 Found 117 hits with Last Name = 'tashiro' and Initial = 'k'
Found 117 hits with Last Name = 'tashiro' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127501
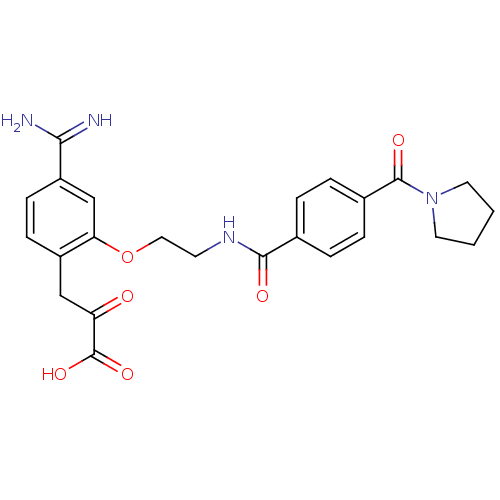
(3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OCCNC(=O)c2ccc(cc2)C(=O)N2CCCC2)c1 Show InChI InChI=1S/C24H26N4O6/c25-21(26)18-8-7-17(13-19(29)24(32)33)20(14-18)34-12-9-27-22(30)15-3-5-16(6-4-15)23(31)28-10-1-2-11-28/h3-8,14H,1-2,9-13H2,(H3,25,26)(H,27,30)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127492
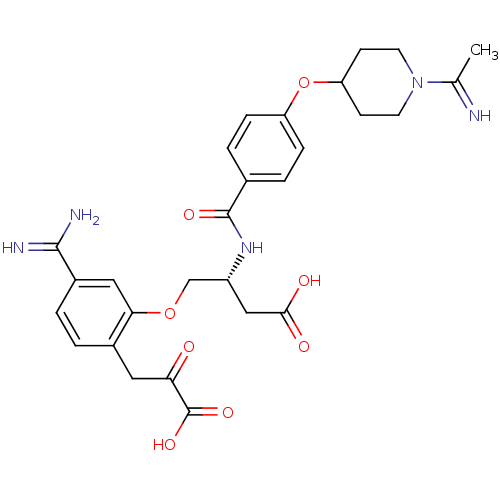
(4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)N[C@@H](COc1cc(ccc1CC(=O)C(O)=O)C(N)=N)CC(O)=O Show InChI InChI=1S/C28H33N5O8/c1-16(29)33-10-8-22(9-11-33)41-21-6-4-17(5-7-21)27(37)32-20(14-25(35)36)15-40-24-13-19(26(30)31)3-2-18(24)12-23(34)28(38)39/h2-7,13,20,22,29H,8-12,14-15H2,1H3,(H3,30,31)(H,32,37)(H,35,36)(H,38,39)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127502
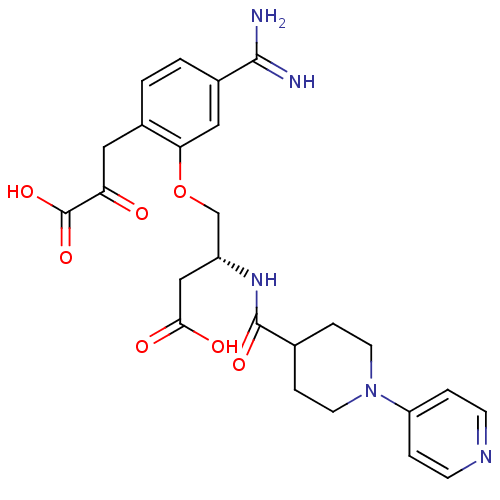
(4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OC[C@@H](CC(O)=O)NC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C25H29N5O7/c26-23(27)17-2-1-16(11-20(31)25(35)36)21(12-17)37-14-18(13-22(32)33)29-24(34)15-5-9-30(10-6-15)19-3-7-28-8-4-19/h1-4,7-8,12,15,18H,5-6,9-11,13-14H2,(H3,26,27)(H,29,34)(H,32,33)(H,35,36)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127504
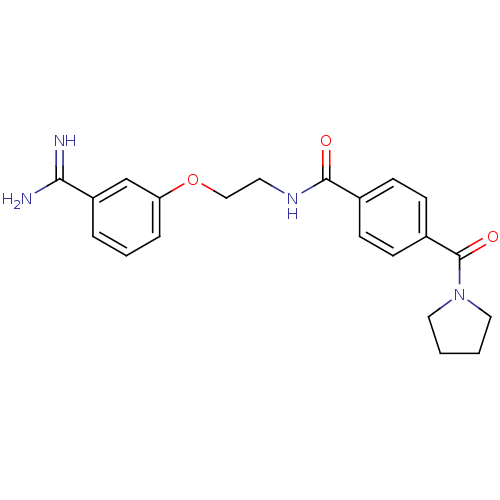
(CHEMBL55770 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show SMILES NC(=N)c1cccc(OCCNC(=O)c2ccc(cc2)C(=O)N2CCCC2)c1 Show InChI InChI=1S/C21H24N4O3/c22-19(23)17-4-3-5-18(14-17)28-13-10-24-20(26)15-6-8-16(9-7-15)21(27)25-11-1-2-12-25/h3-9,14H,1-2,10-13H2,(H3,22,23)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127495

