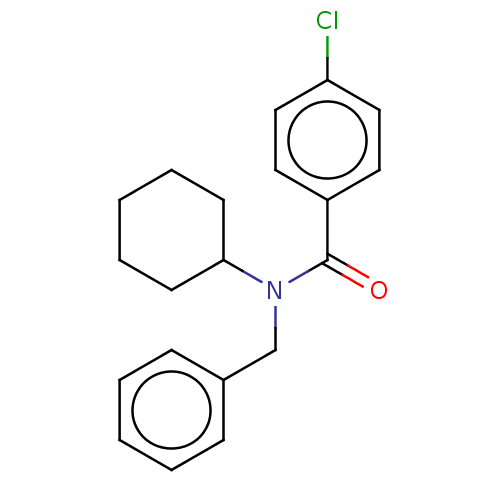 (CHEMBL4075936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114347 BindingDB Entry DOI: 10.7270/Q2PG1WTJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297859 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893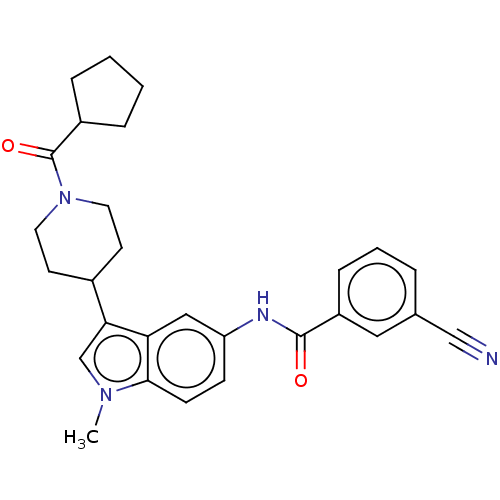 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297864 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297853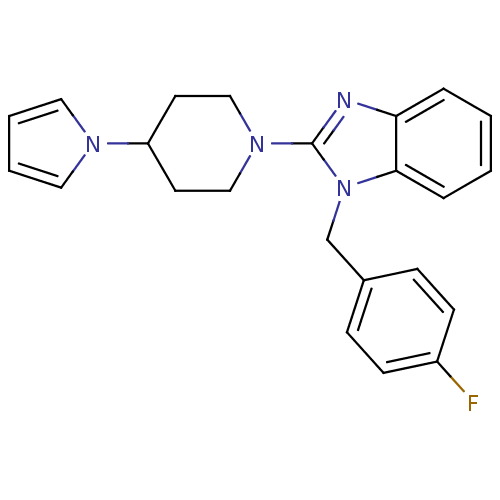 (2-(4-(1H-pyrrol-1-yl)piperidin-1-yl)-1-(4-fluorobe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297863 (CHEMBL563451 | N,N-dimethyl-1-(1-(4-methylbenzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297872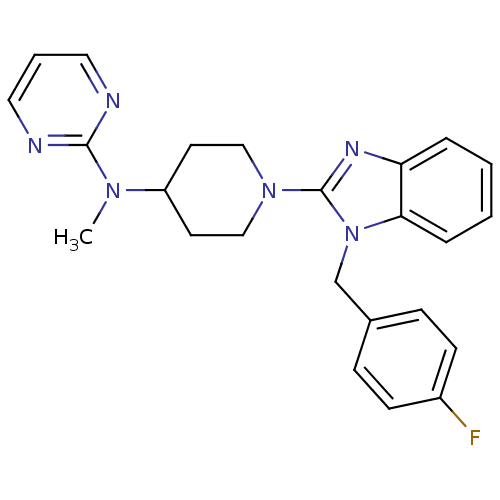 (CHEMBL561489 | N-(1-(1-(4-fluorobenzyl)-1H-benzo[d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50297869 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297860 (1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297858 (2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297852 (2-(4-(1H-imidazol-1-yl)piperidin-1-yl)-1-(4-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297853 (2-(4-(1H-pyrrol-1-yl)piperidin-1-yl)-1-(4-fluorobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297862 (1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297857 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50297869 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297855 (CHEMBL559061 | N1-(1-(1-(4-fluorobenzyl)-1H-benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50297870 (CHEMBL551888 | N-benzyl-1-(1-(4-fluorobenzyl)-1H-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 805 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50297866 (1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 988 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50297872 (CHEMBL561489 | N-(1-(1-(4-fluorobenzyl)-1H-benzo[d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466892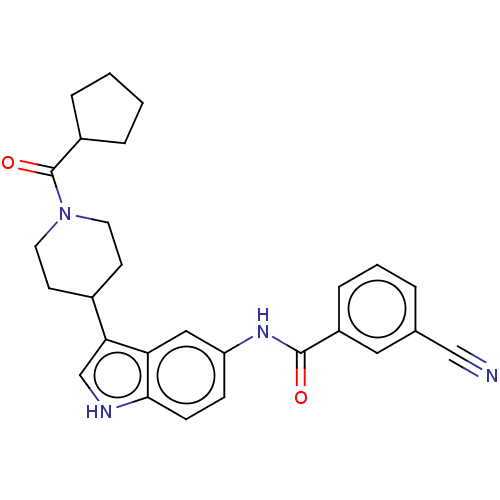 (CHEMBL4283871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297856 (1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50297867 (CHEMBL558933 | N-cyclohexyl-1-(1-(4-fluorobenzyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50297867 (CHEMBL558933 | N-cyclohexyl-1-(1-(4-fluorobenzyl)-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50241570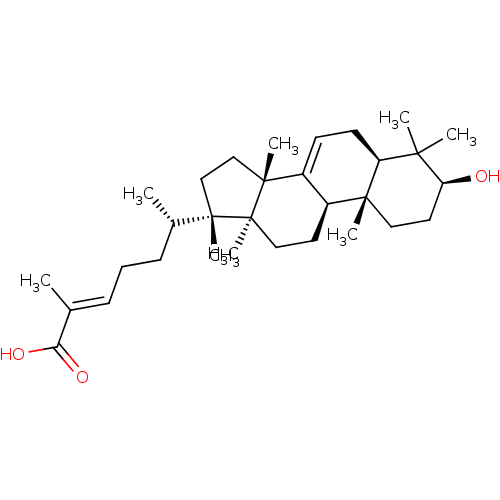 ((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta after 20 mins by uncompetitive inhibition assay in presence of activated calf thymus DNA and 0.1 mg... | J Nat Prod 63: 1356-60 (2000) BindingDB Entry DOI: 10.7270/Q2X066S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297851 (2-(4-(1H-pyrazol-1-yl)piperidin-1-yl)-1-(4-fluorob...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50297870 (CHEMBL551888 | N-benzyl-1-(1-(4-fluorobenzyl)-1H-b...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50297850 (1-(4-fluorobenzyl)-2-(4-(4-methyl-1H-pyrazol-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay | Bioorg Med Chem Lett 19: 4380-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.086 BindingDB Entry DOI: 10.7270/Q2KS6RM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2669 total ) | Next | Last >> |