 Found 98 hits with Last Name = 'swenson' and Initial = 're'
Found 98 hits with Last Name = 'swenson' and Initial = 're' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Progonadoliberin-1
(RAT) | BDBM84726
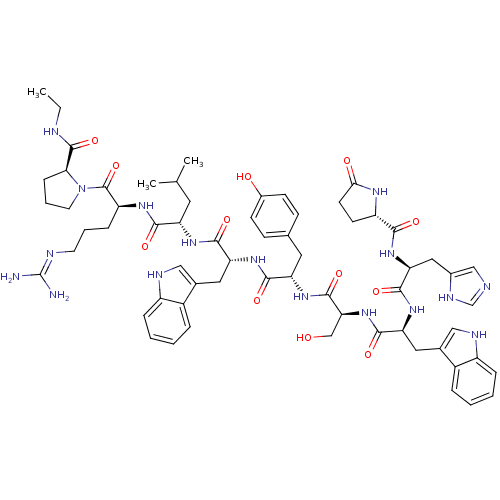
(deslorelin)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,63.79,5.4,77.90,87.93,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C64H83N17O12/c1-4-68-62(92)53-16-10-24-81(53)63(93)46(15-9-23-69-64(65)66)74-56(86)47(25-35(2)3)75-58(88)49(27-37-30-70-43-13-7-5-11-41(37)43)77-57(87)48(26-36-17-19-40(83)20-18-36)76-61(91)52(33-82)80-59(89)50(28-38-31-71-44-14-8-6-12-42(38)44)78-60(90)51(29-39-32-67-34-72-39)79-55(85)45-21-22-54(84)73-45/h5-8,11-14,17-20,30-32,34-35,45-53,70-71,82-83H,4,9-10,15-16,21-29,33H2,1-3H3,(H,67,72)(H,68,92)(H,73,84)(H,74,86)(H,75,88)(H,76,91)(H,77,87)(H,78,90)(H,79,85)(H,80,89)(H4,65,66,69)/t45-,46-,47-,48-,49+,50-,51-,52-,53-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84707

(nafarelin)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:41.56,65.70,23.34,74.80,88.96,wD:35.40,55.67,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.6,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C66H83N17O13/c1-36(2)25-48(58(89)76-47(13-7-23-71-66(68)69)65(96)83-24-8-14-54(83)64(95)73-33-55(67)86)77-60(91)50(28-38-15-18-39-9-3-4-10-40(39)26-38)78-59(90)49(27-37-16-19-43(85)20-17-37)79-63(94)53(34-84)82-61(92)51(29-41-31-72-45-12-6-5-11-44(41)45)80-62(93)52(30-42-32-70-35-74-42)81-57(88)46-21-22-56(87)75-46/h3-6,9-12,15-20,26,31-32,35-36,46-54,72,84-85H,7-8,13-14,21-25,27-30,33-34H2,1-2H3,(H2,67,86)(H,70,74)(H,73,95)(H,75,87)(H,76,89)(H,77,91)(H,78,90)(H,79,94)(H,80,93)(H,81,88)(H,82,92)(H4,68,69,71)/t46-,47-,48-,49-,50+,51-,52-,53-,54-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50469862
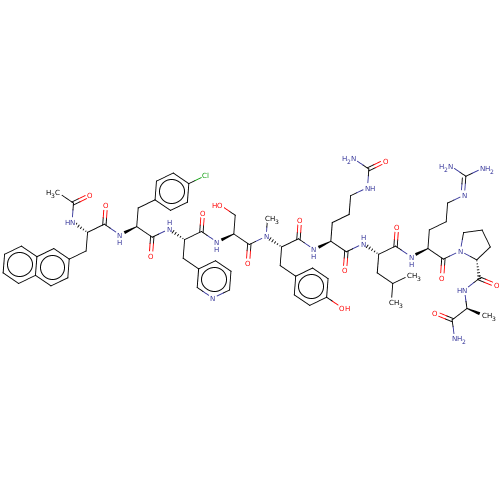
(CHEMBL409219)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-c1cccnc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#7])=O Show InChI InChI=1S/C71H94ClN17O14/c1-40(2)32-53(62(95)83-52(16-10-29-78-70(74)75)69(102)89-31-11-17-58(89)66(99)80-41(3)60(73)93)84-61(94)51(15-9-30-79-71(76)103)82-67(100)59(37-44-21-26-50(92)27-22-44)88(5)68(101)57(39-90)87-65(98)56(36-46-12-8-28-77-38-46)86-64(97)55(34-43-19-24-49(72)25-20-43)85-63(96)54(81-42(4)91)35-45-18-23-47-13-6-7-14-48(47)33-45/h6-8,12-14,18-28,33,38,40-41,51-59,90,92H,9-11,15-17,29-32,34-37,39H2,1-5H3,(H2,73,93)(H,80,99)(H,81,91)(H,82,100)(H,83,95)(H,84,94)(H,85,96)(H,86,97)(H,87,98)(H4,74,75,78)(H3,76,79,103)/t41-,51-,52-,53-,54-,55-,56-,57-,58+,59-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... |
J Med Chem 36: 928-33 (1993)
Article DOI: 10.1021/jm00059a020
BindingDB Entry DOI: 10.7270/Q2Z03BW7 |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84712
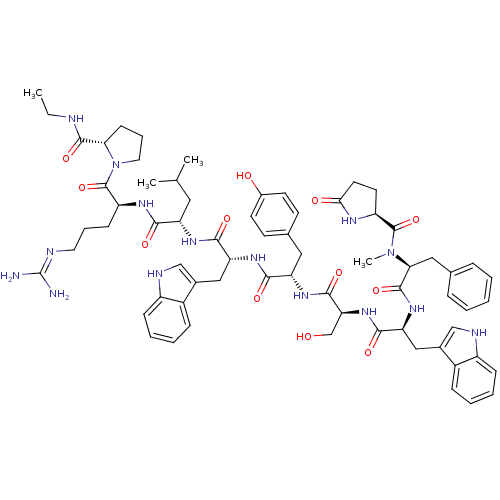
(deslorelin 2NMePhe | deslorelin 2Phe)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,63.79,5.4,77.91,89.95,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.53,2.14,;13.13,.66,;14.22,-.43,;15.71,-.03,;16.11,1.45,;15.02,2.54,;10.35,4.51,;11.12,5.85,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C68H87N15O12/c1-5-71-64(92)56-22-14-30-83(56)67(95)49(21-13-29-72-68(69)70)76-59(87)51(31-39(2)3)77-61(89)53(34-42-36-73-47-19-11-9-17-45(42)47)79-60(88)52(32-41-23-25-44(85)26-24-41)78-63(91)55(38-84)81-62(90)54(35-43-37-74-48-20-12-10-18-46(43)48)80-65(93)57(33-40-15-7-6-8-16-40)82(4)66(94)50-27-28-58(86)75-50/h6-12,15-20,23-26,36-37,39,49-57,73-74,84-85H,5,13-14,21-22,27-35,38H2,1-4H3,(H,71,92)(H,75,86)(H,76,87)(H,77,89)(H,78,91)(H,79,88)(H,80,93)(H,81,90)(H4,69,70,72)/t49-,50-,51-,52-,53+,54-,55-,56-,57-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84708
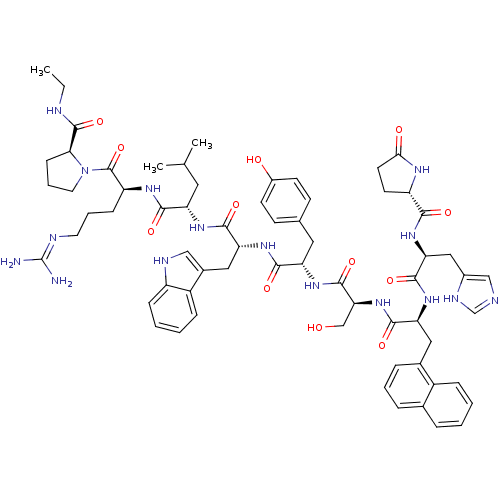
(deslorelin 31Nal)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,63.80,5.4,78.91,88.94,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.27,-3.14,;8.73,-3.13,;7.96,-4.47,;8.73,-5.8,;10.27,-5.8,;11.04,-7.14,;12.58,-7.14,;13.35,-5.8,;12.58,-4.47,;11.04,-4.47,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C66H84N16O12/c1-4-70-64(93)55-19-11-27-82(55)65(94)48(18-10-26-71-66(67)68)75-58(87)49(28-37(2)3)76-60(89)52(31-41-33-72-46-17-8-7-16-45(41)46)79-59(88)50(29-38-20-22-43(84)23-21-38)77-63(92)54(35-83)81-61(90)51(30-40-14-9-13-39-12-5-6-15-44(39)40)78-62(91)53(32-42-34-69-36-73-42)80-57(86)47-24-25-56(85)74-47/h5-9,12-17,20-23,33-34,36-37,47-55,72,83-84H,4,10-11,18-19,24-32,35H2,1-3H3,(H,69,73)(H,70,93)(H,74,85)(H,75,87)(H,76,89)(H,77,92)(H,78,91)(H,79,88)(H,80,86)(H,81,90)(H4,67,68,71)/t47-,48-,49-,50-,51-,52+,53-,54-,55-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50469858
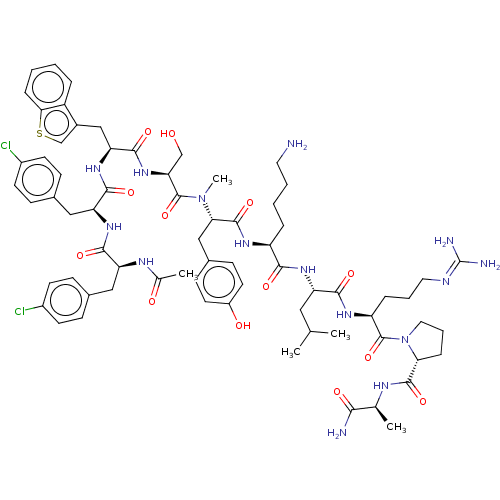
(CHEMBL268397)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-c1csc2ccccc12)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#7])=O Show InChI InChI=1S/C70H93Cl2N15O13S/c1-39(2)32-52(62(93)81-51(14-10-30-77-70(75)76)69(100)87-31-11-15-57(87)66(97)78-40(3)60(74)91)82-61(92)50(13-8-9-29-73)80-67(98)58(35-44-21-27-48(90)28-22-44)86(5)68(99)56(37-88)85-65(96)55(36-45-38-101-59-16-7-6-12-49(45)59)84-64(95)54(34-43-19-25-47(72)26-20-43)83-63(94)53(79-41(4)89)33-42-17-23-46(71)24-18-42/h6-7,12,16-28,38-40,50-58,88,90H,8-11,13-15,29-37,73H2,1-5H3,(H2,74,91)(H,78,97)(H,79,89)(H,80,98)(H,81,93)(H,82,92)(H,83,94)(H,84,95)(H,85,96)(H4,75,76,77)/t40-,50-,51-,52-,53-,54-,55-,56-,57+,58-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... |
J Med Chem 36: 928-33 (1993)
Article DOI: 10.1021/jm00059a020
BindingDB Entry DOI: 10.7270/Q2Z03BW7 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230127
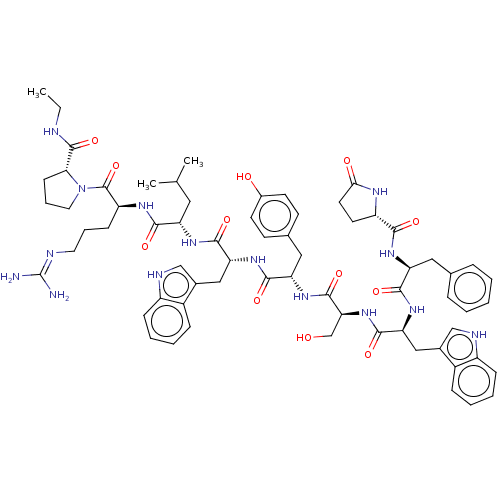
(CHEMBL407606)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1 |wU:45.57,31.44,63.79,23.28,5.4,88.94,wD:57.63,77.91,12.20,(27.09,-8.95,;27.47,-10.44,;28.96,-10.86,;30.04,-11.96,;31.53,-11.6,;29.61,-13.45,;30.56,-14.67,;29.69,-15.95,;28.21,-15.53,;28.16,-13.97,;26.88,-13.11,;26.98,-11.58,;25.49,-13.78,;25.38,-15.33,;26.66,-16.2,;26.55,-17.73,;27.83,-18.59,;27.72,-20.13,;29,-21,;26.33,-20.8,;24.22,-12.92,;22.82,-13.6,;22.72,-15.13,;21.54,-12.73,;21.65,-11.2,;23.05,-10.52,;24.32,-11.38,;23.15,-8.98,;20.15,-13.41,;18.89,-12.55,;18.99,-11.01,;17.49,-13.23,;17.39,-14.76,;18.14,-16.08,;19.64,-16.43,;19.78,-17.94,;18.38,-18.56,;17.91,-20.01,;16.43,-20.33,;15.39,-19.17,;15.88,-17.73,;17.36,-17.42,;16.22,-12.36,;14.83,-13.03,;14.71,-14.57,;13.55,-12.17,;13.66,-10.62,;15.04,-9.95,;15.16,-8.42,;16.54,-7.74,;17.82,-8.6,;19.2,-7.93,;17.71,-10.14,;16.32,-10.81,;12.16,-12.85,;10.88,-11.97,;10.99,-10.44,;9.49,-12.65,;9.39,-14.19,;10.67,-15.06,;8.21,-11.79,;6.83,-12.46,;6.71,-14.01,;5.54,-11.6,;5.65,-10.07,;7.05,-9.39,;8.58,-9.18,;8.85,-7.67,;7.49,-6.93,;7.12,-5.44,;5.63,-5,;4.53,-6.07,;4.89,-7.56,;6.37,-8,;4.17,-12.27,;2.88,-11.41,;3,-9.87,;1.49,-12.09,;1.38,-13.62,;2.15,-14.95,;3.66,-14.95,;4.41,-16.27,;3.65,-17.59,;2.12,-17.59,;1.37,-16.26,;.22,-11.23,;-1.18,-11.9,;-1.28,-13.44,;-2.34,-11.11,;-2.34,-9.69,;-3.87,-9.46,;-4.55,-10.83,;-6.06,-11.09,;-3.45,-11.92,)| Show InChI InChI=1S/C67H85N15O12/c1-4-70-65(93)56-21-13-29-82(56)66(94)49(20-12-28-71-67(68)69)75-59(87)50(30-38(2)3)76-62(90)53(33-41-35-72-46-18-10-8-16-44(41)46)79-61(89)52(32-40-22-24-43(84)25-23-40)78-64(92)55(37-83)81-63(91)54(34-42-36-73-47-19-11-9-17-45(42)47)80-60(88)51(31-39-14-6-5-7-15-39)77-58(86)48-26-27-57(85)74-48/h5-11,14-19,22-25,35-36,38,48-56,72-73,83-84H,4,12-13,20-21,26-34,37H2,1-3H3,(H,70,93)(H,74,85)(H,75,87)(H,76,90)(H,77,86)(H,78,92)(H,79,89)(H,80,88)(H,81,91)(H4,68,69,71)/t48-,49-,50-,51-,52-,53+,54-,55-,56+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84703
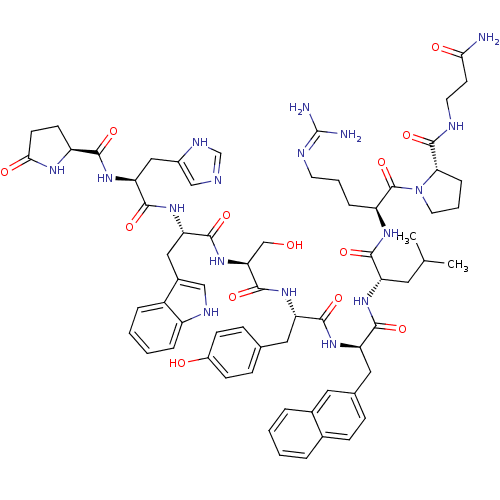
(nafarelin 10SarNH2)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCCC(N)=O |r,wU:41.56,65.70,23.34,74.80,88.96,wD:35.40,55.67,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.6,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.42,-7.65,;4.57,-8.59,;3.26,-7.74,;4.46,-10.13,)| Show InChI InChI=1S/C67H85N17O13/c1-37(2)27-49(59(90)77-48(13-7-24-73-67(69)70)66(97)84-26-8-14-55(84)65(96)72-25-23-56(68)87)78-61(92)51(30-39-15-18-40-9-3-4-10-41(40)28-39)79-60(91)50(29-38-16-19-44(86)20-17-38)80-64(95)54(35-85)83-62(93)52(31-42-33-74-46-12-6-5-11-45(42)46)81-63(94)53(32-43-34-71-36-75-43)82-58(89)47-21-22-57(88)76-47/h3-6,9-12,15-20,28,33-34,36-37,47-55,74,85-86H,7-8,13-14,21-27,29-32,35H2,1-2H3,(H2,68,87)(H,71,75)(H,72,96)(H,76,88)(H,77,90)(H,78,92)(H,79,91)(H,80,95)(H,81,94)(H,82,89)(H,83,93)(H4,69,70,73)/t47-,48-,49-,50-,51+,52-,53-,54-,55-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84717
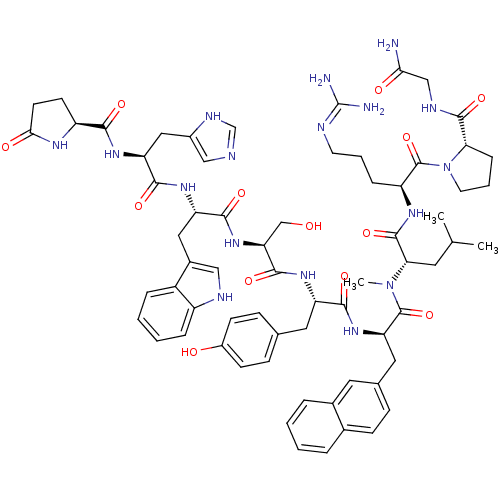
(nafarelin 7NMeLeu)Show SMILES CC(C)C[C@H](N(C)C(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:42.57,66.71,24.35,75.81,89.97,wD:36.41,56.68,9.22,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;8.78,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.6,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C67H85N17O13/c1-37(2)26-55(64(95)77-48(14-8-24-72-67(69)70)66(97)84-25-9-15-54(84)63(94)74-34-56(68)87)83(3)65(96)52(29-39-16-19-40-10-4-5-11-41(40)27-39)81-59(90)49(28-38-17-20-44(86)21-18-38)78-62(93)53(35-85)82-60(91)50(30-42-32-73-46-13-7-6-12-45(42)46)79-61(92)51(31-43-33-71-36-75-43)80-58(89)47-22-23-57(88)76-47/h4-7,10-13,16-21,27,32-33,36-37,47-55,73,85-86H,8-9,14-15,22-26,28-31,34-35H2,1-3H3,(H2,68,87)(H,71,75)(H,74,94)(H,76,88)(H,77,95)(H,78,93)(H,79,92)(H,80,89)(H,81,90)(H,82,91)(H4,69,70,72)/t47-,48-,49-,50-,51-,52+,53-,54-,55-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84728
(nafarelin 5NMeTyr)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:42.57,66.71,23.34,75.81,89.97,wD:36.41,56.68,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;.5,2.4,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.6,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C67H85N17O13/c1-37(2)26-49(59(90)77-48(14-8-24-72-67(69)70)66(97)84-25-9-15-54(84)63(94)74-34-56(68)87)78-60(91)50(28-39-16-19-40-10-4-5-11-41(40)27-39)81-64(95)55(29-38-17-20-44(86)21-18-38)83(3)65(96)53(35-85)82-61(92)51(30-42-32-73-46-13-7-6-12-45(42)46)79-62(93)52(31-43-33-71-36-75-43)80-58(89)47-22-23-57(88)76-47/h4-7,10-13,16-21,27,32-33,36-37,47-55,73,85-86H,8-9,14-15,22-26,28-31,34-35H2,1-3H3,(H2,68,87)(H,71,75)(H,74,94)(H,76,88)(H,77,90)(H,78,91)(H,79,93)(H,80,89)(H,81,95)(H,82,92)(H4,69,70,72)/t47-,48-,49-,50+,51-,52-,53-,54-,55-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84711