(3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cc(ccc1CC(=O)C(O)=O)C(N)=N Show InChI InChI=1S/C26H31N5O6/c1-16(27)31-11-8-21(9-12-31)37-20-6-4-17(5-7-20)25(33)30-10-13-36-23-15-19(24(28)29)3-2-18(23)14-22(32)26(34)35/h2-7,15,21,27H,8-14H2,1H3,(H3,28,29)(H,30,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127494
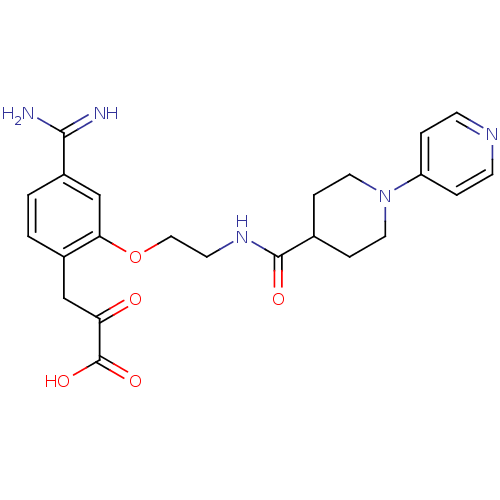
(3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C23H27N5O5/c24-21(25)17-2-1-16(13-19(29)23(31)32)20(14-17)33-12-9-27-22(30)15-5-10-28(11-6-15)18-3-7-26-8-4-18/h1-4,7-8,14-15H,5-6,9-13H2,(H3,24,25)(H,27,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127498
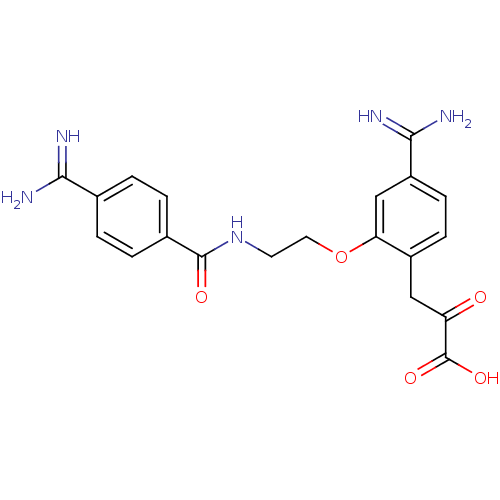
(3-{4-Carbamimidoyl-2-[2-(4-carbamimidoyl-benzoylam...)Show SMILES NC(=N)c1ccc(cc1)C(=O)NCCOc1cc(ccc1CC(=O)C(O)=O)C(N)=N Show InChI InChI=1S/C20H21N5O5/c21-17(22)11-1-3-12(4-2-11)19(27)25-7-8-30-16-10-14(18(23)24)6-5-13(16)9-15(26)20(28)29/h1-6,10H,7-9H2,(H3,21,22)(H3,23,24)(H,25,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127503
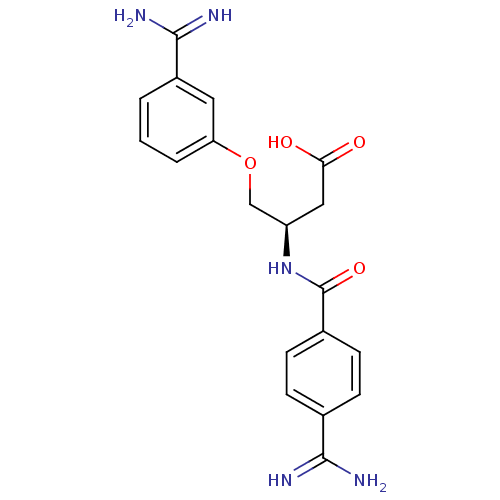
(3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@@H](COc1cccc(c1)C(N)=N)CC(O)=O Show InChI InChI=1S/C19H21N5O4/c20-17(21)11-4-6-12(7-5-11)19(27)24-14(9-16(25)26)10-28-15-3-1-2-13(8-15)18(22)23/h1-8,14H,9-10H2,(H3,20,21)(H3,22,23)(H,24,27)(H,25,26)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127493
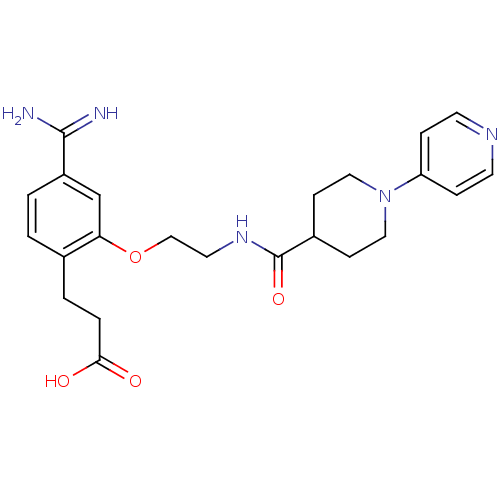
(3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...)Show SMILES NC(=N)c1ccc(CCC(O)=O)c(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C23H29N5O4/c24-22(25)18-2-1-16(3-4-21(29)30)20(15-18)32-14-11-27-23(31)17-7-12-28(13-8-17)19-5-9-26-10-6-19/h1-2,5-6,9-10,15,17H,3-4,7-8,11-14H2,(H3,24,25)(H,27,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288745
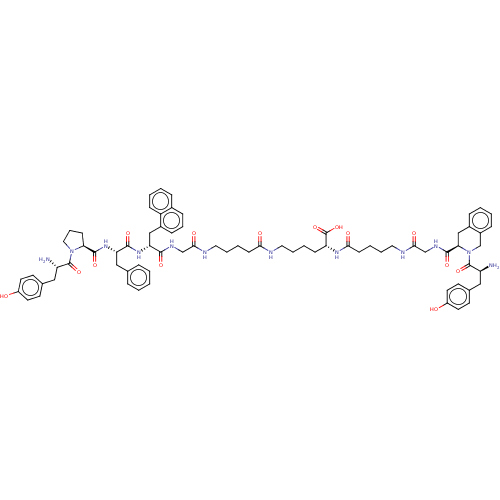
(CHEMBL4159810)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCCC(=O)NCCCC[C@@H](NC(=O)CCCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C75H92N12O14/c76-58(40-49-28-32-55(88)33-29-49)73(98)86-39-15-25-63(86)72(97)85-61(42-48-16-2-1-3-17-48)70(95)84-62(43-53-22-14-21-51-18-6-7-23-57(51)53)69(94)81-45-67(92)79-37-12-9-26-65(90)78-36-11-8-24-60(75(100)101)83-66(91)27-10-13-38-80-68(93)46-82-71(96)64-44-52-19-4-5-20-54(52)47-87(64)74(99)59(77)41-50-30-34-56(89)35-31-50/h1-7,14,16-23,28-35,58-64,88-89H,8-13,15,24-27,36-47,76-77H2,(H,78,90)(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,91)(H,84,95)(H,85,97)(H,100,101)/t58-,59-,60+,61-,62+,63-,64+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288740
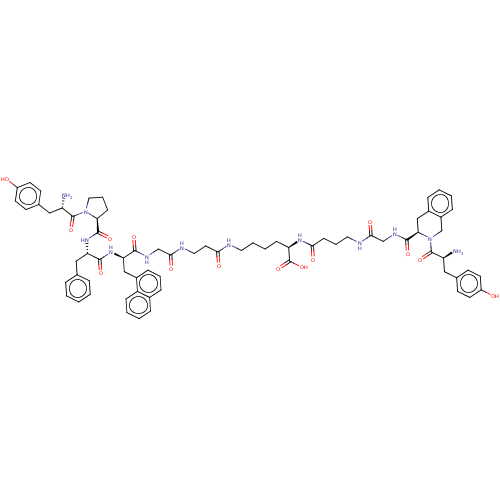
(CHEMBL4167726)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCC(=O)NCCCC[C@@H](NC(=O)CCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C72H86N12O14/c73-55(37-46-24-28-52(85)29-25-46)70(95)83-36-12-22-60(83)69(94)82-58(39-45-13-2-1-3-14-45)67(92)81-59(40-50-19-10-18-48-15-6-7-20-54(48)50)66(91)78-42-65(90)77-35-32-62(87)75-33-9-8-21-57(72(97)98)80-63(88)23-11-34-76-64(89)43-79-68(93)61-41-49-16-4-5-17-51(49)44-84(61)71(96)56(74)38-47-26-30-53(86)31-27-47/h1-7,10,13-20,24-31,55-61,85-86H,8-9,11-12,21-23,32-44,73-74H2,(H,75,87)(H,76,89)(H,77,90)(H,78,91)(H,79,93)(H,80,88)(H,81,92)(H,82,94)(H,97,98)/t55-,56-,57+,58-,59+,60-,61+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288741
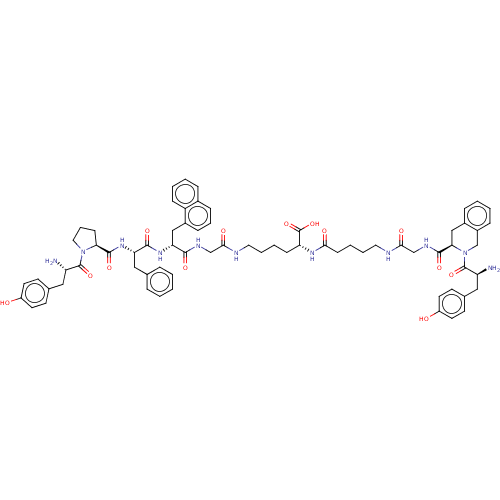
(CHEMBL4174926)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCC[C@@H](NC(=O)CCCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C70H83N11O13/c71-54(36-45-25-29-51(82)30-26-45)68(91)80-35-13-23-59(80)67(90)79-57(38-44-14-2-1-3-15-44)65(88)78-58(39-49-20-12-19-47-16-6-7-21-53(47)49)64(87)75-41-62(85)73-33-10-8-22-56(70(93)94)77-61(84)24-9-11-34-74-63(86)42-76-66(89)60-40-48-17-4-5-18-50(48)43-81(60)69(92)55(72)37-46-27-31-52(83)32-28-46/h1-7,12,14-21,25-32,54-60,82-83H,8-11,13,22-24,33-43,71-72H2,(H,73,85)(H,74,86)(H,75,87)(H,76,89)(H,77,84)(H,78,88)(H,79,90)(H,93,94)/t54-,55-,56+,57-,58+,59-,60+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127496
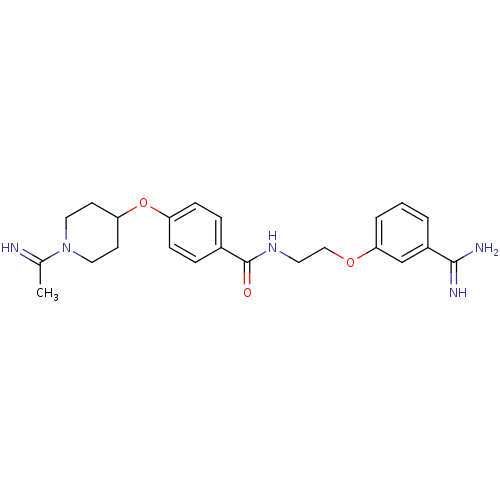
(CHEMBL51796 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cccc(c1)C(N)=N Show InChI InChI=1S/C23H29N5O3/c1-16(24)28-12-9-20(10-13-28)31-19-7-5-17(6-8-19)23(29)27-11-14-30-21-4-2-3-18(15-21)22(25)26/h2-8,15,20,24H,9-14H2,1H3,(H3,25,26)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127506
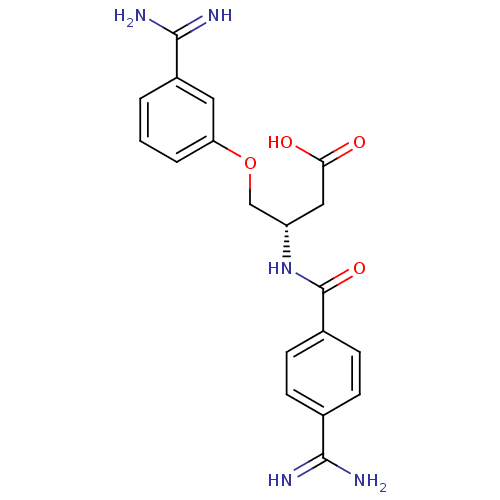
(3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@H](COc1cccc(c1)C(N)=N)CC(O)=O Show InChI InChI=1S/C19H21N5O4/c20-17(21)11-4-6-12(7-5-11)19(27)24-14(9-16(25)26)10-28-15-3-1-2-13(8-15)18(22)23/h1-8,14H,9-10H2,(H3,20,21)(H3,22,23)(H,24,27)(H,25,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127497