(nafarelin 31Nal)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:41.57,66.71,23.34,75.81,89.97,wD:35.40,56.68,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.59,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.03,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.53,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.1,;-6.59,7.15,;-5.5,8.24,;-5.9,9.73,;-7.38,10.12,;-8.47,9.04,;-9.96,9.43,;-11.05,8.35,;-10.65,6.86,;-9.16,6.46,;-8.07,7.55,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.59,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.34,-2.2,;12.39,-3.01,;13.81,-2.21,;15.16,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.37,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C68H84N16O13/c1-38(2)28-50(60(90)77-49(16-8-26-73-68(70)71)67(97)84-27-9-17-56(84)66(96)74-35-57(69)87)78-62(92)52(31-40-18-21-41-10-3-4-12-43(41)29-40)79-61(91)51(30-39-19-22-46(86)23-20-39)80-65(95)55(36-85)83-63(93)53(32-44-14-7-13-42-11-5-6-15-47(42)44)81-64(94)54(33-45-34-72-37-75-45)82-59(89)48-24-25-58(88)76-48/h3-7,10-15,18-23,29,34,37-38,48-56,85-86H,8-9,16-17,24-28,30-33,35-36H2,1-2H3,(H2,69,87)(H,72,75)(H,74,96)(H,76,88)(H,77,90)(H,78,92)(H,79,91)(H,80,95)(H,81,94)(H,82,89)(H,83,93)(H4,70,71,73)/t48-,49-,50-,51-,52+,53-,54-,55-,56-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50469859
(A-75998 | CHEMBL263839)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCC(N)C(=O)c1ccncc1)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1cccnc1)NC(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(C)=O)C(=O)N[C@@H](CCCC(N)C(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](C)C(N)=O Show InChI InChI=1S/C80H104ClN15O14/c1-46(2)38-63(73(103)90-62(20-10-17-59(82)47(3)4)80(110)96-37-13-21-68(96)77(107)87-48(5)71(84)101)91-72(102)61(19-11-18-60(83)70(100)55-32-35-85-36-33-55)89-78(108)69(43-51-25-30-58(99)31-26-51)95(7)79(109)67(45-97)94-76(106)66(42-53-14-12-34-86-44-53)93-75(105)65(40-50-23-28-57(81)29-24-50)92-74(104)64(88-49(6)98)41-52-22-27-54-15-8-9-16-56(54)39-52/h8-9,12,14-16,22-36,39,44,46-48,59-69,97,99H,10-11,13,17-21,37-38,40-43,45,82-83H2,1-7H3,(H2,84,101)(H,87,107)(H,88,98)(H,89,108)(H,90,103)(H,91,102)(H,92,104)(H,93,105)(H,94,106)/t48-,59?,60?,61-,62-,63-,64-,65-,66-,67-,68+,69-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... |
J Med Chem 36: 928-33 (1993)
Article DOI: 10.1021/jm00059a020
BindingDB Entry DOI: 10.7270/Q2Z03BW7 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50469856

(CHEMBL263840 | ORG-30850)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-c1csc2ccccc12)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#7])=O Show InChI InChI=1S/C69H91Cl2N15O13S/c1-38(2)31-51(61(92)80-50(13-9-29-76-69(74)75)68(99)86-30-10-14-57(86)67(98)77-39(3)59(73)90)81-60(91)49(12-7-8-28-72)79-63(94)53(34-43-20-26-47(89)27-21-43)83-66(97)56(36-87)85-65(96)55(35-44-37-100-58-15-6-5-11-48(44)58)84-64(95)54(33-42-18-24-46(71)25-19-42)82-62(93)52(78-40(4)88)32-41-16-22-45(70)23-17-41/h5-6,11,15-27,37-39,49-57,87,89H,7-10,12-14,28-36,72H2,1-4H3,(H2,73,90)(H,77,98)(H,78,88)(H,79,94)(H,80,92)(H,81,91)(H,82,93)(H,83,97)(H,84,95)(H,85,96)(H4,74,75,76)/t39-,49-,50-,51-,52-,53-,54-,55-,56-,57+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... |
J Med Chem 36: 928-33 (1993)
Article DOI: 10.1021/jm00059a020
BindingDB Entry DOI: 10.7270/Q2Z03BW7 |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84713
(nafarelin 4NMeSer)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:42.57,66.71,23.34,75.81,89.97,wD:35.40,56.68,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-3.45,6.18,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.6,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C67H85N17O13/c1-37(2)26-49(59(90)77-48(14-8-24-72-67(69)70)66(97)84-25-9-15-54(84)63(94)74-34-56(68)87)78-61(92)51(29-39-16-19-40-10-4-5-11-41(40)27-39)79-60(91)50(28-38-17-20-44(86)21-18-38)81-64(95)55(35-85)83(3)65(96)53(30-42-32-73-46-13-7-6-12-45(42)46)82-62(93)52(31-43-33-71-36-75-43)80-58(89)47-22-23-57(88)76-47/h4-7,10-13,16-21,27,32-33,36-37,47-55,73,85-86H,8-9,14-15,22-26,28-31,34-35H2,1-3H3,(H2,68,87)(H,71,75)(H,74,94)(H,76,88)(H,77,90)(H,78,92)(H,79,91)(H,80,89)(H,81,95)(H,82,93)(H4,69,70,72)/t47-,48-,49-,50-,51+,52-,53-,54-,55-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230129