(4-Carbamimidoyl-N-[2-(3-carbamimidoyl-phenoxy)-eth...)Show InChI InChI=1S/C17H19N5O2/c18-15(19)11-4-6-12(7-5-11)17(23)22-8-9-24-14-3-1-2-13(10-14)16(20)21/h1-7,10H,8-9H2,(H3,18,19)(H3,20,21)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288744
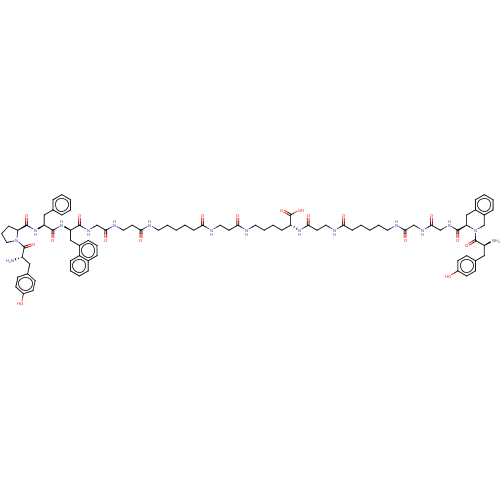
(CHEMBL4163178)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCC(=O)NCCCCCC(=O)NCCC(=O)NCCCC[C@@H](NC(=O)CCNC(=O)CCCCCNC(=O)CNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C88H114N16O18/c89-67(48-58-30-34-64(105)35-31-58)86(119)103-47-17-27-72(103)85(118)102-70(50-57-18-4-1-5-19-57)83(116)101-71(51-62-24-16-23-60-20-10-11-25-66(60)62)82(115)98-54-80(113)96-45-39-77(110)91-41-13-2-6-28-74(107)94-44-38-76(109)92-43-15-12-26-69(88(121)122)100-78(111)40-46-95-75(108)29-7-3-14-42-93-79(112)53-97-81(114)55-99-84(117)73-52-61-21-8-9-22-63(61)56-104(73)87(120)68(90)49-59-32-36-65(106)37-33-59/h1,4-5,8-11,16,18-25,30-37,67-73,105-106H,2-3,6-7,12-15,17,26-29,38-56,89-90H2,(H,91,110)(H,92,109)(H,93,112)(H,94,107)(H,95,108)(H,96,113)(H,97,114)(H,98,115)(H,99,117)(H,100,111)(H,101,116)(H,102,118)(H,121,122)/t67-,68-,69+,70-,71+,72-,73+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288746
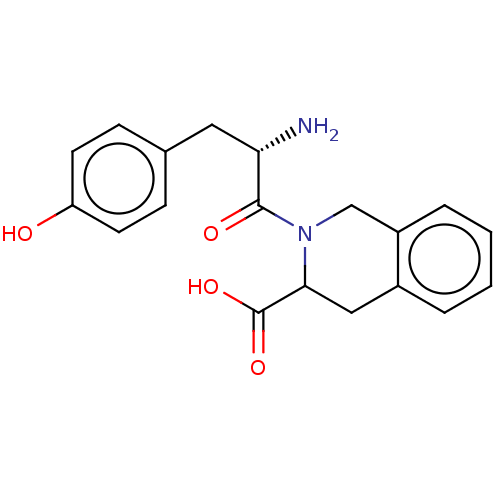
(CHEMBL4166622)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1Cc2ccccc2CC1C(O)=O |r| Show InChI InChI=1S/C19H20N2O4/c20-16(9-12-5-7-15(22)8-6-12)18(23)21-11-14-4-2-1-3-13(14)10-17(21)19(24)25/h1-8,16-17,22H,9-11,20H2,(H,24,25)/t16-,17?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288788
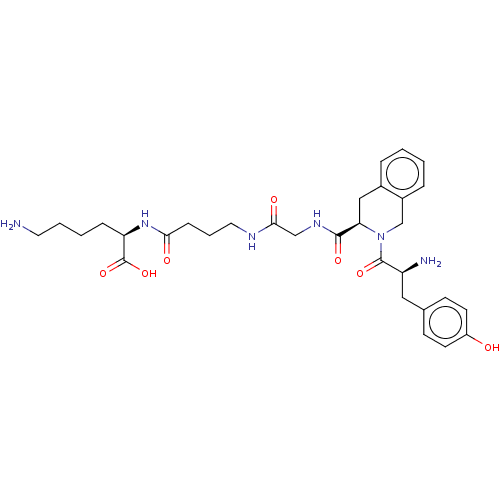
(CHEMBL4174537)Show SMILES NCCCC[C@@H](NC(=O)CCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C31H42N6O7/c32-14-4-3-8-25(31(43)44)36-27(39)9-5-15-34-28(40)18-35-29(41)26-17-21-6-1-2-7-22(21)19-37(26)30(42)24(33)16-20-10-12-23(38)13-11-20/h1-2,6-7,10-13,24-26,38H,3-5,8-9,14-19,32-33H2,(H,34,40)(H,35,41)(H,36,39)(H,43,44)/t24-,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288742
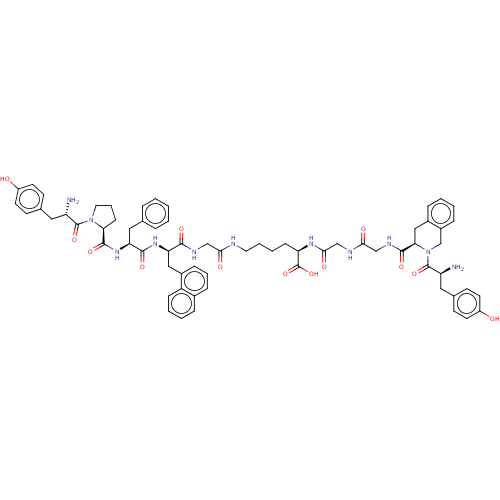
(CHEMBL4164277)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCC[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C67H77N11O13/c68-51(32-42-22-26-48(79)27-23-42)65(88)77-31-11-21-56(77)64(87)76-54(34-41-12-2-1-3-13-41)62(85)75-55(35-46-18-10-17-44-14-6-7-19-50(44)46)61(84)72-37-58(81)70-30-9-8-20-53(67(90)91)74-60(83)39-71-59(82)38-73-63(86)57-36-45-15-4-5-16-47(45)40-78(57)66(89)52(69)33-43-24-28-49(80)29-25-43/h1-7,10,12-19,22-29,51-57,79-80H,8-9,11,20-21,30-40,68-69H2,(H,70,81)(H,71,82)(H,72,84)(H,73,86)(H,74,83)(H,75,85)(H,76,87)(H,90,91)/t51-,52-,53+,54-,55+,56-,57+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288743
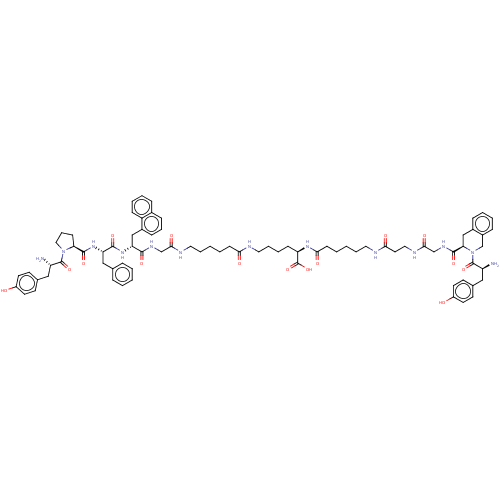
(CHEMBL4171099)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCCCC(=O)NCCCC[C@@H](NC(=O)CCCCCNC(=O)CCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C80H101N13O15/c81-62(44-53-30-34-59(94)35-31-53)78(105)92-43-17-27-67(92)77(104)91-65(46-52-18-4-1-5-19-52)75(102)90-66(47-57-24-16-23-55-20-10-11-25-61(55)57)74(101)87-49-72(99)85-40-14-2-6-28-69(96)83-41-15-12-26-64(80(107)108)89-71(98)29-7-3-13-39-84-70(97)38-42-86-73(100)50-88-76(103)68-48-56-21-8-9-22-58(56)51-93(68)79(106)63(82)45-54-32-36-60(95)37-33-54/h1,4-5,8-11,16,18-25,30-37,62-68,94-95H,2-3,6-7,12-15,17,26-29,38-51,81-82H2,(H,83,96)(H,84,97)(H,85,99)(H,86,100)(H,87,101)(H,88,103)(H,89,98)(H,90,102)(H,91,104)(H,107,108)/t62-,63-,64+,65-,66+,67-,68+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127500
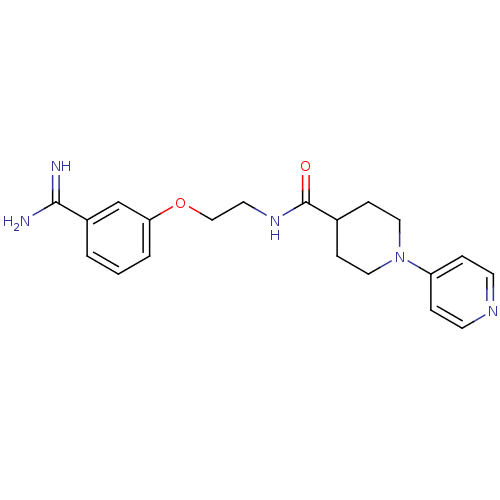
(3,4,5,6-Tetrahydro-2H-[1,4']bipyridinyl-4-carboxyl...)Show SMILES NC(=N)c1cccc(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C20H25N5O2/c21-19(22)16-2-1-3-18(14-16)27-13-10-24-20(26)15-6-11-25(12-7-15)17-4-8-23-9-5-17/h1-5,8-9,14-15H,6-7,10-13H2,(H3,21,22)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127499
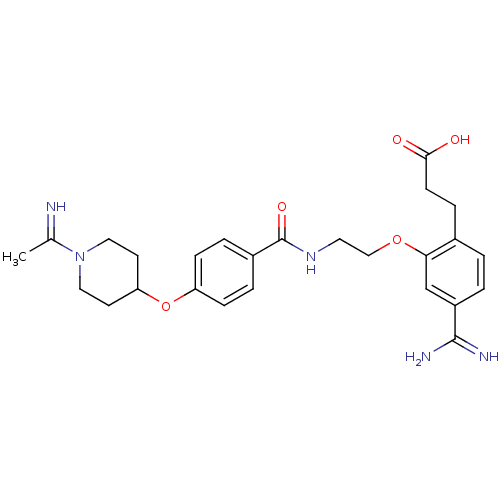
(3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cc(ccc1CCC(O)=O)C(N)=N Show InChI InChI=1S/C26H33N5O5/c1-17(27)31-13-10-22(11-14-31)36-21-7-4-19(5-8-21)26(34)30-12-15-35-23-16-20(25(28)29)3-2-18(23)6-9-24(32)33/h2-5,7-8,16,22,27H,6,9-15H2,1H3,(H3,28,29)(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50288740

(CHEMBL4167726)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCC(=O)NCCCC[C@@H](NC(=O)CCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C72H86N12O14/c73-55(37-46-24-28-52(85)29-25-46)70(95)83-36-12-22-60(83)69(94)82-58(39-45-13-2-1-3-14-45)67(92)81-59(40-50-19-10-18-48-15-6-7-20-54(48)50)66(91)78-42-65(90)77-35-32-62(87)75-33-9-8-21-57(72(97)98)80-63(88)23-11-34-76-64(89)43-79-68(93)61-41-49-16-4-5-17-51(49)44-84(61)71(96)56(74)38-47-26-30-53(86)31-27-47/h1-7,10,13-20,24-31,55-61,85-86H,8-9,11-12,21-23,32-44,73-74H2,(H,75,87)(H,76,89)(H,77,90)(H,78,91)(H,79,93)(H,80,88)(H,81,92)(H,82,94)(H,97,98)/t55-,56-,57+,58-,59+,60-,61+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50288743

(CHEMBL4171099)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCCCC(=O)NCCCC[C@@H](NC(=O)CCCCCNC(=O)CCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C80H101N13O15/c81-62(44-53-30-34-59(94)35-31-53)78(105)92-43-17-27-67(92)77(104)91-65(46-52-18-4-1-5-19-52)75(102)90-66(47-57-24-16-23-55-20-10-11-25-61(55)57)74(101)87-49-72(99)85-40-14-2-6-28-69(96)83-41-15-12-26-64(80(107)108)89-71(98)29-7-3-13-39-84-70(97)38-42-86-73(100)50-88-76(103)68-48-56-21-8-9-22-58(56)51-93(68)79(106)63(82)45-54-32-36-60(95)37-33-54/h1,4-5,8-11,16,18-25,30-37,62-68,94-95H,2-3,6-7,12-15,17,26-29,38-51,81-82H2,(H,83,96)(H,84,97)(H,85,99)(H,86,100)(H,87,101)(H,88,103)(H,89,98)(H,90,102)(H,91,104)(H,107,108)/t62-,63-,64+,65-,66+,67-,68+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50288744