(CHEMBL407123)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(=O)NCC(O)=O |wU:42.57,23.34,8.21,4.4,89.97,66.71,wD:56.68,75.81,35.38,(22.87,-8.85,;21.6,-7.99,;21.71,-6.46,;20.2,-8.67,;20.11,-10.2,;18.72,-10.89,;17.44,-10.03,;17.55,-8.48,;16.05,-10.7,;15.95,-12.23,;16.7,-13.56,;18.23,-13.58,;19,-14.91,;18.21,-16.25,;18.95,-17.58,;18.18,-18.9,;16.64,-18.88,;15.89,-17.55,;16.68,-16.23,;15.91,-14.89,;14.77,-9.83,;13.39,-10.5,;13.28,-12.05,;12.12,-9.64,;12.22,-8.11,;13.61,-7.44,;14.89,-8.3,;16.27,-7.62,;16.37,-6.08,;17.77,-5.41,;15.1,-5.21,;13.72,-5.9,;10.73,-10.31,;9.45,-9.46,;9.55,-7.9,;8.06,-10.13,;7.95,-11.66,;9.24,-12.54,;6.79,-9.27,;6.88,-7.74,;5.39,-9.94,;5.28,-11.48,;4.12,-9.08,;4.23,-7.54,;5.62,-6.87,;7.15,-6.65,;7.41,-5.14,;6.06,-4.41,;5.7,-2.91,;4.21,-2.48,;3.1,-3.55,;3.47,-5.05,;4.94,-5.48,;2.74,-9.75,;1.45,-8.89,;1.56,-7.34,;.08,-9.56,;-.04,-11.1,;1.24,-11.96,;1.28,-13.51,;2.77,-13.94,;3.63,-12.66,;2.68,-11.43,;-1.21,-8.71,;-2.59,-9.38,;-2.71,-10.92,;-3.76,-8.59,;-3.76,-7.16,;-5.29,-6.93,;-5.97,-8.31,;-7.49,-8.57,;-4.87,-9.39,;21.39,-11.07,;21.27,-12.61,;22.77,-10.4,;24.05,-11.27,;23.94,-12.8,;25.22,-13.67,;25.1,-15.21,;26.38,-16.06,;26.26,-17.6,;27.56,-18.46,;24.89,-18.28,;25.43,-10.59,;25.54,-9.05,;26.7,-11.45,;26.77,-13,;28.24,-13.43,;29.11,-12.14,;28.17,-10.92,;28.58,-9.43,;30.09,-9.08,;27.52,-8.34,;26.03,-7.92,;25.64,-6.44,;24.17,-6.02,;26.73,-5.36,)| Show InChI InChI=1S/C67H84N16O14/c1-37(2)26-49(59(90)76-48(14-8-24-71-67(68)69)66(97)83-25-9-15-54(83)63(94)73-34-57(87)88)77-61(92)51(29-39-16-19-40-10-4-5-11-41(40)27-39)78-60(91)50(28-38-17-20-44(85)21-18-38)80-64(95)55(35-84)82(3)65(96)53(30-42-32-72-46-13-7-6-12-45(42)46)81-62(93)52(31-43-33-70-36-74-43)79-58(89)47-22-23-56(86)75-47/h4-7,10-13,16-21,27,32-33,36-37,47-55,72,84-85H,8-9,14-15,22-26,28-31,34-35H2,1-3H3,(H,70,74)(H,73,94)(H,75,86)(H,76,90)(H,77,92)(H,78,91)(H,79,89)(H,80,95)(H,81,93)(H,87,88)(H4,68,69,71)/t47-,48-,49-,50-,51+,52-,53-,54+,55-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50469857
(CHEMBL268319)Show SMILES [#6]-[#8]-c1ccc(cc1)-[#6](=O)-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-c1cccnc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#7])=O Show InChI InChI=1S/C73H97ClN18O14/c1-41(2)34-55(65(99)87-54(16-10-32-82-73(78)79)71(105)92-33-11-17-60(92)70(104)83-42(3)62(75)96)88-64(98)53(28-29-61(95)48-22-26-51(106-5)27-23-48)86-63(97)52(15-9-31-81-72(76)77)85-69(103)59(40-93)91-68(102)58(38-46-12-8-30-80-39-46)90-67(101)57(36-44-19-24-50(74)25-20-44)89-66(100)56(84-43(4)94)37-45-18-21-47-13-6-7-14-49(47)35-45/h6-8,12-14,18-27,30,35,39,41-42,52-60,93H,9-11,15-17,28-29,31-34,36-38,40H2,1-5H3,(H2,75,96)(H,83,104)(H,84,94)(H,85,103)(H,86,97)(H,87,99)(H,88,98)(H,89,100)(H,90,101)(H,91,102)(H4,76,77,81)(H4,78,79,82)/t42-,52-,53-,54-,55-,56-,57-,58-,59-,60+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... |
J Med Chem 36: 928-33 (1993)
Article DOI: 10.1021/jm00059a020
BindingDB Entry DOI: 10.7270/Q2Z03BW7 |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84715
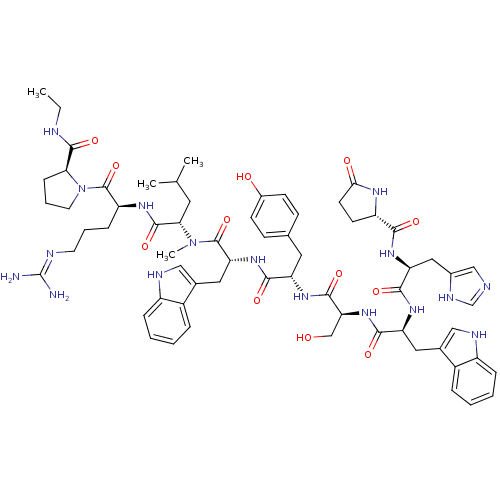
(deslorelin 7NMeLeu)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:32.45,23.28,12.20,58.64,64.80,5.4,78.91,88.94,wD:46.58,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-1.3,-1.91,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C65H85N17O12/c1-5-69-61(91)53-17-11-25-82(53)64(94)47(16-10-24-70-65(66)67)75-62(92)54(26-36(2)3)81(4)63(93)51(29-39-32-72-45-15-9-7-13-43(39)45)79-57(87)48(27-37-18-20-41(84)21-19-37)76-60(90)52(34-83)80-58(88)49(28-38-31-71-44-14-8-6-12-42(38)44)77-59(89)50(30-40-33-68-35-73-40)78-56(86)46-22-23-55(85)74-46/h6-9,12-15,18-21,31-33,35-36,46-54,71-72,83-84H,5,10-11,16-17,22-30,34H2,1-4H3,(H,68,73)(H,69,91)(H,74,85)(H,75,92)(H,76,90)(H,77,89)(H,78,86)(H,79,87)(H,80,88)(H4,66,67,70)/t46-,47-,48-,49-,50-,51+,52-,53-,54-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50469860
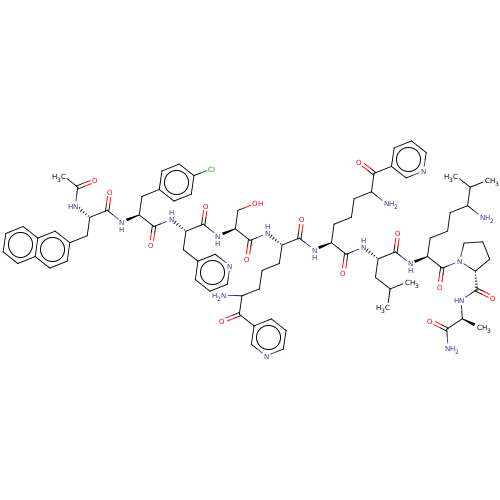
(CHEMBL409990)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCC(N)C(=O)c1cccnc1)NC(=O)[C@H](CCCC(N)C(=O)c1cccnc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccnc1)NC(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(C)=O)C(=O)N[C@@H](CCCC(N)C(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](C)C(N)=O Show InChI InChI=1S/C82H108ClN17O14/c1-47(2)38-65(76(108)95-64(26-9-21-59(84)48(3)4)82(114)100-37-15-27-70(100)81(113)91-49(5)73(87)105)96-75(107)63(25-11-23-61(86)72(104)57-20-14-36-90-45-57)93-74(106)62(24-10-22-60(85)71(103)56-19-13-35-89-44-56)94-80(112)69(46-101)99-79(111)68(42-53-16-12-34-88-43-53)98-78(110)67(40-51-29-32-58(83)33-30-51)97-77(109)66(92-50(6)102)41-52-28-31-54-17-7-8-18-55(54)39-52/h7-8,12-14,16-20,28-36,39,43-45,47-49,59-70,101H,9-11,15,21-27,37-38,40-42,46,84-86H2,1-6H3,(H2,87,105)(H,91,113)(H,92,102)(H,93,106)(H,94,112)(H,95,108)(H,96,107)(H,97,109)(H,98,110)(H,99,111)/t49-,59?,60?,61?,62-,63-,64-,65-,66-,67-,68-,69-,70+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... |
J Med Chem 36: 928-33 (1993)
Article DOI: 10.1021/jm00059a020
BindingDB Entry DOI: 10.7270/Q2Z03BW7 |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84730
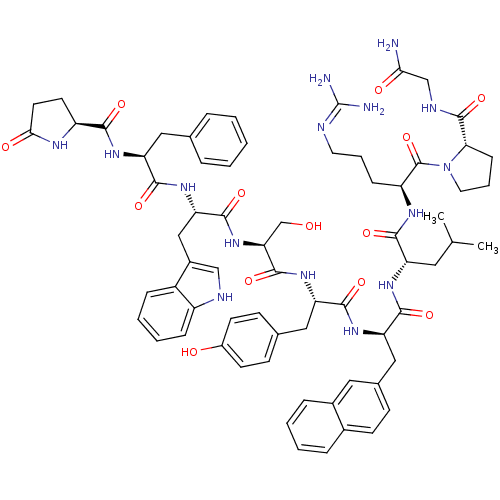
(nafarelin 2Phe)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:41.56,66.71,23.34,75.81,89.97,wD:35.40,55.68,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.65,1.65,;-8.65,.11,;-7.32,-.66,;-5.98,.11,;-5.98,1.65,;-7.32,2.42,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C69H85N15O13/c1-39(2)30-51(61(90)77-50(18-10-28-73-69(71)72)68(97)84-29-11-19-57(84)67(96)75-37-58(70)87)78-64(93)54(34-42-20-23-43-14-6-7-15-44(43)31-42)80-62(91)53(33-41-21-24-46(86)25-22-41)81-66(95)56(38-85)83-65(94)55(35-45-36-74-48-17-9-8-16-47(45)48)82-63(92)52(32-40-12-4-3-5-13-40)79-60(89)49-26-27-59(88)76-49/h3-9,12-17,20-25,31,36,39,49-57,74,85-86H,10-11,18-19,26-30,32-35,37-38H2,1-2H3,(H2,70,87)(H,75,96)(H,76,88)(H,77,90)(H,78,93)(H,79,89)(H,80,91)(H,81,95)(H,82,92)(H,83,94)(H4,71,72,73)/t49-,50-,51-,52-,53-,54+,55-,56-,57-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230120
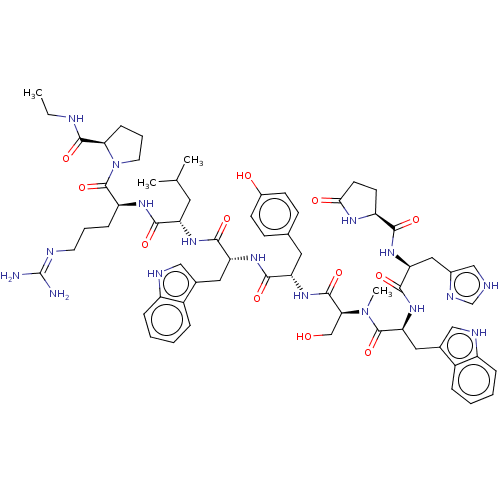
(CHEMBL405737)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:64.80,45.57,31.44,23.28,5.4,88.94,wD:78.91,12.20,57.61,(25.64,-6.44,;26.03,-7.92,;27.52,-8.34,;28.58,-9.43,;30.09,-9.08,;28.17,-10.92,;29.11,-12.14,;28.24,-13.43,;26.77,-13,;26.7,-11.45,;25.43,-10.59,;25.54,-9.05,;24.05,-11.27,;23.94,-12.8,;25.22,-13.67,;25.1,-15.21,;26.38,-16.06,;26.26,-17.6,;27.56,-18.46,;24.89,-18.28,;22.77,-10.4,;21.39,-11.07,;21.27,-12.61,;20.11,-10.2,;20.2,-8.67,;21.6,-7.99,;22.87,-8.85,;21.71,-6.46,;18.72,-10.89,;17.44,-10.03,;17.55,-8.48,;16.05,-10.7,;15.95,-12.23,;16.7,-13.56,;18.2,-13.91,;18.35,-15.42,;16.95,-16.04,;16.47,-17.49,;14.98,-17.81,;13.96,-16.65,;14.44,-15.21,;15.93,-14.9,;14.77,-9.83,;13.39,-10.5,;13.28,-12.05,;12.12,-9.64,;12.22,-8.11,;13.61,-7.44,;13.72,-5.9,;15.1,-5.21,;16.37,-6.08,;17.77,-5.41,;16.27,-7.62,;14.89,-8.3,;10.73,-10.31,;9.45,-9.46,;9.55,-7.9,;8.06,-10.13,;7.95,-11.66,;9.24,-12.54,;6.79,-9.27,;6.88,-7.74,;5.39,-9.94,;5.28,-11.48,;4.12,-9.08,;4.23,-7.54,;5.62,-6.87,;7.15,-6.65,;7.41,-5.14,;6.06,-4.41,;5.7,-2.91,;4.21,-2.48,;3.1,-3.55,;3.47,-5.05,;4.94,-5.48,;2.74,-9.75,;1.45,-8.89,;1.56,-7.34,;.08,-9.56,;-.04,-11.1,;1.24,-11.96,;1.28,-13.51,;2.77,-13.94,;3.63,-12.66,;2.68,-11.43,;-1.21,-8.71,;-2.59,-9.38,;-2.71,-10.92,;-3.76,-8.59,;-3.76,-7.16,;-5.29,-6.93,;-5.97,-8.31,;-7.49,-8.57,;-4.87,-9.39,)| Show InChI InChI=1S/C65H85N17O12/c1-5-69-61(91)53-17-11-25-82(53)64(94)47(16-10-24-70-65(66)67)75-57(87)48(26-36(2)3)76-59(89)50(28-38-31-71-44-14-8-6-12-42(38)44)77-58(88)49(27-37-18-20-41(84)21-19-37)79-62(92)54(34-83)81(4)63(93)52(29-39-32-72-45-15-9-7-13-43(39)45)80-60(90)51(30-40-33-68-35-73-40)78-56(86)46-22-23-55(85)74-46/h6-9,12-15,18-21,31-33,35-36,46-54,71-72,83-84H,5,10-11,16-17,22-30,34H2,1-4H3,(H,68,73)(H,69,91)(H,74,85)(H,75,87)(H,76,89)(H,77,88)(H,78,86)(H,79,92)(H,80,90)(H4,66,67,70)/t46-,47-,48-,49-,50+,51-,52-,53+,54-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84704
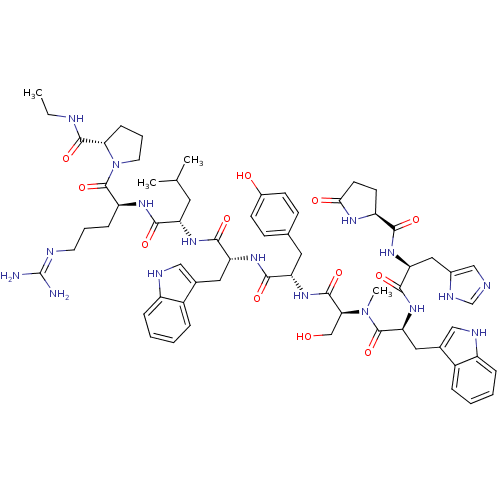
(deslorelin 4NMeSer)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,64.80,5.4,78.91,88.94,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.96,-2.95,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C65H85N17O12/c1-5-69-61(91)53-17-11-25-82(53)64(94)47(16-10-24-70-65(66)67)75-57(87)48(26-36(2)3)76-59(89)50(28-38-31-71-44-14-8-6-12-42(38)44)77-58(88)49(27-37-18-20-41(84)21-19-37)79-62(92)54(34-83)81(4)63(93)52(29-39-32-72-45-15-9-7-13-43(39)45)80-60(90)51(30-40-33-68-35-73-40)78-56(86)46-22-23-55(85)74-46/h6-9,12-15,18-21,31-33,35-36,46-54,71-72,83-84H,5,10-11,16-17,22-30,34H2,1-4H3,(H,68,73)(H,69,91)(H,74,85)(H,75,87)(H,76,89)(H,77,88)(H,78,86)(H,79,92)(H,80,90)(H4,66,67,70)/t46-,47-,48-,49-,50+,51-,52-,53-,54-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84721

(deslorelin 5NMeTyr)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,58.64,64.80,5.4,78.91,88.94,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;3.7,1.09,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C65H85N17O12/c1-5-69-61(91)53-17-11-25-82(53)64(94)47(16-10-24-70-65(66)67)75-57(87)48(26-36(2)3)76-58(88)50(29-39-32-72-45-15-9-7-13-43(39)45)79-62(92)54(27-37-18-20-41(84)21-19-37)81(4)63(93)52(34-83)80-59(89)49(28-38-31-71-44-14-8-6-12-42(38)44)77-60(90)51(30-40-33-68-35-73-40)78-56(86)46-22-23-55(85)74-46/h6-9,12-15,18-21,31-33,35-36,46-54,71-72,83-84H,5,10-11,16-17,22-30,34H2,1-4H3,(H,68,73)(H,69,91)(H,74,85)(H,75,87)(H,76,88)(H,77,90)(H,78,86)(H,79,92)(H,80,89)(H4,66,67,70)/t46-,47-,48-,49-,50+,51-,52-,53-,54-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84700
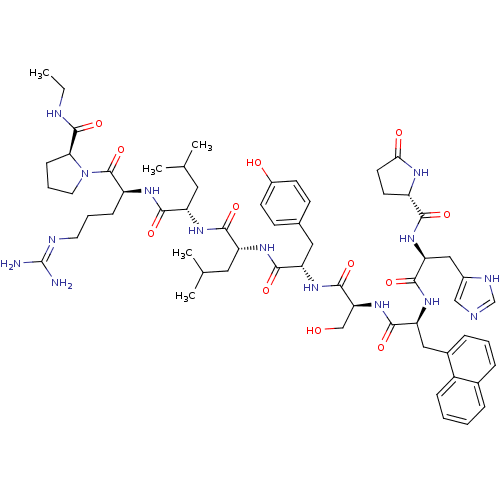
(leuprolide 31Nal)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:51.55,72.83,82.86,31.36,5.4,wD:57.72,39.49,23.28,12.20,(10.22,12.44,;9.73,11.04,;10.69,9.91,;10.16,8.49,;11.09,7.36,;8.69,8.25,;7.58,9.33,;6.25,8.6,;6.47,7.14,;8.01,6.89,;8.69,5.56,;10.34,5.13,;7.76,4.7,;8.05,3.19,;9.4,2.76,;10.56,3.77,;11.98,3.34,;13.07,4.34,;14.52,3.91,;12.73,5.81,;6.36,5.25,;5.17,4.27,;3.75,4.73,;5.43,2.76,;6.79,2.3,;7.11,.79,;6.64,-.49,;8.54,.28,;4.27,1.76,;2.87,2.26,;2.59,3.77,;1.73,1.23,;1.97,-.25,;3.45,-.72,;3.77,-2.23,;4.1,.15,;.26,1.68,;-.83,.68,;-2.3,1.12,;-.5,-.83,;.97,-1.29,;1.33,-2.76,;.22,-3.83,;.66,-5.34,;2.12,-5.74,;2.48,-7.21,;3.27,-4.67,;2.81,-3.11,;-1.58,-1.87,;-2.98,-1.43,;-3.37,.08,;-4.09,-2.51,;-5.56,-2.12,;-6.7,-3.19,;-3.76,-3.99,;-4.84,-5.02,;-6.31,-4.63,;-4.45,-6.49,;-2.94,-6.89,;-2.63,-8.53,;-1.14,-8.92,;-.74,-10.41,;-1.83,-11.5,;-3.32,-11.1,;-4.41,-12.19,;-5.89,-11.79,;-6.29,-10.3,;-5.2,-9.22,;-3.72,-9.61,;-5.56,-7.56,;-7.01,-7.21,;-7.41,-5.74,;-8.1,-8.33,;-9.59,-7.89,;-9.99,-6.42,;-11.44,-5.87,;-11.3,-4.34,;-9.79,-3.94,;-9,-5.27,;-7.71,-9.82,;-8.81,-10.93,;-8.46,-12.44,;-10.37,-10.57,;-10.93,-9.1,;-12.5,-9.1,;-12.97,-10.64,;-14.52,-11.2,;-11.66,-11.59,)| Show InChI InChI=1S/C61H85N15O12/c1-6-65-59(87)50-17-11-25-76(50)60(88)43(16-10-24-66-61(62)63)69-53(81)44(26-34(2)3)70-54(82)45(27-35(4)5)71-55(83)46(28-36-18-20-40(78)21-19-36)72-58(86)49(32-77)75-56(84)47(29-38-14-9-13-37-12-7-8-15-41(37)38)73-57(85)48(30-39-31-64-33-67-39)74-52(80)42-22-23-51(79)68-42/h7-9,12-15,18-21,31,33-35,42-50,77-78H,6,10-11,16-17,22-30,32H2,1-5H3,(H,64,67)(H,65,87)(H,68,79)(H,69,81)(H,70,82)(H,71,83)(H,72,86)(H,73,85)(H,74,80)(H,75,84)(H4,62,63,66)/t42-,43-,44-,45+,46-,47-,48-,49-,50-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230123

(CHEMBL385042)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.72,31.36,23.28,5.4,82.86,wD:51.55,72.83,12.20,(29.65,-8.55,;30.04,-10.04,;31.53,-10.45,;32.6,-11.55,;34.09,-11.18,;32.18,-13.04,;33.11,-14.25,;32.25,-15.54,;30.77,-15.11,;30.72,-13.57,;29.44,-12.71,;29.55,-11.16,;28.05,-13.38,;27.95,-14.92,;29.23,-15.78,;29.12,-17.32,;30.4,-18.18,;30.28,-19.72,;31.56,-20.59,;28.9,-20.4,;26.77,-12.51,;25.39,-13.18,;25.28,-14.73,;24.12,-12.32,;24.22,-10.79,;25.61,-10.11,;26.89,-10.97,;25.72,-8.57,;22.72,-12.99,;21.45,-12.14,;21.54,-10.6,;20.06,-12.81,;19.94,-14.34,;20.71,-15.68,;22.24,-15.69,;19.93,-17.01,;18.78,-11.95,;17.39,-12.62,;17.27,-14.16,;16.11,-11.76,;16.22,-10.22,;17.61,-9.55,;17.71,-8,;19.1,-7.33,;20.38,-8.19,;21.77,-7.51,;20.27,-9.73,;18.89,-10.41,;14.73,-12.44,;13.44,-11.57,;13.55,-10.02,;12.06,-12.24,;11.95,-13.78,;13.23,-14.64,;10.78,-11.38,;9.39,-12.06,;9.28,-13.6,;8.11,-11.18,;8.22,-9.65,;9.3,-8.56,;10.79,-8.95,;11.87,-7.86,;11.46,-6.37,;9.97,-5.99,;9.56,-4.51,;8.07,-4.14,;7,-5.24,;7.42,-6.71,;8.9,-7.09,;6.72,-11.86,;5.44,-11,;5.56,-9.46,;4.07,-11.67,;3.95,-13.22,;5.23,-14.08,;5.28,-15.62,;6.76,-16.05,;7.63,-14.78,;6.68,-13.55,;2.77,-10.81,;1.39,-11.48,;1.29,-13.03,;.22,-10.69,;.22,-9.28,;-1.3,-9.05,;-1.99,-10.42,;-3.5,-10.67,;-.9,-11.51,)| Show InChI InChI=1S/C61H85N15O12/c1-6-65-59(87)50-17-11-25-76(50)60(88)43(16-10-24-66-61(62)63)69-53(81)44(26-34(2)3)70-54(82)45(27-35(4)5)71-55(83)46(28-36-18-20-40(78)21-19-36)72-58(86)49(32-77)75-56(84)47(29-38-14-9-13-37-12-7-8-15-41(37)38)73-57(85)48(30-39-31-64-33-67-39)74-52(80)42-22-23-51(79)68-42/h7-9,12-15,18-21,31,33-35,42-50,77-78H,6,10-11,16-17,22-30,32H2,1-5H3,(H,64,67)(H,65,87)(H,68,79)(H,69,81)(H,70,82)(H,71,83)(H,72,86)(H,73,85)(H,74,80)(H,75,84)(H4,62,63,66)/t42-,43-,44-,45+,46-,47-,48-,49-,50+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50469861
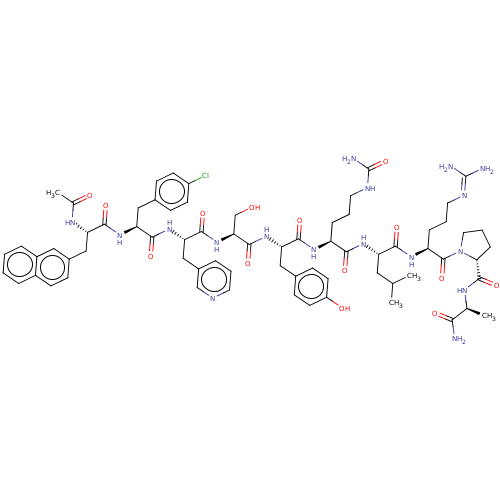
(CHEMBL438829)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-c1cccnc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#7])=O Show InChI InChI=1S/C70H92ClN17O14/c1-39(2)31-52(61(94)82-51(15-9-28-77-69(73)74)68(101)88-30-10-16-58(88)67(100)79-40(3)59(72)92)83-60(93)50(14-8-29-78-70(75)102)81-63(96)54(34-43-20-25-49(91)26-21-43)86-66(99)57(38-89)87-65(98)56(36-45-11-7-27-76-37-45)85-64(97)55(33-42-18-23-48(71)24-19-42)84-62(95)53(80-41(4)90)35-44-17-22-46-12-5-6-13-47(46)32-44/h5-7,11-13,17-27,32,37,39-40,50-58,89,91H,8-10,14-16,28-31,33-36,38H2,1-4H3,(H2,72,92)(H,79,100)(H,80,90)(H,81,96)(H,82,94)(H,83,93)(H,84,95)(H,85,97)(H,86,99)(H,87,98)(H4,73,74,77)(H3,75,78,102)/t40-,50-,51-,52-,53-,54-,55-,56-,57-,58+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... |
J Med Chem 36: 928-33 (1993)
Article DOI: 10.1021/jm00059a020
BindingDB Entry DOI: 10.7270/Q2Z03BW7 |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230122

(CHEMBL415571)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.69,23.28,31.36,5.4,80.83,wD:51.55,70.80,12.20,(24.19,-10.58,;24.58,-12.06,;26.07,-12.48,;27.14,-13.57,;28.64,-13.22,;26.72,-15.06,;27.66,-16.28,;26.79,-17.57,;25.32,-17.14,;25.25,-15.59,;23.98,-14.73,;24.09,-13.19,;22.6,-15.41,;22.49,-16.94,;23.77,-17.81,;23.65,-19.35,;24.93,-20.21,;24.81,-21.75,;26.11,-22.61,;23.44,-22.42,;21.32,-14.54,;19.94,-15.21,;19.82,-16.75,;18.66,-14.34,;18.75,-12.81,;20.14,-12.13,;21.42,-12.99,;20.26,-10.6,;17.26,-15.03,;15.99,-14.17,;16.1,-12.62,;14.59,-14.84,;14.49,-16.37,;15.26,-17.71,;16.79,-17.72,;14.48,-19.03,;13.32,-13.97,;11.94,-14.64,;11.82,-16.19,;10.66,-13.78,;10.76,-12.25,;12.15,-11.57,;13.43,-12.43,;14.82,-11.76,;14.92,-10.22,;16.31,-9.55,;13.64,-9.34,;12.26,-10.03,;9.27,-14.45,;7.99,-13.59,;8.09,-12.05,;6.6,-14.27,;6.49,-15.8,;7.78,-16.68,;5.33,-13.41,;3.93,-14.08,;3.82,-15.62,;2.66,-13.22,;2.77,-11.68,;3.84,-10.59,;5.34,-10.97,;6.42,-9.88,;6.02,-8.39,;7.08,-7.32,;6.67,-5.84,;4.51,-8.01,;3.45,-9.11,;1.28,-13.89,;-.02,-13.03,;.1,-11.48,;-1.39,-13.7,;-1.5,-15.24,;-.22,-16.1,;-.18,-17.65,;1.31,-18.08,;2.17,-16.8,;1.22,-15.57,;-2.67,-12.84,;-4.06,-13.52,;-4.17,-15.06,;-5.23,-12.73,;-5.23,-11.3,;-6.75,-11.06,;-7.43,-12.45,;-8.96,-12.7,;-6.34,-13.53,)| Show InChI InChI=1S/C58H85N15O13/c1-7-62-56(84)47-11-9-23-73(47)57(85)40(10-8-22-63-58(59)60)66-50(78)41(24-32(2)3)67-51(79)42(25-33(4)5)68-52(80)43(26-34-12-16-37(75)17-13-34)70-55(83)46(30-74)72-53(81)44(27-35-14-18-38(86-6)19-15-35)69-54(82)45(28-36-29-61-31-64-36)71-49(77)39-20-21-48(76)65-39/h12-19,29,31-33,39-47,74-75H,7-11,20-28,30H2,1-6H3,(H,61,64)(H,62,84)(H,65,76)(H,66,78)(H,67,79)(H,68,80)(H,69,82)(H,70,83)(H,71,77)(H,72,81)(H4,59,60,63)/t39-,40-,41-,42+,43-,44-,45-,46-,47+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84727

(leuprolide 7NMeLeu)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:52.56,72.83,82.86,32.37,5.4,wD:58.72,40.50,23.28,12.20,(10.22,12.44,;9.73,11.04,;10.69,9.91,;10.16,8.49,;11.09,7.36,;8.69,8.25,;7.58,9.33,;6.25,8.6,;6.47,7.14,;8.01,6.89,;8.69,5.56,;10.34,5.13,;7.76,4.7,;8.05,3.19,;9.4,2.76,;10.56,3.77,;11.98,3.34,;13.07,4.34,;14.52,3.91,;12.73,5.81,;6.36,5.25,;5.17,4.27,;3.75,4.73,;5.43,2.76,;6.79,2.3,;7.11,.79,;6.64,-.49,;8.54,.28,;4.27,1.76,;5.36,.67,;2.87,2.26,;2.59,3.77,;1.73,1.23,;1.97,-.25,;3.45,-.72,;3.77,-2.23,;4.1,.15,;.26,1.68,;-.83,.68,;-2.3,1.12,;-.5,-.83,;.97,-1.29,;1.33,-2.76,;.22,-3.83,;.66,-5.34,;2.12,-5.74,;2.48,-7.21,;3.27,-4.67,;2.81,-3.11,;-1.58,-1.87,;-2.98,-1.43,;-3.37,.08,;-4.09,-2.51,;-5.56,-2.12,;-6.7,-3.19,;-3.76,-3.99,;-4.84,-5.02,;-6.31,-4.63,;-4.45,-6.49,;-2.94,-6.89,;-2.51,-8.36,;-1.14,-8.86,;-1.18,-10.4,;-2.61,-10.84,;-3.29,-12.23,;-4.91,-12.31,;-5.73,-10.97,;-5.02,-9.57,;-3.46,-9.5,;-5.56,-7.56,;-7.01,-7.21,;-7.41,-5.74,;-8.1,-8.33,;-9.59,-7.89,;-9.99,-6.42,;-11.44,-5.87,;-11.3,-4.34,;-9.79,-3.94,;-9,-5.27,;-7.71,-9.82,;-8.81,-10.93,;-8.46,-12.44,;-10.37,-10.57,;-10.93,-9.1,;-12.5,-9.1,;-12.97,-10.64,;-14.52,-11.2,;-11.66,-11.59,)| Show InChI InChI=1S/C60H86N16O12/c1-7-64-56(85)48-15-11-23-76(48)59(88)42(14-10-22-65-60(61)62)69-57(86)49(25-34(4)5)75(6)58(87)46(24-33(2)3)73-52(81)43(26-35-16-18-38(78)19-17-35)70-55(84)47(31-77)74-53(82)44(27-36-29-66-40-13-9-8-12-39(36)40)71-54(83)45(28-37-30-63-32-67-37)72-51(80)41-20-21-50(79)68-41/h8-9,12-13,16-19,29-30,32-34,41-49,66,77-78H,7,10-11,14-15,20-28,31H2,1-6H3,(H,63,67)(H,64,85)(H,68,79)(H,69,86)(H,70,84)(H,71,83)(H,72,80)(H,73,81)(H,74,82)(H4,61,62,65)/t41-,42-,43-,44-,45-,46+,47-,48-,49-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230126

(CHEMBL385468)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.71,31.36,23.28,5.4,81.85,wD:51.55,71.82,12.20,(25.64,-6.44,;26.03,-7.92,;27.52,-8.34,;28.58,-9.43,;30.09,-9.08,;28.17,-10.92,;29.11,-12.14,;28.24,-13.43,;26.77,-13,;26.7,-11.45,;25.43,-10.59,;25.54,-9.05,;24.05,-11.27,;23.94,-12.8,;25.22,-13.67,;25.1,-15.21,;26.38,-16.06,;26.26,-17.6,;27.56,-18.46,;24.89,-18.28,;22.77,-10.4,;21.39,-11.07,;21.27,-12.61,;20.11,-10.2,;20.2,-8.67,;21.6,-7.99,;22.87,-8.85,;21.71,-6.46,;18.72,-10.89,;17.44,-10.03,;17.55,-8.48,;16.05,-10.7,;15.95,-12.23,;16.71,-13.56,;18.25,-13.58,;15.93,-14.89,;14.77,-9.83,;13.39,-10.5,;13.28,-12.05,;12.12,-9.64,;12.22,-8.11,;13.61,-7.44,;14.89,-8.3,;16.27,-7.62,;16.37,-6.08,;17.77,-5.41,;15.1,-5.21,;13.72,-5.9,;10.73,-10.31,;9.45,-9.46,;9.55,-7.9,;8.06,-10.13,;7.95,-11.66,;9.24,-12.54,;6.79,-9.27,;5.39,-9.94,;5.28,-11.48,;4.12,-9.08,;4.23,-7.54,;5.62,-6.87,;7.15,-6.65,;7.41,-5.14,;6.06,-4.41,;5.7,-2.91,;4.21,-2.48,;3.1,-3.55,;3.47,-5.05,;4.94,-5.48,;2.74,-9.75,;1.45,-8.89,;1.56,-7.34,;.08,-9.56,;-.04,-11.1,;1.24,-11.96,;1.28,-13.51,;2.77,-13.94,;3.63,-12.66,;2.68,-11.43,;-1.21,-8.71,;-2.59,-9.38,;-2.71,-10.92,;-3.76,-8.59,;-3.76,-7.16,;-5.29,-6.93,;-5.97,-8.31,;-7.49,-8.57,;-4.87,-9.39,)| Show InChI InChI=1S/C59H84N16O12/c1-6-63-57(86)48-14-10-22-75(48)58(87)41(13-9-21-64-59(60)61)68-51(80)42(23-32(2)3)69-52(81)43(24-33(4)5)70-53(82)44(25-34-15-17-37(77)18-16-34)71-56(85)47(30-76)74-54(83)45(26-35-28-65-39-12-8-7-11-38(35)39)72-55(84)46(27-36-29-62-31-66-36)73-50(79)40-19-20-49(78)67-40/h7-8,11-12,15-18,28-29,31-33,40-48,65,76-77H,6,9-10,13-14,19-27,30H2,1-5H3,(H,62,66)(H,63,86)(H,67,78)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,79)(H,74,83)(H4,60,61,64)/t40-,41-,42-,43+,44-,45-,46-,47-,48+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230124