(CHEMBL4163178)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCC(=O)NCCCCCC(=O)NCCC(=O)NCCCC[C@@H](NC(=O)CCNC(=O)CCCCCNC(=O)CNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C88H114N16O18/c89-67(48-58-30-34-64(105)35-31-58)86(119)103-47-17-27-72(103)85(118)102-70(50-57-18-4-1-5-19-57)83(116)101-71(51-62-24-16-23-60-20-10-11-25-66(60)62)82(115)98-54-80(113)96-45-39-77(110)91-41-13-2-6-28-74(107)94-44-38-76(109)92-43-15-12-26-69(88(121)122)100-78(111)40-46-95-75(108)29-7-3-14-42-93-79(112)53-97-81(114)55-99-84(117)73-52-61-21-8-9-22-63(61)56-104(73)87(120)68(90)49-59-32-36-65(106)37-33-59/h1,4-5,8-11,16,18-25,30-37,67-73,105-106H,2-3,6-7,12-15,17,26-29,38-56,89-90H2,(H,91,110)(H,92,109)(H,93,112)(H,94,107)(H,95,108)(H,96,113)(H,97,114)(H,98,115)(H,99,117)(H,100,111)(H,101,116)(H,102,118)(H,121,122)/t67-,68-,69+,70-,71+,72-,73+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50203825
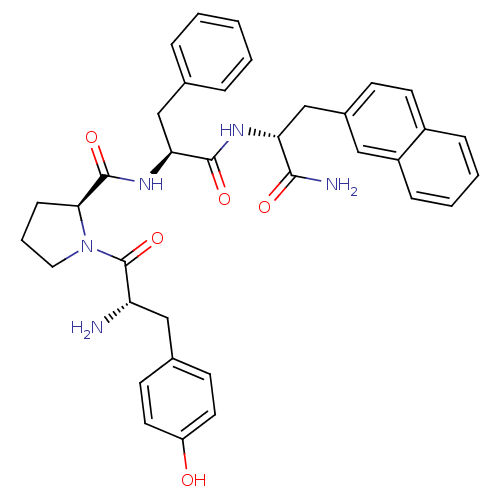
(CHEMBL375070 | Tyr-Pro-Phe-D-2-Nal-NH2)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(N)=O Show InChI InChI=1S/C36H39N5O5/c37-29(20-24-13-16-28(42)17-14-24)36(46)41-18-6-11-32(41)35(45)40-31(21-23-7-2-1-3-8-23)34(44)39-30(33(38)43)22-25-12-15-26-9-4-5-10-27(26)19-25/h1-5,7-10,12-17,19,29-32,42H,6,11,18,20-22,37H2,(H2,38,43)(H,39,44)(H,40,45)/t29-,30+,31-,32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50288741

(CHEMBL4174926)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCC[C@@H](NC(=O)CCCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C70H83N11O13/c71-54(36-45-25-29-51(82)30-26-45)68(91)80-35-13-23-59(80)67(90)79-57(38-44-14-2-1-3-15-44)65(88)78-58(39-49-20-12-19-47-16-6-7-21-53(47)49)64(87)75-41-62(85)73-33-10-8-22-56(70(93)94)77-61(84)24-9-11-34-74-63(86)42-76-66(89)60-40-48-17-4-5-18-50(48)43-81(60)69(92)55(72)37-46-27-31-52(83)32-28-46/h1-7,12,14-21,25-32,54-60,82-83H,8-11,13,22-24,33-43,71-72H2,(H,73,85)(H,74,86)(H,75,87)(H,76,89)(H,77,84)(H,78,88)(H,79,90)(H,93,94)/t54-,55-,56+,57-,58+,59-,60+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50288745

(CHEMBL4159810)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCCC(=O)NCCCC[C@@H](NC(=O)CCCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C75H92N12O14/c76-58(40-49-28-32-55(88)33-29-49)73(98)86-39-15-25-63(86)72(97)85-61(42-48-16-2-1-3-17-48)70(95)84-62(43-53-22-14-21-51-18-6-7-23-57(51)53)69(94)81-45-67(92)79-37-12-9-26-65(90)78-36-11-8-24-60(75(100)101)83-66(91)27-10-13-38-80-68(93)46-82-71(96)64-44-52-19-4-5-20-54(52)47-87(64)74(99)59(77)41-50-30-34-56(89)35-31-50/h1-7,14,16-23,28-35,58-64,88-89H,8-13,15,24-27,36-47,76-77H2,(H,78,90)(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,91)(H,84,95)(H,85,97)(H,100,101)/t58-,59-,60+,61-,62+,63-,64+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50288742