(CHEMBL429240)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@@H]1CCC(=O)N1 |wU:89.95,63.79,45.57,31.44,23.28,5.4,wD:57.63,12.20,77.83,(27.09,-8.95,;27.47,-10.44,;28.96,-10.86,;30.04,-11.96,;31.53,-11.6,;29.61,-13.45,;30.56,-14.67,;29.69,-15.95,;28.21,-15.53,;28.16,-13.97,;26.88,-13.11,;26.98,-11.58,;25.49,-13.78,;25.38,-15.33,;26.66,-16.2,;26.55,-17.73,;27.83,-18.59,;27.72,-20.13,;29,-21,;26.33,-20.8,;24.22,-12.92,;22.82,-13.6,;22.72,-15.13,;21.54,-12.73,;21.65,-11.2,;23.05,-10.52,;23.15,-8.98,;24.32,-11.38,;20.15,-13.41,;18.89,-12.55,;18.99,-11.01,;17.49,-13.23,;17.39,-14.76,;18.14,-16.08,;19.64,-16.43,;19.78,-17.94,;18.38,-18.56,;17.91,-20.01,;16.43,-20.33,;15.39,-19.17,;15.88,-17.73,;17.36,-17.42,;16.22,-12.36,;14.83,-13.03,;14.71,-14.57,;13.55,-12.17,;13.66,-10.62,;15.04,-9.95,;16.32,-10.81,;17.71,-10.14,;17.82,-8.6,;19.2,-7.93,;16.54,-7.74,;15.16,-8.42,;12.16,-12.85,;10.88,-11.97,;10.99,-10.44,;9.49,-12.65,;9.39,-14.19,;10.67,-15.06,;8.21,-11.79,;6.83,-12.46,;6.71,-14.01,;5.54,-11.6,;5.65,-10.07,;7.05,-9.39,;8.58,-9.18,;8.85,-7.67,;7.49,-6.93,;7.12,-5.44,;5.63,-5,;4.53,-6.07,;4.89,-7.56,;6.37,-8,;4.17,-12.27,;2.88,-11.41,;3,-9.87,;1.49,-12.09,;1.38,-13.62,;2.15,-14.95,;1.37,-16.26,;2.12,-17.59,;3.65,-17.59,;4.41,-16.27,;3.66,-14.95,;.22,-11.23,;.31,-9.7,;-1.18,-11.9,;-1.28,-13.44,;-2.34,-11.11,;-2.34,-9.69,;-3.87,-9.46,;-4.55,-10.83,;-6.06,-11.09,;-3.45,-11.92,)| Show InChI InChI=1S/C68H87N15O12/c1-5-71-64(92)56-22-14-30-83(56)67(95)49(21-13-29-72-68(69)70)76-59(87)51(31-39(2)3)77-61(89)53(34-42-36-73-47-19-11-9-17-45(42)47)79-60(88)52(32-41-23-25-44(85)26-24-41)78-63(91)55(38-84)81-62(90)54(35-43-37-74-48-20-12-10-18-46(43)48)80-65(93)57(33-40-15-7-6-8-16-40)82(4)66(94)50-27-28-58(86)75-50/h6-12,15-20,23-26,36-37,39,49-57,73-74,84-85H,5,13-14,21-22,27-35,38H2,1-4H3,(H,71,92)(H,75,86)(H,76,87)(H,77,89)(H,78,91)(H,79,88)(H,80,93)(H,81,90)(H4,69,70,72)/t49-,50-,51-,52-,53+,54-,55-,56+,57-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84712

(deslorelin 2NMePhe | deslorelin 2Phe)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,63.79,5.4,77.91,89.95,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.53,2.14,;13.13,.66,;14.22,-.43,;15.71,-.03,;16.11,1.45,;15.02,2.54,;10.35,4.51,;11.12,5.85,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C68H87N15O12/c1-5-71-64(92)56-22-14-30-83(56)67(95)49(21-13-29-72-68(69)70)76-59(87)51(31-39(2)3)77-61(89)53(34-42-36-73-47-19-11-9-17-45(42)47)79-60(88)52(32-41-23-25-44(85)26-24-41)78-63(91)55(38-84)81-62(90)54(35-43-37-74-48-20-12-10-18-46(43)48)80-65(93)57(33-40-15-7-6-8-16-40)82(4)66(94)50-27-28-58(86)75-50/h6-12,15-20,23-26,36-37,39,49-57,73-74,84-85H,5,13-14,21-22,27-35,38H2,1-4H3,(H,71,92)(H,75,86)(H,76,87)(H,77,89)(H,78,91)(H,79,88)(H,80,93)(H,81,90)(H4,69,70,72)/t49-,50-,51-,52-,53+,54-,55-,56-,57-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84731

(nafarelin 2NMePhe)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:41.56,67.72,23.34,76.82,90.98,wD:35.40,55.68,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.65,1.65,;-8.65,.11,;-7.32,-.66,;-5.98,.11,;-5.98,1.65,;-7.32,2.42,;-11.26,4.41,;-12.03,5.75,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C70H87N15O13/c1-40(2)31-52(61(90)78-50(19-11-29-74-70(72)73)69(98)85-30-12-20-57(85)66(95)76-38-59(71)88)79-63(92)54(34-43-21-24-44-15-7-8-16-45(44)32-43)80-62(91)53(33-42-22-25-47(87)26-23-42)81-65(94)56(39-86)83-64(93)55(36-46-37-75-49-18-10-9-17-48(46)49)82-67(96)58(35-41-13-5-4-6-14-41)84(3)68(97)51-27-28-60(89)77-51/h4-10,13-18,21-26,32,37,40,50-58,75,86-87H,11-12,19-20,27-31,33-36,38-39H2,1-3H3,(H2,71,88)(H,76,95)(H,77,89)(H,78,90)(H,79,92)(H,80,91)(H,81,94)(H,82,96)(H,83,93)(H4,72,73,74)/t50-,51-,52-,53-,54+,55-,56-,57-,58-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84714
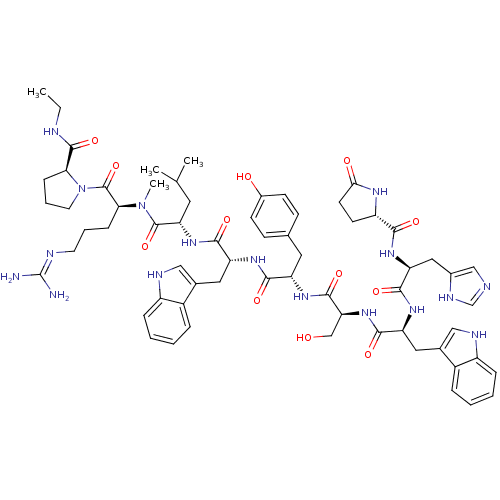
(deslorelin 8NMeArg)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:32.45,24.29,12.20,58.64,64.80,5.4,78.91,88.94,wD:46.58,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-5.75,-5.45,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C65H85N17O12/c1-5-69-62(92)53-17-11-25-82(53)64(94)54(16-10-24-70-65(66)67)81(4)63(93)51(26-36(2)3)79-58(88)48(28-38-31-71-44-14-8-6-12-42(38)44)76-57(87)47(27-37-18-20-41(84)21-19-37)75-61(91)52(34-83)80-59(89)49(29-39-32-72-45-15-9-7-13-43(39)45)77-60(90)50(30-40-33-68-35-73-40)78-56(86)46-22-23-55(85)74-46/h6-9,12-15,18-21,31-33,35-36,46-54,71-72,83-84H,5,10-11,16-17,22-30,34H2,1-4H3,(H,68,73)(H,69,92)(H,74,85)(H,75,91)(H,76,87)(H,77,90)(H,78,86)(H,79,88)(H,80,89)(H4,66,67,70)/t46-,47-,48+,49-,50-,51-,52-,53-,54-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84720

(deslorelin 10SarNH2)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N(C)[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:8.20,4.4,74.80,34.39,40.55,88.96,54.66,64.69,wD:22.33,(-2.79,-8.25,;-2.46,-6.71,;-.84,-7.09,;-3.52,-5.56,;-3.12,-4.06,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,;-4.4,-3.25,;-4.38,-1.66,;-5.75,-3.91,;-5.75,-5.45,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-6.97,-1.49,;-5.62,-.85,;-8.21,-.6,;-9.77,-1.09,;-10.59,.25,;-9.55,1.3,;-8.21,.86,;-6.91,1.76,;-5.56,.97,;-6.91,3.28,;-8.21,4.01,;-8.25,5.5,;-6.92,6.27,;-9.58,6.27,)| Show InChI InChI=1S/C65H84N18O13/c1-35(2)24-50(63(95)82(3)53(14-8-22-70-65(67)68)64(96)83-23-9-15-52(83)62(94)73-32-54(66)86)80-58(90)47(26-37-29-71-43-12-6-4-10-41(37)43)77-57(89)46(25-36-16-18-40(85)19-17-36)76-61(93)51(33-84)81-59(91)48(27-38-30-72-44-13-7-5-11-42(38)44)78-60(92)49(28-39-31-69-34-74-39)79-56(88)45-20-21-55(87)75-45/h4-7,10-13,16-19,29-31,34-35,45-53,71-72,84-85H,8-9,14-15,20-28,32-33H2,1-3H3,(H2,66,86)(H,69,74)(H,73,94)(H,75,87)(H,76,93)(H,77,89)(H,78,92)(H,79,88)(H,80,90)(H,81,91)(H4,67,68,70)/t45-,46-,47+,48-,49-,50-,51-,52-,53-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230128
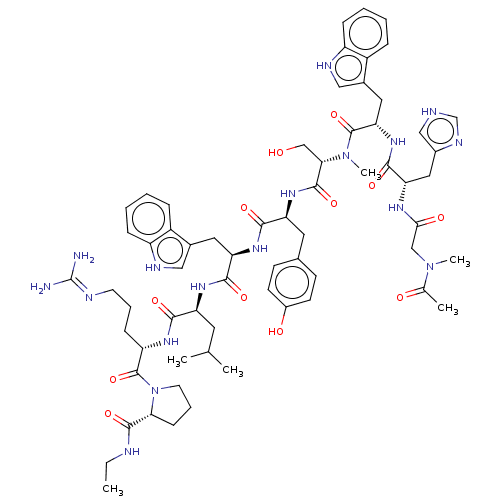
(CHEMBL437798)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(C)C(C)=O |wU:64.80,45.57,31.44,23.28,5.4,wD:12.20,78.91,57.61,(25.45,-7.16,;25.84,-8.64,;27.33,-9.06,;28.4,-10.16,;29.9,-9.79,;27.98,-11.65,;28.92,-12.86,;28.05,-14.15,;26.58,-13.71,;26.52,-12.17,;25.24,-11.31,;25.35,-9.77,;23.86,-11.99,;23.75,-13.53,;25.03,-14.39,;24.91,-15.92,;26.19,-16.78,;26.07,-18.33,;27.37,-19.2,;24.7,-19,;22.58,-11.11,;21.2,-11.79,;21.08,-13.34,;19.92,-10.93,;20.01,-9.39,;21.4,-8.72,;21.52,-7.18,;22.68,-9.58,;18.52,-11.6,;17.25,-10.74,;17.35,-9.21,;15.85,-11.41,;15.75,-12.95,;16.5,-14.29,;18,-14.62,;18.15,-16.15,;16.76,-16.76,;16.27,-18.2,;14.78,-18.52,;13.76,-17.38,;14.24,-15.92,;15.74,-15.62,;14.57,-10.56,;13.2,-11.23,;13.08,-12.76,;11.92,-10.37,;12.02,-8.83,;13.41,-8.14,;13.52,-6.61,;14.9,-5.93,;16.18,-6.79,;17.57,-6.12,;16.08,-8.34,;14.69,-9.01,;10.53,-11.04,;9.25,-10.18,;9.35,-8.63,;7.86,-10.85,;7.74,-12.39,;9.04,-13.25,;6.58,-9.99,;6.68,-8.46,;5.19,-10.66,;5.07,-12.2,;3.91,-9.79,;4.03,-8.26,;5.41,-7.58,;6.95,-7.37,;7.21,-5.85,;5.85,-5.12,;5.49,-3.63,;4,-3.19,;2.89,-4.26,;3.26,-5.76,;4.74,-6.19,;2.53,-10.46,;1.24,-9.6,;1.36,-8.07,;-.13,-10.28,;-.25,-11.83,;1.03,-12.68,;1.08,-14.23,;2.56,-14.66,;3.42,-13.38,;2.47,-12.16,;-1.42,-9.42,;-2.8,-10.09,;-2.92,-11.64,;-3.97,-9.3,;-5.09,-10.11,;-5.2,-11.65,;-6.18,-9.02,;-5.5,-7.65,;-7.71,-9.28,)| Show InChI InChI=1S/C65H87N17O12/c1-7-69-61(91)54-19-13-25-82(54)64(94)48(18-12-24-70-65(66)67)75-57(87)49(26-37(2)3)76-59(89)51(28-40-31-71-46-16-10-8-14-44(40)46)77-58(88)50(27-39-20-22-43(85)23-21-39)78-62(92)55(35-83)81(6)63(93)53(29-41-32-72-47-17-11-9-15-45(41)47)79-60(90)52(30-42-33-68-36-73-42)74-56(86)34-80(5)38(4)84/h8-11,14-17,20-23,31-33,36-37,48-55,71-72,83,85H,7,12-13,18-19,24-30,34-35H2,1-6H3,(H,68,73)(H,69,91)(H,74,86)(H,75,87)(H,76,89)(H,77,88)(H,78,92)(H,79,90)(H4,66,67,70)/t48-,49-,50-,51+,52-,53-,54+,55-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84702
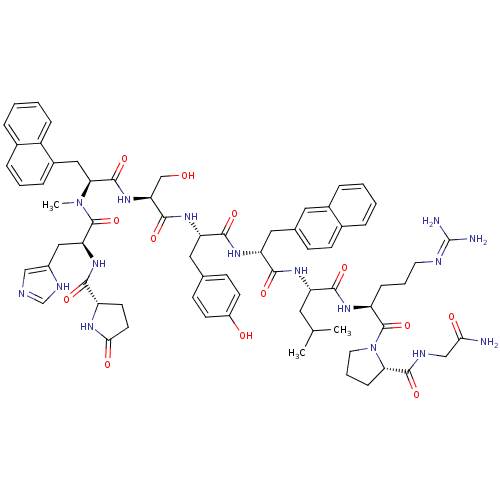
(nafarelin 3NMe1Nal)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)N(C)C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:41.57,67.72,23.34,76.82,90.98,wD:35.40,57.69,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.59,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.03,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.53,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.1,;-6.59,7.15,;-5.5,8.24,;-5.9,9.73,;-7.38,10.12,;-8.47,9.04,;-9.96,9.43,;-11.05,8.35,;-10.65,6.86,;-9.16,6.46,;-8.07,7.55,;-7.5,3.63,;-7.1,2.14,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.59,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.34,-2.2,;12.39,-3.01,;13.81,-2.21,;15.16,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.37,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C69H86N16O13/c1-39(2)29-51(61(91)78-50(17-9-27-74-69(71)72)68(98)85-28-10-18-56(85)65(95)75-36-58(70)88)79-63(93)53(32-41-19-22-42-11-4-5-13-44(42)30-41)80-62(92)52(31-40-20-23-47(87)24-21-40)81-64(94)55(37-86)83-66(96)57(33-45-15-8-14-43-12-6-7-16-48(43)45)84(3)67(97)54(34-46-35-73-38-76-46)82-60(90)49-25-26-59(89)77-49/h4-8,11-16,19-24,30,35,38-39,49-57,86-87H,9-10,17-18,25-29,31-34,36-37H2,1-3H3,(H2,70,88)(H,73,76)(H,75,95)(H,77,89)(H,78,91)(H,79,93)(H,80,92)(H,81,94)(H,82,90)(H,83,96)(H4,71,72,74)/t49-,50-,51-,52-,53+,54-,55-,56-,57-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84719