(CHEMBL4164277)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCC[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C67H77N11O13/c68-51(32-42-22-26-48(79)27-23-42)65(88)77-31-11-21-56(77)64(87)76-54(34-41-12-2-1-3-13-41)62(85)75-55(35-46-18-10-17-44-14-6-7-19-50(44)46)61(84)72-37-58(81)70-30-9-8-20-53(67(90)91)74-60(83)39-71-59(82)38-73-63(86)57-36-45-15-4-5-16-47(45)40-78(57)66(89)52(69)33-43-24-28-49(80)29-25-43/h1-7,10,12-19,22-29,51-57,79-80H,8-9,11,20-21,30-40,68-69H2,(H,70,81)(H,71,82)(H,72,84)(H,73,86)(H,74,83)(H,75,85)(H,76,87)(H,90,91)/t51-,52-,53+,54-,55+,56-,57+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysis |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127505
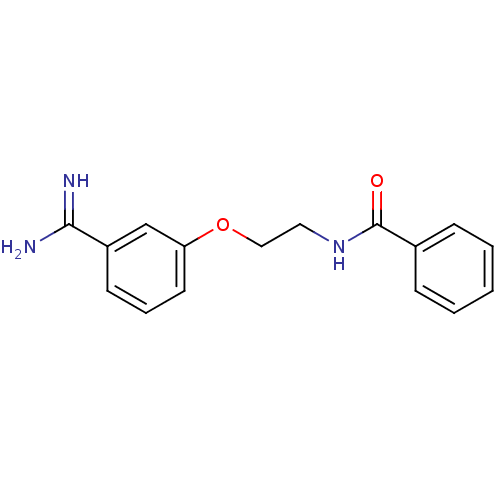
(CHEMBL55364 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show InChI InChI=1S/C16H17N3O2/c17-15(18)13-7-4-8-14(11-13)21-10-9-19-16(20)12-5-2-1-3-6-12/h1-8,11H,9-10H2,(H3,17,18)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127501

(3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OCCNC(=O)c2ccc(cc2)C(=O)N2CCCC2)c1 Show InChI InChI=1S/C24H26N4O6/c25-21(26)18-8-7-17(13-19(29)24(32)33)20(14-18)34-12-9-27-22(30)15-3-5-16(6-4-15)23(31)28-10-1-2-11-28/h3-8,14H,1-2,9-13H2,(H3,25,26)(H,27,30)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127498

(3-{4-Carbamimidoyl-2-[2-(4-carbamimidoyl-benzoylam...)Show SMILES NC(=N)c1ccc(cc1)C(=O)NCCOc1cc(ccc1CC(=O)C(O)=O)C(N)=N Show InChI InChI=1S/C20H21N5O5/c21-17(22)11-1-3-12(4-2-11)19(27)25-7-8-30-16-10-14(18(23)24)6-5-13(16)9-15(26)20(28)29/h1-6,10H,7-9H2,(H3,21,22)(H3,23,24)(H,25,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127495

(3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cc(ccc1CC(=O)C(O)=O)C(N)=N Show InChI InChI=1S/C26H31N5O6/c1-16(27)31-11-8-21(9-12-31)37-20-6-4-17(5-7-20)25(33)30-10-13-36-23-15-19(24(28)29)3-2-18(23)14-22(32)26(34)35/h2-7,15,21,27H,8-14H2,1H3,(H3,28,29)(H,30,33)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127504

(CHEMBL55770 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show SMILES NC(=N)c1cccc(OCCNC(=O)c2ccc(cc2)C(=O)N2CCCC2)c1 Show InChI InChI=1S/C21H24N4O3/c22-19(23)17-4-3-5-18(14-17)28-13-10-24-20(26)15-6-8-16(9-7-15)21(27)25-11-1-2-12-25/h3-9,14H,1-2,10-13H2,(H3,22,23)(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127494

(3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C23H27N5O5/c24-21(25)17-2-1-16(13-19(29)23(31)32)20(14-17)33-12-9-27-22(30)15-5-10-28(11-6-15)18-3-7-26-8-4-18/h1-4,7-8,14-15H,5-6,9-13H2,(H3,24,25)(H,27,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127496

(CHEMBL51796 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cccc(c1)C(N)=N Show InChI InChI=1S/C23H29N5O3/c1-16(24)28-12-9-20(10-13-28)31-19-7-5-17(6-8-19)23(29)27-11-14-30-21-4-2-3-18(15-21)22(25)26/h2-8,15,20,24H,9-14H2,1H3,(H3,25,26)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127492

(4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)N[C@@H](COc1cc(ccc1CC(=O)C(O)=O)C(N)=N)CC(O)=O Show InChI InChI=1S/C28H33N5O8/c1-16(29)33-10-8-22(9-11-33)41-21-6-4-17(5-7-21)27(37)32-20(14-25(35)36)15-40-24-13-19(26(30)31)3-2-18(24)12-23(34)28(38)39/h2-7,13,20,22,29H,8-12,14-15H2,1H3,(H3,30,31)(H,32,37)(H,35,36)(H,38,39)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127502

(4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OC[C@@H](CC(O)=O)NC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C25H29N5O7/c26-23(27)17-2-1-16(11-20(31)25(35)36)21(12-17)37-14-18(13-22(32)33)29-24(34)15-5-9-30(10-6-15)19-3-7-28-8-4-19/h1-4,7-8,12,15,18H,5-6,9-11,13-14H2,(H3,26,27)(H,29,34)(H,32,33)(H,35,36)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127497

(4-Carbamimidoyl-N-[2-(3-carbamimidoyl-phenoxy)-eth...)Show InChI InChI=1S/C17H19N5O2/c18-15(19)11-4-6-12(7-5-11)17(23)22-8-9-24-14-3-1-2-13(10-14)16(20)21/h1-7,10H,8-9H2,(H3,18,19)(H3,20,21)(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127505

(CHEMBL55364 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show InChI InChI=1S/C16H17N3O2/c17-15(18)13-7-4-8-14(11-13)21-10-9-19-16(20)12-5-2-1-3-6-12/h1-8,11H,9-10H2,(H3,17,18)(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127500

(3,4,5,6-Tetrahydro-2H-[1,4']bipyridinyl-4-carboxyl...)Show SMILES NC(=N)c1cccc(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C20H25N5O2/c21-19(22)16-2-1-3-18(14-16)27-13-10-24-20(26)15-6-11-25(12-7-15)17-4-8-23-9-5-17/h1-5,8-9,14-15H,6-7,10-13H2,(H3,21,22)(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127503