(deslorelin 3NMe1Nal)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)N(C)C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,63.80,5.4,79.92,89.95,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.27,-3.14,;8.73,-3.13,;7.96,-4.47,;8.73,-5.8,;10.27,-5.8,;11.04,-7.14,;12.58,-7.14,;13.35,-5.8,;12.58,-4.47,;11.04,-4.47,;10.95,.6,;12.29,-.17,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C67H86N16O12/c1-5-71-63(92)55-20-12-28-83(55)66(95)49(19-11-27-72-67(68)69)76-59(88)50(29-38(2)3)77-61(90)52(31-42-34-73-47-18-9-8-17-46(42)47)79-60(89)51(30-39-21-23-44(85)24-22-39)78-62(91)54(36-84)81-64(93)56(32-41-15-10-14-40-13-6-7-16-45(40)41)82(4)65(94)53(33-43-35-70-37-74-43)80-58(87)48-25-26-57(86)75-48/h6-10,13-18,21-24,34-35,37-38,48-56,73,84-85H,5,11-12,19-20,25-33,36H2,1-4H3,(H,70,74)(H,71,92)(H,75,86)(H,76,88)(H,77,90)(H,78,91)(H,79,89)(H,80,87)(H,81,93)(H4,68,69,72)/t48-,49-,50-,51-,52+,53-,54-,55-,56-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84701

(CAS_35263-73-1 | LHRH)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O |r,wU:30.43,12.21,4.4,wD:24.27,44.54,54.57,63.67,77.83,(8.46,2.13,;7.11,2.91,;7.08,4.45,;5.79,2.1,;5.81,.57,;4.48,-.19,;3.13,.55,;3.1,2.06,;1.8,-.22,;.47,.51,;-.84,-.24,;-.82,-1.77,;-2.18,.49,;-2.2,2.02,;-.86,2.81,;-.89,4.35,;.42,5.13,;1.75,4.37,;3.09,5.15,;1.77,2.83,;.44,2.03,;-3.51,-.27,;-4.87,.45,;-4.87,2,;-6.18,-.29,;-6.18,-1.83,;-4.83,-2.59,;-7.51,.44,;-8.85,-.3,;-8.82,-1.84,;-10.18,.41,;-10.2,1.94,;-8.86,2.73,;-7.58,1.91,;-6.39,2.91,;-6.95,4.28,;-6.27,5.67,;-7.13,7.02,;-8.67,6.9,;-9.39,5.5,;-8.52,4.2,;-11.49,-.34,;-12.84,.39,;-12.84,1.94,;-14.19,-.36,;-14.18,-1.88,;-12.81,-2.66,;-11.41,-2.01,;-10.38,-3.2,;-11.13,-4.5,;-12.64,-4.19,;-15.52,.37,;-16.85,-.37,;-16.85,-1.91,;-18.18,.34,;-17.2,-.73,;-17.89,-2.19,;-19.42,-1.88,;-20.72,-2.66,;-19.42,-.37,;7.14,-.19,;7.16,-1.69,;8.47,.6,;9.81,-.14,;9.82,-1.65,;11.14,-2.41,;11.17,-3.96,;12.52,-4.7,;12.53,-6.24,;11.18,-7.02,;13.88,-6.99,;11.13,.63,;11.11,2.17,;12.45,-.14,;12.48,-1.54,;14.07,-1.8,;14.65,-.45,;13.93,.71,;15.39,-.14,;15.39,-1.77,;16.71,.64,;18.06,-.13,;19.38,.66,;20.72,-.1,;19.38,2.18,)| Show InChI InChI=1S/C55H74N16O14/c1-29(2)19-38(49(80)66-37(9-5-17-59-55(56)57)54(85)71-18-6-10-43(71)53(84)62-26-46(76)77)65-45(75)25-61-47(78)39(20-30-11-13-33(73)14-12-30)67-52(83)42(27-72)70-50(81)40(21-31-23-60-35-8-4-3-7-34(31)35)68-51(82)41(22-32-24-58-28-63-32)69-48(79)36-15-16-44(74)64-36/h3-4,7-8,11-14,23-24,28-29,36-43,60,72-73H,5-6,9-10,15-22,25-27H2,1-2H3,(H,58,63)(H,61,78)(H,62,84)(H,64,74)(H,65,75)(H,66,80)(H,67,83)(H,68,82)(H,69,79)(H,70,81)(H,76,77)(H4,56,57,59)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230130

(CHEMBL266205)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:58.72,39.49,31.36,23.28,5.4,82.86,wD:72.83,12.20,51.53,(25.64,-6.44,;26.03,-7.92,;27.52,-8.34,;28.58,-9.43,;30.09,-9.08,;28.17,-10.92,;29.11,-12.14,;28.24,-13.43,;26.77,-13,;26.7,-11.45,;25.43,-10.59,;25.54,-9.05,;24.05,-11.27,;23.94,-12.8,;25.22,-13.67,;25.1,-15.21,;26.38,-16.06,;26.26,-17.6,;27.56,-18.46,;24.89,-18.28,;22.77,-10.4,;21.39,-11.07,;21.27,-12.61,;20.11,-10.2,;20.2,-8.67,;21.6,-7.99,;21.71,-6.46,;22.87,-8.85,;18.72,-10.89,;17.44,-10.03,;17.55,-8.48,;16.05,-10.7,;15.95,-12.23,;16.71,-13.56,;18.25,-13.58,;15.93,-14.89,;14.77,-9.83,;13.39,-10.5,;13.28,-12.05,;12.12,-9.64,;12.22,-8.11,;13.61,-7.44,;13.72,-5.9,;15.1,-5.21,;16.37,-6.08,;17.77,-5.41,;16.27,-7.62,;14.89,-8.3,;10.73,-10.31,;9.45,-9.46,;9.55,-7.9,;8.06,-10.13,;7.95,-11.66,;9.24,-12.54,;6.79,-9.27,;6.88,-7.74,;5.39,-9.94,;5.28,-11.48,;4.12,-9.08,;4.23,-7.54,;5.62,-6.87,;7.15,-6.65,;7.41,-5.14,;6.06,-4.41,;5.7,-2.91,;4.21,-2.48,;3.1,-3.55,;3.47,-5.05,;4.94,-5.48,;2.74,-9.75,;1.45,-8.89,;1.56,-7.34,;.08,-9.56,;-.04,-11.1,;1.24,-11.96,;1.28,-13.51,;2.77,-13.94,;3.63,-12.66,;2.68,-11.43,;-1.21,-8.71,;-2.59,-9.38,;-2.71,-10.92,;-3.76,-8.59,;-3.76,-7.16,;-5.29,-6.93,;-5.97,-8.31,;-7.49,-8.57,;-4.87,-9.39,)| Show InChI InChI=1S/C60H86N16O12/c1-7-64-56(85)48-15-11-23-76(48)59(88)42(14-10-22-65-60(61)62)69-52(81)43(24-33(2)3)70-53(82)44(25-34(4)5)71-54(83)45(26-35-16-18-38(78)19-17-35)73-57(86)49(31-77)75(6)58(87)47(27-36-29-66-40-13-9-8-12-39(36)40)74-55(84)46(28-37-30-63-32-67-37)72-51(80)41-20-21-50(79)68-41/h8-9,12-13,16-19,29-30,32-34,41-49,66,77-78H,7,10-11,14-15,20-28,31H2,1-6H3,(H,63,67)(H,64,85)(H,68,79)(H,69,81)(H,70,82)(H,71,83)(H,72,80)(H,73,86)(H,74,84)(H4,61,62,65)/t41-,42-,43-,44+,45-,46-,47-,48+,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84732

(deslorelin 2NMeHis)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@@H]1CCC(=O)N1 |r,wU:31.44,23.28,12.20,57.63,63.79,5.4,77.90,88.94,wD:45.57,(-8.25,5.5,;-8.21,4.01,;-6.91,3.28,;-6.91,1.76,;-5.56,.97,;-8.21,.86,;-9.55,1.3,;-10.59,.25,;-9.77,-1.09,;-8.21,-.6,;-6.97,-1.49,;-5.62,-.85,;-7.01,-3.06,;-8.43,-3.76,;-9.77,-2.88,;-11.13,-3.55,;-12.37,-2.75,;-13.77,-3.43,;-15.09,-2.58,;-13.89,-4.95,;-5.75,-3.91,;-4.4,-3.25,;-4.38,-1.66,;-3.12,-4.06,;-3.52,-5.56,;-2.46,-6.71,;-2.79,-8.25,;-.84,-7.09,;-1.7,-3.39,;-.45,-4.25,;-.33,-5.83,;.61,-3.28,;.76,-1.8,;-.23,-.66,;.18,.7,;-.73,1.73,;-2.18,1.46,;-3.12,2.52,;-4.51,2.21,;-4.98,.86,;-4.03,-.18,;-2.61,.1,;2.04,-3.86,;3.22,-2.91,;4.71,-3.45,;2.98,-1.4,;1.57,-.7,;1.7,.88,;.39,1.92,;.68,3.61,;2.13,4.32,;2.37,5.95,;3.43,3.26,;3.26,1.51,;4.09,-.39,;5.66,-.45,;6.38,.82,;6.32,-1.82,;5.59,-3.1,;6.26,-4.46,;7.87,-1.86,;8.66,-.54,;7.93,.8,;10.17,-.6,;10.89,-1.99,;10.45,-3.49,;9,-4.01,;9.07,-5.58,;10.52,-6,;11.2,-7.39,;12.75,-7.49,;13.62,-6.2,;12.93,-4.81,;11.38,-4.71,;10.95,.6,;10.29,1.94,;8.75,1.98,;11.11,3.26,;12.59,3.16,;13.35,1.86,;12.66,.45,;13.72,-.54,;15.09,.1,;14.84,1.64,;10.35,4.51,;11.12,5.85,;8.83,4.51,;8.02,3.22,;8.02,5.91,;8.59,7.19,;7.54,8.25,;6.16,7.59,;4.77,8.19,;6.53,5.95,)| Show InChI InChI=1S/C65H85N17O12/c1-5-69-61(91)53-17-11-25-82(53)64(94)46(16-10-24-70-65(66)67)75-56(86)48(26-36(2)3)76-58(88)50(28-38-31-71-44-14-8-6-12-42(38)44)78-57(87)49(27-37-18-20-41(84)21-19-37)77-60(90)52(34-83)80-59(89)51(29-39-32-72-45-15-9-7-13-43(39)45)79-62(92)54(30-40-33-68-35-73-40)81(4)63(93)47-22-23-55(85)74-47/h6-9,12-15,18-21,31-33,35-36,46-54,71-72,83-84H,5,10-11,16-17,22-30,34H2,1-4H3,(H,68,73)(H,69,91)(H,74,85)(H,75,86)(H,76,88)(H,77,90)(H,78,87)(H,79,92)(H,80,89)(H4,66,67,70)/t46-,47-,48-,49-,50+,51-,52-,53-,54-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84718
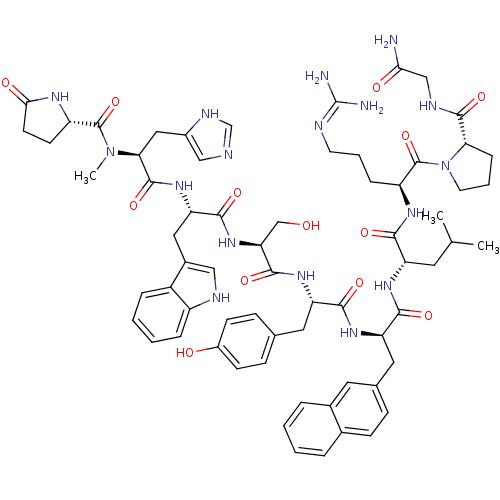
(nafarelin 2NMeHis)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:41.56,66.71,23.34,75.81,89.97,wD:35.40,55.67,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.6,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.03,5.75,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C67H85N17O13/c1-37(2)26-49(58(89)77-47(14-8-24-72-67(69)70)66(97)84-25-9-15-54(84)63(94)74-34-56(68)87)78-60(91)51(29-39-16-19-40-10-4-5-11-41(40)27-39)79-59(90)50(28-38-17-20-44(86)21-18-38)80-62(93)53(35-85)82-61(92)52(30-42-32-73-46-13-7-6-12-45(42)46)81-64(95)55(31-43-33-71-36-75-43)83(3)65(96)48-22-23-57(88)76-48/h4-7,10-13,16-21,27,32-33,36-37,47-55,73,85-86H,8-9,14-15,22-26,28-31,34-35H2,1-3H3,(H2,68,87)(H,71,75)(H,74,94)(H,76,88)(H,77,89)(H,78,91)(H,79,90)(H,80,93)(H,81,95)(H,82,92)(H4,69,70,72)/t47-,48-,49-,50-,51+,52-,53-,54-,55-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84722
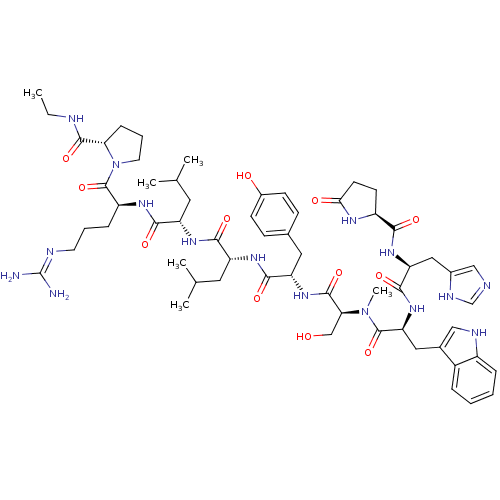
(leuprolide 4NMeSer)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:51.55,72.83,82.86,31.36,5.4,wD:58.72,39.49,23.28,12.20,(10.22,12.44,;9.73,11.04,;10.69,9.91,;10.16,8.5,;11.09,7.36,;8.69,8.25,;7.58,9.33,;6.25,8.6,;6.47,7.14,;8.01,6.89,;8.69,5.56,;10.34,5.13,;7.76,4.7,;8.05,3.19,;9.4,2.76,;10.56,3.77,;11.98,3.34,;13.07,4.34,;14.52,3.91,;12.73,5.81,;6.36,5.25,;5.17,4.27,;3.75,4.73,;5.43,2.76,;6.79,2.3,;7.11,.79,;6.64,-.49,;8.54,.28,;4.27,1.76,;2.87,2.26,;2.59,3.77,;1.73,1.23,;1.97,-.25,;3.45,-.72,;3.77,-2.23,;4.1,.15,;.26,1.68,;-.83,.68,;-2.3,1.12,;-.5,-.83,;.97,-1.29,;1.33,-2.76,;.22,-3.83,;.66,-5.34,;2.12,-5.74,;2.48,-7.22,;3.27,-4.67,;2.81,-3.11,;-1.58,-1.87,;-2.98,-1.43,;-3.37,.08,;-4.09,-2.51,;-5.56,-2.12,;-6.7,-3.19,;-3.76,-3.99,;-2.27,-4.39,;-4.84,-5.02,;-6.31,-4.63,;-4.45,-6.49,;-2.94,-6.89,;-2.51,-8.36,;-1.14,-8.86,;-1.18,-10.4,;-2.61,-10.84,;-3.29,-12.23,;-4.91,-12.31,;-5.73,-10.97,;-5.02,-9.57,;-3.46,-9.5,;-5.56,-7.57,;-7.01,-7.22,;-7.41,-5.74,;-8.1,-8.33,;-9.59,-7.89,;-9.99,-6.42,;-11.44,-5.87,;-11.3,-4.34,;-9.79,-3.94,;-9,-5.27,;-7.71,-9.82,;-8.81,-10.93,;-8.46,-12.44,;-10.37,-10.57,;-10.93,-9.1,;-12.5,-9.1,;-12.98,-10.64,;-14.52,-11.2,;-11.66,-11.59,)| Show InChI InChI=1S/C60H86N16O12/c1-7-64-56(85)48-15-11-23-76(48)59(88)42(14-10-22-65-60(61)62)69-52(81)43(24-33(2)3)70-53(82)44(25-34(4)5)71-54(83)45(26-35-16-18-38(78)19-17-35)73-57(86)49(31-77)75(6)58(87)47(27-36-29-66-40-13-9-8-12-39(36)40)74-55(84)46(28-37-30-63-32-67-37)72-51(80)41-20-21-50(79)68-41/h8-9,12-13,16-19,29-30,32-34,41-49,66,77-78H,7,10-11,14-15,20-28,31H2,1-6H3,(H,63,67)(H,64,85)(H,68,79)(H,69,81)(H,70,82)(H,71,83)(H,72,80)(H,73,86)(H,74,84)(H4,61,62,65)/t41-,42-,43-,44+,45-,46-,47-,48-,49-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84710
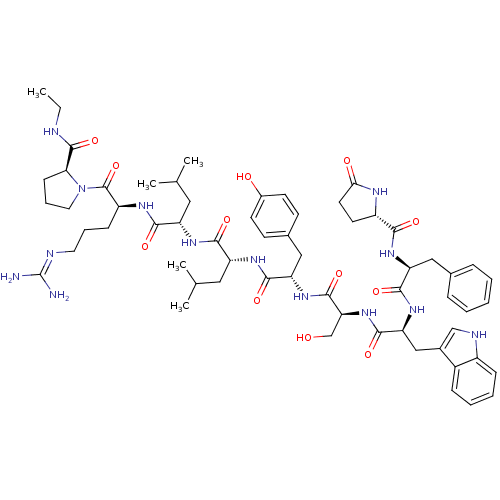
(leuprolide 2 Phe)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:51.55,71.83,82.86,31.36,5.4,wD:57.71,39.49,23.28,12.20,(10.22,12.44,;9.73,11.04,;10.69,9.91,;10.16,8.49,;11.09,7.36,;8.69,8.25,;7.58,9.33,;6.25,8.6,;6.47,7.14,;8.01,6.89,;8.69,5.56,;10.34,5.13,;7.76,4.7,;8.05,3.19,;9.4,2.76,;10.56,3.77,;11.98,3.34,;13.07,4.34,;14.52,3.91,;12.73,5.81,;6.36,5.25,;5.17,4.27,;3.75,4.73,;5.43,2.76,;6.79,2.3,;7.11,.79,;6.64,-.49,;8.54,.28,;4.27,1.76,;2.87,2.26,;2.59,3.77,;1.73,1.23,;1.97,-.25,;3.45,-.72,;3.77,-2.23,;4.1,.15,;.26,1.68,;-.83,.68,;-2.3,1.12,;-.5,-.83,;.97,-1.29,;1.33,-2.76,;.22,-3.83,;.66,-5.34,;2.12,-5.74,;2.48,-7.21,;3.27,-4.67,;2.81,-3.11,;-1.58,-1.87,;-2.98,-1.43,;-3.37,.08,;-4.09,-2.51,;-5.56,-2.12,;-6.7,-3.19,;-3.76,-3.99,;-4.84,-5.02,;-6.31,-4.63,;-4.45,-6.49,;-2.94,-6.89,;-2.51,-8.36,;-1.14,-8.86,;-1.18,-10.4,;-2.61,-10.84,;-3.29,-12.23,;-4.91,-12.31,;-5.73,-10.97,;-5.02,-9.57,;-3.46,-9.5,;-5.56,-7.56,;-7.01,-7.21,;-7.41,-5.74,;-8.1,-8.33,;-9.59,-7.89,;-10.07,-6.27,;-11.55,-5.87,;-11.95,-4.38,;-10.86,-3.29,;-9.37,-3.69,;-8.98,-5.18,;-7.71,-9.82,;-8.81,-10.93,;-8.46,-12.44,;-10.37,-10.57,;-10.93,-9.1,;-12.5,-9.1,;-12.97,-10.64,;-14.52,-11.2,;-11.66,-11.59,)| Show InChI InChI=1S/C62H86N14O12/c1-6-65-60(87)51-19-13-27-76(51)61(88)44(18-12-26-66-62(63)64)69-54(81)45(28-35(2)3)70-55(82)46(29-36(4)5)71-56(83)48(31-38-20-22-40(78)23-21-38)73-59(86)50(34-77)75-58(85)49(32-39-33-67-42-17-11-10-16-41(39)42)74-57(84)47(30-37-14-8-7-9-15-37)72-53(80)43-24-25-52(79)68-43/h7-11,14-17,20-23,33,35-36,43-51,67,77-78H,6,12-13,18-19,24-32,34H2,1-5H3,(H,65,87)(H,68,79)(H,69,81)(H,70,82)(H,71,83)(H,72,80)(H,73,86)(H,74,84)(H,75,85)(H4,63,64,66)/t43-,44-,45-,46+,47-,48-,49-,50-,51-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230121

(CHEMBL217405)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.71,31.36,23.28,5.4,82.86,wD:51.55,71.83,12.20,(27.09,-8.95,;27.46,-10.44,;28.95,-10.86,;30.04,-11.96,;31.53,-11.6,;29.6,-13.45,;30.55,-14.67,;29.69,-15.94,;28.21,-15.52,;28.15,-13.97,;26.88,-13.11,;26.98,-11.58,;25.49,-13.78,;25.37,-15.33,;26.65,-16.2,;26.55,-17.73,;27.83,-18.59,;27.71,-20.12,;29,-21,;26.33,-20.8,;24.21,-12.92,;22.82,-13.6,;22.72,-15.13,;21.54,-12.73,;21.65,-11.2,;23.04,-10.52,;24.32,-11.38,;23.14,-8.97,;20.15,-13.41,;18.89,-12.55,;18.99,-11.01,;17.49,-13.23,;17.38,-14.76,;18.15,-16.1,;17.36,-17.42,;19.68,-16.11,;16.21,-12.36,;14.83,-13.03,;14.71,-14.57,;13.55,-12.17,;13.66,-10.62,;15.04,-9.95,;15.15,-8.42,;16.54,-7.74,;17.82,-8.6,;19.2,-7.93,;17.71,-10.14,;16.31,-10.81,;12.16,-12.85,;10.88,-11.97,;10.99,-10.44,;9.49,-12.64,;9.38,-14.19,;10.67,-15.06,;8.21,-11.79,;6.83,-12.46,;6.71,-14.01,;5.54,-11.6,;5.65,-10.07,;7.05,-9.39,;8.58,-9.18,;8.85,-7.67,;7.49,-6.93,;7.12,-5.44,;5.63,-5,;4.53,-6.07,;4.89,-7.56,;6.37,-8,;4.17,-12.27,;2.88,-11.41,;3,-9.87,;1.49,-12.09,;1.38,-13.62,;2.14,-14.94,;3.65,-14.94,;4.41,-16.27,;3.65,-17.59,;2.12,-17.58,;1.37,-16.26,;.22,-11.23,;-1.18,-11.9,;-1.28,-13.43,;-2.34,-11.11,;-2.34,-9.69,;-3.87,-9.45,;-4.55,-10.83,;-6.06,-11.09,;-3.45,-11.92,)| Show InChI InChI=1S/C62H86N14O12/c1-6-65-60(87)51-19-13-27-76(51)61(88)44(18-12-26-66-62(63)64)69-54(81)45(28-35(2)3)70-55(82)46(29-36(4)5)71-56(83)48(31-38-20-22-40(78)23-21-38)73-59(86)50(34-77)75-58(85)49(32-39-33-67-42-17-11-10-16-41(39)42)74-57(84)47(30-37-14-8-7-9-15-37)72-53(80)43-24-25-52(79)68-43/h7-11,14-17,20-23,33,35-36,43-51,67,77-78H,6,12-13,18-19,24-32,34H2,1-5H3,(H,65,87)(H,68,79)(H,69,81)(H,70,82)(H,71,83)(H,72,80)(H,73,86)(H,74,84)(H,75,85)(H4,63,64,66)/t43-,44-,45-,46+,47-,48-,49-,50-,51+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84724
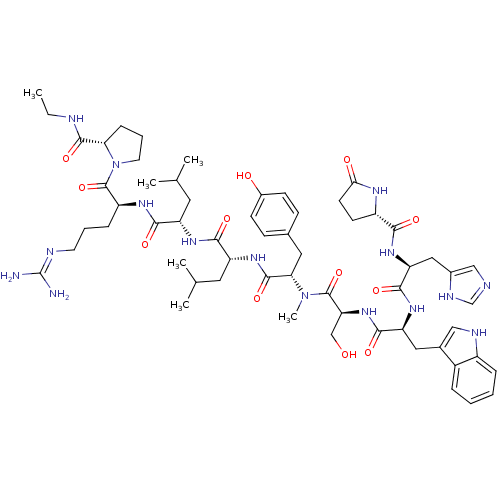
(leuprolide 5NMeTyr)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:52.56,72.83,82.86,31.36,5.4,wD:58.72,39.49,23.28,12.20,(10.22,12.44,;9.73,11.04,;10.69,9.91,;10.16,8.5,;11.09,7.36,;8.69,8.25,;7.58,9.33,;6.25,8.6,;6.47,7.14,;8.01,6.89,;8.69,5.56,;10.34,5.13,;7.76,4.7,;8.05,3.19,;9.4,2.76,;10.56,3.77,;11.98,3.34,;13.07,4.34,;14.52,3.91,;12.73,5.81,;6.36,5.25,;5.17,4.27,;3.75,4.73,;5.43,2.76,;6.79,2.3,;7.11,.79,;6.64,-.49,;8.54,.28,;4.27,1.76,;2.87,2.26,;2.59,3.77,;1.73,1.23,;1.97,-.25,;3.45,-.72,;3.77,-2.23,;4.1,.15,;.26,1.68,;-.83,.68,;-2.3,1.12,;-.5,-.83,;.97,-1.29,;1.33,-2.76,;.22,-3.83,;.66,-5.34,;2.12,-5.74,;2.48,-7.22,;3.27,-4.67,;2.81,-3.11,;-1.58,-1.87,;-1.18,-3.35,;-2.98,-1.43,;-3.37,.08,;-4.09,-2.51,;-5.56,-2.12,;-6.7,-3.19,;-3.76,-3.99,;-4.84,-5.02,;-6.31,-4.63,;-4.45,-6.49,;-2.94,-6.89,;-2.51,-8.36,;-1.14,-8.86,;-1.18,-10.4,;-2.61,-10.84,;-3.29,-12.23,;-4.91,-12.31,;-5.73,-10.97,;-5.02,-9.57,;-3.46,-9.5,;-5.56,-7.57,;-7.01,-7.22,;-7.41,-5.74,;-8.1,-8.33,;-9.59,-7.89,;-9.99,-6.42,;-11.44,-5.87,;-11.3,-4.34,;-9.79,-3.94,;-9,-5.27,;-7.71,-9.82,;-8.81,-10.93,;-8.46,-12.44,;-10.37,-10.57,;-10.93,-9.1,;-12.5,-9.1,;-12.98,-10.64,;-14.52,-11.2,;-11.66,-11.59,)| Show InChI InChI=1S/C60H86N16O12/c1-7-64-56(85)48-15-11-23-76(48)59(88)42(14-10-22-65-60(61)62)69-52(81)43(24-33(2)3)70-53(82)44(25-34(4)5)73-57(86)49(26-35-16-18-38(78)19-17-35)75(6)58(87)47(31-77)74-54(83)45(27-36-29-66-40-13-9-8-12-39(36)40)71-55(84)46(28-37-30-63-32-67-37)72-51(80)41-20-21-50(79)68-41/h8-9,12-13,16-19,29-30,32-34,41-49,66,77-78H,7,10-11,14-15,20-28,31H2,1-6H3,(H,63,67)(H,64,85)(H,68,79)(H,69,81)(H,70,82)(H,71,84)(H,72,80)(H,73,86)(H,74,83)(H4,61,62,65)/t41-,42-,43-,44+,45-,46-,47-,48-,49-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230125