(3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@@H](COc1cccc(c1)C(N)=N)CC(O)=O Show InChI InChI=1S/C19H21N5O4/c20-17(21)11-4-6-12(7-5-11)19(27)24-14(9-16(25)26)10-28-15-3-1-2-13(8-15)18(22)23/h1-8,14H,9-10H2,(H3,20,21)(H3,22,23)(H,24,27)(H,25,26)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127506

(3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@H](COc1cccc(c1)C(N)=N)CC(O)=O Show InChI InChI=1S/C19H21N5O4/c20-17(21)11-4-6-12(7-5-11)19(27)24-14(9-16(25)26)10-28-15-3-1-2-13(8-15)18(22)23/h1-8,14H,9-10H2,(H3,20,21)(H3,22,23)(H,24,27)(H,25,26)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127493

(3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...)Show SMILES NC(=N)c1ccc(CCC(O)=O)c(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C23H29N5O4/c24-22(25)18-2-1-16(3-4-21(29)30)20(15-18)32-14-11-27-23(31)17-7-12-28(13-8-17)19-5-9-26-10-6-19/h1-2,5-6,9-10,15,17H,3-4,7-8,11-14H2,(H3,24,25)(H,27,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127499

(3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cc(ccc1CCC(O)=O)C(N)=N Show InChI InChI=1S/C26H33N5O5/c1-17(27)31-13-10-22(11-14-31)36-21-7-4-19(5-8-21)26(34)30-12-15-35-23-16-20(25(28)29)3-2-18(23)6-9-24(32)33/h2-5,7-8,16,22,27H,6,9-15H2,1H3,(H3,28,29)(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human KOR expressed in CHO cell membranes assessed as inhibition of U50488-induced [35S]-GTPgammaS binding preincubated for 5 ... |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288742

(CHEMBL4164277)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCC[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C67H77N11O13/c68-51(32-42-22-26-48(79)27-23-42)65(88)77-31-11-21-56(77)64(87)76-54(34-41-12-2-1-3-13-41)62(85)75-55(35-46-18-10-17-44-14-6-7-19-50(44)46)61(84)72-37-58(81)70-30-9-8-20-53(67(90)91)74-60(83)39-71-59(82)38-73-63(86)57-36-45-15-4-5-16-47(45)40-78(57)66(89)52(69)33-43-24-28-49(80)29-25-43/h1-7,10,12-19,22-29,51-57,79-80H,8-9,11,20-21,30-40,68-69H2,(H,70,81)(H,71,82)(H,72,84)(H,73,86)(H,74,83)(H,75,85)(H,76,87)(H,90,91)/t51-,52-,53+,54-,55+,56-,57+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human DOR expressed in CHO cell membranes assessed as inhibition of CYM51010-induced [35S]-GTPgammaS binding preincubated for ... |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288741

(CHEMBL4174926)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCC[C@@H](NC(=O)CCCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C70H83N11O13/c71-54(36-45-25-29-51(82)30-26-45)68(91)80-35-13-23-59(80)67(90)79-57(38-44-14-2-1-3-15-44)65(88)78-58(39-49-20-12-19-47-16-6-7-21-53(47)49)64(87)75-41-62(85)73-33-10-8-22-56(70(93)94)77-61(84)24-9-11-34-74-63(86)42-76-66(89)60-40-48-17-4-5-18-50(48)43-81(60)69(92)55(72)37-46-27-31-52(83)32-28-46/h1-7,12,14-21,25-32,54-60,82-83H,8-11,13,22-24,33-43,71-72H2,(H,73,85)(H,74,86)(H,75,87)(H,76,89)(H,77,84)(H,78,88)(H,79,90)(H,93,94)/t54-,55-,56+,57-,58+,59-,60+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human DOR expressed in CHO cell membranes assessed as inhibition of CYM51010-induced [35S]-GTPgammaS binding preincubated for ... |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288740

(CHEMBL4167726)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCC(=O)NCCCC[C@@H](NC(=O)CCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C72H86N12O14/c73-55(37-46-24-28-52(85)29-25-46)70(95)83-36-12-22-60(83)69(94)82-58(39-45-13-2-1-3-14-45)67(92)81-59(40-50-19-10-18-48-15-6-7-20-54(48)50)66(91)78-42-65(90)77-35-32-62(87)75-33-9-8-21-57(72(97)98)80-63(88)23-11-34-76-64(89)43-79-68(93)61-41-49-16-4-5-17-51(49)44-84(61)71(96)56(74)38-47-26-30-53(86)31-27-47/h1-7,10,13-20,24-31,55-61,85-86H,8-9,11-12,21-23,32-44,73-74H2,(H,75,87)(H,76,89)(H,77,90)(H,78,91)(H,79,93)(H,80,88)(H,81,92)(H,82,94)(H,97,98)/t55-,56-,57+,58-,59+,60-,61+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human DOR expressed in CHO cell membranes assessed as inhibition of CYM51010-induced [35S]-GTPgammaS binding preincubated for ... |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50288745

(CHEMBL4159810)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(=O)NCC(=O)NCCCCC(=O)NCCCC[C@@H](NC(=O)CCCCNC(=O)CNC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C75H92N12O14/c76-58(40-49-28-32-55(88)33-29-49)73(98)86-39-15-25-63(86)72(97)85-61(42-48-16-2-1-3-17-48)70(95)84-62(43-53-22-14-21-51-18-6-7-23-57(51)53)69(94)81-45-67(92)79-37-12-9-26-65(90)78-36-11-8-24-60(75(100)101)83-66(91)27-10-13-38-80-68(93)46-82-71(96)64-44-52-19-4-5-20-54(52)47-87(64)74(99)59(77)41-50-30-34-56(89)35-31-50/h1-7,14,16-23,28-35,58-64,88-89H,8-13,15,24-27,36-47,76-77H2,(H,78,90)(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,91)(H,84,95)(H,85,97)(H,100,101)/t58-,59-,60+,61-,62+,63-,64+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human DOR expressed in CHO cell membranes assessed as inhibition of CYM51010-induced [35S]-GTPgammaS binding preincubated for ... |
J Med Chem 61: 6075-6086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00403
BindingDB Entry DOI: 10.7270/Q25B0514 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data