(CHEMBL274682)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(C)C(C)=O |wU:39.49,57.71,31.36,23.28,wD:51.55,12.20,5.4,71.82,(26.08,-5.67,;26.47,-7.16,;27.96,-7.58,;29.03,-8.67,;30.53,-8.31,;28.61,-10.15,;29.55,-11.37,;28.68,-12.66,;27.21,-12.23,;27.15,-10.69,;25.87,-9.83,;25.98,-8.29,;24.49,-10.5,;24.38,-12.03,;25.66,-12.91,;25.54,-14.44,;26.82,-15.3,;26.7,-16.84,;28,-17.7,;25.33,-17.51,;23.21,-9.63,;21.83,-10.31,;21.71,-11.85,;20.55,-9.43,;20.65,-7.9,;22.04,-7.23,;23.31,-8.09,;22.15,-5.7,;19.16,-10.12,;17.88,-9.26,;17.99,-7.71,;16.49,-9.94,;16.39,-11.47,;17.14,-12.8,;18.67,-12.82,;16.37,-14.14,;15.21,-9.06,;13.84,-9.73,;13.72,-11.28,;12.56,-8.88,;12.66,-7.34,;14.05,-6.67,;15.33,-7.53,;16.71,-6.86,;16.81,-5.32,;18.21,-4.64,;15.54,-4.44,;14.16,-5.13,;11.17,-9.55,;9.89,-8.69,;9.99,-7.15,;8.5,-9.36,;8.39,-10.9,;9.68,-11.77,;7.23,-8.5,;5.83,-9.18,;5.72,-10.71,;4.56,-8.31,;4.67,-6.78,;6.06,-6.11,;7.59,-5.88,;7.85,-4.37,;6.5,-3.64,;6.14,-2.14,;4.65,-1.72,;3.54,-2.79,;3.91,-4.28,;5.39,-4.71,;3.18,-8.98,;1.89,-8.12,;2,-6.58,;.52,-8.8,;.4,-10.34,;1.68,-11.2,;1.73,-12.74,;3.21,-13.17,;4.07,-11.89,;3.12,-10.66,;-.77,-7.94,;-2.15,-8.61,;-2.27,-10.15,;-3.32,-7.82,;-4.43,-8.62,;-4.55,-10.15,;-5.52,-7.54,;-7,-7.95,;-5.13,-6.07,)| Show InChI InChI=1S/C59H86N16O12/c1-8-63-57(86)49-16-12-22-75(49)58(87)42(15-11-21-64-59(60)61)68-51(80)43(23-33(2)3)69-52(81)44(24-34(4)5)70-53(82)45(25-36-17-19-39(78)20-18-36)71-56(85)48(31-76)73-54(83)46(26-37-28-65-41-14-10-9-13-40(37)41)72-55(84)47(27-38-29-62-32-66-38)67-50(79)30-74(7)35(6)77/h9-10,13-14,17-20,28-29,32-34,42-49,65,76,78H,8,11-12,15-16,21-27,30-31H2,1-7H3,(H,62,66)(H,63,86)(H,67,79)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,83)(H4,60,61,64)/t42-,43-,44+,45-,46-,47-,48-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84705
(nafarelin 6NMe2Nal)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)N(C)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:42.57,66.71,24.35,75.81,89.97,wD:36.41,56.68,8.21,4.4,(4.31,-3.28,;5.62,-2.47,;6.98,-3.2,;5.58,-.92,;7.27,.05,;7.24,1.6,;5.89,2.38,;4.25,1.42,;5.89,3.78,;7.3,4.67,;8.68,4.11,;8.64,2.57,;9.96,1.77,;11.33,2.51,;12.66,1.72,;14.02,2.47,;14.04,4.01,;12.72,4.83,;11.36,4.07,;10.03,4.87,;4.65,4.7,;5.05,6.19,;3.3,3.93,;3.3,2.31,;1.92,4.76,;1.92,6.36,;3.25,7.09,;4.54,6.36,;5.85,7.07,;5.85,8.62,;7.01,9.29,;4.55,9.38,;3.25,8.62,;.5,3.94,;-.84,4.7,;-.87,6.2,;-2.13,3.93,;-2.1,2.42,;-.79,1.7,;-3.45,4.64,;-4.69,3.8,;-4.62,2.07,;-6.14,4.56,;-6.14,6.66,;-7.38,7.5,;-7.54,9.01,;-9,9.32,;-9.76,8.03,;-11.24,7.72,;-11.7,6.27,;-10.72,5.17,;-9.23,5.47,;-8.74,6.92,;-7.5,3.63,;-8.71,4.41,;-8.64,5.92,;-9.94,3.67,;-9.94,2.17,;-8.64,1.41,;-7.14,1.48,;-6.6,.06,;-7.77,-.88,;-9.04,-.05,;-11.26,4.41,;-12.55,3.67,;-12.53,2.1,;-13.85,4.43,;-14.01,5.87,;-15.44,6.27,;-16.37,5.01,;-17.87,4.96,;-15.32,3.81,;8.64,-.67,;9.96,.12,;8.68,-2.21,;10,-2.98,;11.35,-2.2,;12.39,-3.01,;13.81,-2.21,;15.17,-3.2,;16.48,-2.17,;17.87,-2.85,;16.38,-.62,;10,-4.51,;8.68,-5.3,;11.36,-5.3,;12.78,-4.67,;13.81,-5.86,;13.05,-7.16,;11.52,-6.83,;10.37,-7.86,;10.7,-9.38,;8.9,-7.38,;7.75,-8.42,;6.28,-7.95,;5.95,-6.42,;5.11,-8.96,)| Show InChI InChI=1S/C67H85N17O13/c1-37(2)26-49(59(90)77-48(14-8-24-72-67(69)70)66(97)84-25-9-15-54(84)63(94)74-34-56(68)87)80-64(95)55(29-39-16-19-40-10-4-5-11-41(40)27-39)83(3)65(96)52(28-38-17-20-44(86)21-18-38)81-62(93)53(35-85)82-60(91)50(30-42-32-73-46-13-7-6-12-45(42)46)78-61(92)51(31-43-33-71-36-75-43)79-58(89)47-22-23-57(88)76-47/h4-7,10-13,16-21,27,32-33,36-37,47-55,73,85-86H,8-9,14-15,22-26,28-31,34-35H2,1-3H3,(H2,68,87)(H,71,75)(H,74,94)(H,76,88)(H,77,90)(H,78,92)(H,79,89)(H,80,95)(H,81,93)(H,82,91)(H4,69,70,72)/t47-,48-,49-,50-,51-,52-,53-,54-,55+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84723
(leuprolide 3NMe1Nal)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)N(C)C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:51.55,73.84,83.87,31.36,5.4,wD:57.72,39.49,23.28,12.20,(10.22,12.44,;9.73,11.04,;10.69,9.91,;10.16,8.49,;11.09,7.36,;8.69,8.25,;7.58,9.33,;6.25,8.6,;6.47,7.14,;8.01,6.89,;8.69,5.56,;10.34,5.13,;7.76,4.7,;8.05,3.19,;9.4,2.76,;10.56,3.77,;11.98,3.34,;13.07,4.34,;14.52,3.91,;12.73,5.81,;6.36,5.25,;5.17,4.27,;3.75,4.73,;5.43,2.76,;6.79,2.3,;7.11,.79,;6.64,-.49,;8.54,.28,;4.27,1.76,;2.87,2.26,;2.59,3.77,;1.73,1.23,;1.97,-.25,;3.45,-.72,;3.77,-2.23,;4.1,.15,;.26,1.68,;-.83,.68,;-2.3,1.12,;-.5,-.83,;.97,-1.29,;1.33,-2.76,;.22,-3.83,;.66,-5.34,;2.12,-5.74,;2.48,-7.21,;3.27,-4.67,;2.81,-3.11,;-1.58,-1.87,;-2.98,-1.43,;-3.37,.08,;-4.09,-2.51,;-5.56,-2.12,;-6.7,-3.19,;-3.76,-3.99,;-4.84,-5.02,;-6.31,-4.63,;-4.45,-6.49,;-2.94,-6.89,;-2.63,-8.53,;-1.14,-8.92,;-.74,-10.41,;-1.83,-11.5,;-3.32,-11.1,;-4.41,-12.19,;-5.89,-11.79,;-6.29,-10.3,;-5.2,-9.22,;-3.72,-9.61,;-5.56,-7.56,;-5.96,-9.05,;-7.01,-7.21,;-7.41,-5.74,;-8.1,-8.33,;-9.59,-7.89,;-9.99,-6.42,;-11.44,-5.87,;-11.3,-4.34,;-9.79,-3.94,;-9,-5.27,;-7.71,-9.82,;-8.81,-10.93,;-8.46,-12.44,;-10.37,-10.57,;-10.93,-9.1,;-12.5,-9.1,;-12.97,-10.64,;-14.52,-11.2,;-11.66,-11.59,)| Show InChI InChI=1S/C62H87N15O12/c1-7-66-58(86)50-18-12-26-77(50)61(89)44(17-11-25-67-62(63)64)70-54(82)45(27-35(2)3)71-55(83)46(28-36(4)5)72-56(84)47(29-37-19-21-41(79)22-20-37)73-57(85)49(33-78)75-59(87)51(30-39-15-10-14-38-13-8-9-16-42(38)39)76(6)60(88)48(31-40-32-65-34-68-40)74-53(81)43-23-24-52(80)69-43/h8-10,13-16,19-22,32,34-36,43-51,78-79H,7,11-12,17-18,23-31,33H2,1-6H3,(H,65,68)(H,66,86)(H,69,80)(H,70,82)(H,71,83)(H,72,84)(H,73,85)(H,74,81)(H,75,87)(H4,63,64,67)/t43-,44-,45-,46+,47-,48-,49-,50-,51-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84729
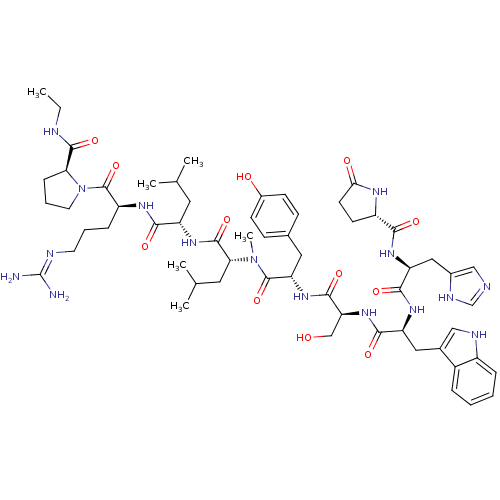
(leuprolide 6NMeDLeu)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r,wU:52.56,72.83,82.86,31.36,5.4,wD:58.72,40.50,23.28,12.20,(10.22,12.44,;9.73,11.04,;10.69,9.91,;10.16,8.5,;11.09,7.36,;8.69,8.25,;7.58,9.33,;6.25,8.6,;6.47,7.14,;8.01,6.89,;8.69,5.56,;10.34,5.13,;7.76,4.7,;8.05,3.19,;9.4,2.76,;10.56,3.77,;11.98,3.34,;13.07,4.34,;14.52,3.91,;12.73,5.81,;6.36,5.25,;5.17,4.27,;3.75,4.73,;5.43,2.76,;6.79,2.3,;7.11,.79,;6.64,-.49,;8.54,.28,;4.27,1.76,;2.87,2.26,;2.59,3.77,;1.73,1.23,;1.97,-.25,;3.45,-.72,;3.77,-2.23,;4.1,.15,;.26,1.68,;-.14,3.17,;-.83,.68,;-2.3,1.12,;-.5,-.83,;.97,-1.29,;1.33,-2.76,;.22,-3.83,;.66,-5.34,;2.12,-5.74,;2.48,-7.22,;3.27,-4.67,;2.81,-3.11,;-1.58,-1.87,;-2.98,-1.43,;-3.37,.08,;-4.09,-2.51,;-5.56,-2.12,;-6.7,-3.19,;-3.76,-3.99,;-4.84,-5.02,;-6.31,-4.63,;-4.45,-6.49,;-2.94,-6.89,;-2.51,-8.36,;-1.14,-8.86,;-1.18,-10.4,;-2.61,-10.84,;-3.29,-12.23,;-4.91,-12.31,;-5.73,-10.97,;-5.02,-9.57,;-3.46,-9.5,;-5.56,-7.57,;-7.01,-7.22,;-7.41,-5.74,;-8.1,-8.33,;-9.59,-7.89,;-9.99,-6.42,;-11.44,-5.87,;-11.3,-4.34,;-9.79,-3.94,;-9,-5.27,;-7.71,-9.82,;-8.81,-10.93,;-8.46,-12.44,;-10.37,-10.57,;-10.93,-9.1,;-12.5,-9.1,;-12.98,-10.64,;-14.52,-11.2,;-11.66,-11.59,)| Show InChI InChI=1S/C60H86N16O12/c1-7-64-56(85)48-15-11-23-76(48)59(88)42(14-10-22-65-60(61)62)69-52(81)43(24-33(2)3)72-57(86)49(25-34(4)5)75(6)58(87)46(26-35-16-18-38(78)19-17-35)73-55(84)47(31-77)74-53(82)44(27-36-29-66-40-13-9-8-12-39(36)40)70-54(83)45(28-37-30-63-32-67-37)71-51(80)41-20-21-50(79)68-41/h8-9,12-13,16-19,29-30,32-34,41-49,66,77-78H,7,10-11,14-15,20-28,31H2,1-6H3,(H,63,67)(H,64,85)(H,68,79)(H,69,81)(H,70,83)(H,71,80)(H,72,86)(H,73,84)(H,74,82)(H4,61,62,65)/t41-,42-,43-,44-,45-,46-,47-,48-,49+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230119
(CHEMBL414071)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.68,23.28,31.36,5.4,79.82,wD:51.55,69.79,12.20,(29.65,-8.55,;30.04,-10.04,;31.53,-10.45,;32.6,-11.55,;34.09,-11.18,;32.18,-13.04,;33.11,-14.25,;32.25,-15.54,;30.77,-15.11,;30.72,-13.57,;29.44,-12.71,;29.55,-11.16,;28.05,-13.38,;27.95,-14.92,;29.23,-15.78,;29.12,-17.32,;30.4,-18.18,;30.28,-19.72,;31.56,-20.59,;28.9,-20.4,;26.77,-12.51,;25.39,-13.18,;25.28,-14.73,;24.12,-12.32,;24.22,-10.79,;25.61,-10.11,;26.89,-10.97,;25.72,-8.57,;22.72,-12.99,;21.45,-12.14,;21.54,-10.6,;20.06,-12.81,;19.94,-14.34,;20.71,-15.68,;19.93,-17.01,;22.24,-15.69,;18.78,-11.95,;17.39,-12.62,;17.27,-14.16,;16.11,-11.76,;16.22,-10.22,;17.61,-9.55,;17.71,-8,;19.1,-7.33,;20.38,-8.19,;21.77,-7.51,;20.27,-9.73,;18.89,-10.41,;14.73,-12.44,;13.44,-11.57,;13.55,-10.02,;12.06,-12.24,;11.95,-13.78,;13.23,-14.64,;10.78,-11.38,;9.39,-12.06,;9.28,-13.6,;8.11,-11.18,;8.22,-9.65,;9.3,-8.56,;10.79,-8.95,;11.87,-7.86,;11.46,-6.37,;12.55,-5.28,;9.97,-5.98,;8.9,-7.09,;6.72,-11.86,;5.44,-11,;5.56,-9.46,;4.07,-11.67,;3.95,-13.22,;5.23,-14.08,;5.28,-15.62,;6.76,-16.05,;7.63,-14.78,;6.68,-13.55,;2.77,-10.81,;1.39,-11.48,;1.29,-13.03,;.22,-10.69,;.22,-9.28,;-1.3,-9.05,;-1.99,-10.42,;-3.5,-10.67,;-.9,-11.51,)| Show InChI InChI=1S/C57H82ClN15O12/c1-6-62-55(84)46-10-8-22-73(46)56(85)39(9-7-21-63-57(59)60)66-49(78)40(23-31(2)3)67-50(79)41(24-32(4)5)68-51(80)42(26-34-13-17-37(75)18-14-34)70-54(83)45(29-74)72-52(81)43(25-33-11-15-35(58)16-12-33)69-53(82)44(27-36-28-61-30-64-36)71-48(77)38-19-20-47(76)65-38/h11-18,28,30-32,38-46,74-75H,6-10,19-27,29H2,1-5H3,(H,61,64)(H,62,84)(H,65,76)(H,66,78)(H,67,79)(H,68,80)(H,69,82)(H,70,83)(H,71,77)(H,72,81)(H4,59,60,63)/t38-,39-,40-,41+,42-,43-,44-,45-,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Progonadoliberin-1
(RAT) | BDBM84706
(leuprolide 10SarNH2)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)NCC(N)=O |r,wU:28.31,48.58,58.61,8.12,81.87,wD:34.47,16.25,4.4,67.71,(6.64,-.49,;7.11,.79,;8.54,.28,;6.79,2.3,;5.43,2.76,;4.27,1.76,;2.87,2.26,;2.59,3.77,;1.73,1.23,;1.97,-.25,;3.45,-.72,;3.77,-2.23,;4.1,.15,;.26,1.68,;-.83,.68,;-2.3,1.12,;-.5,-.83,;.97,-1.29,;1.33,-2.76,;.22,-3.83,;.66,-5.34,;2.12,-5.74,;2.48,-7.22,;3.27,-4.67,;2.81,-3.11,;-1.58,-1.87,;-2.98,-1.43,;-3.37,.08,;-4.09,-2.51,;-5.56,-2.12,;-6.7,-3.19,;-3.76,-3.99,;-4.84,-5.02,;-6.31,-4.63,;-4.45,-6.49,;-2.94,-6.89,;-2.51,-8.36,;-1.14,-8.86,;-1.18,-10.4,;-2.61,-10.84,;-3.29,-12.23,;-4.91,-12.31,;-5.73,-10.97,;-5.02,-9.57,;-3.46,-9.5,;-5.56,-7.57,;-7.01,-7.22,;-7.41,-5.74,;-8.1,-8.33,;-9.59,-7.89,;-9.99,-6.42,;-11.44,-5.87,;-11.3,-4.34,;-9.79,-3.94,;-9,-5.27,;-7.71,-9.82,;-8.81,-10.93,;-8.46,-12.44,;-10.37,-10.57,;-10.93,-9.1,;-12.5,-9.1,;-12.98,-10.64,;-14.52,-11.2,;-11.66,-11.59,;5.17,4.27,;3.75,4.73,;6.36,5.25,;7.76,4.7,;8.05,3.19,;9.4,2.76,;10.56,3.77,;11.99,3.34,;13.07,4.34,;14.52,3.91,;12.73,5.81,;8.69,5.56,;10.34,5.13,;8.01,6.89,;6.47,7.14,;6.25,8.6,;7.58,9.33,;8.69,8.25,;10.16,8.5,;11.09,7.36,;10.69,9.91,;9.73,11.04,;10.22,12.44,;11.71,12.84,;9.45,13.78,)| Show InChI InChI=1S/C59H83N17O13/c1-31(2)21-41(51(82)69-40(11-7-19-64-59(61)62)58(89)76-20-8-12-47(76)57(88)66-28-48(60)79)70-52(83)42(22-32(3)4)71-53(84)43(23-33-13-15-36(78)16-14-33)72-56(87)46(29-77)75-54(85)44(24-34-26-65-38-10-6-5-9-37(34)38)73-55(86)45(25-35-27-63-30-67-35)74-50(81)39-17-18-49(80)68-39/h5-6,9-10,13-16,26-27,30-32,39-47,65,77-78H,7-8,11-12,17-25,28-29H2,1-4H3,(H2,60,79)(H,63,67)(H,66,88)(H,68,80)(H,69,82)(H,70,83)(H,71,84)(H,72,87)(H,73,86)(H,74,81)(H,75,85)(H4,61,62,64)/t39-,40-,41-,42+,43-,44-,45-,46-,47-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Med Chem 36: 363-9 (1993)
Article DOI: 10.1021/jm00055a007
BindingDB Entry DOI: 10.7270/Q2GQ6W8T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data