 Found 1032 hits with Last Name = 'thewlis' and Initial = 'k'
Found 1032 hits with Last Name = 'thewlis' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant rat OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Rattus norvegicus (Rat)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant rat OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419142
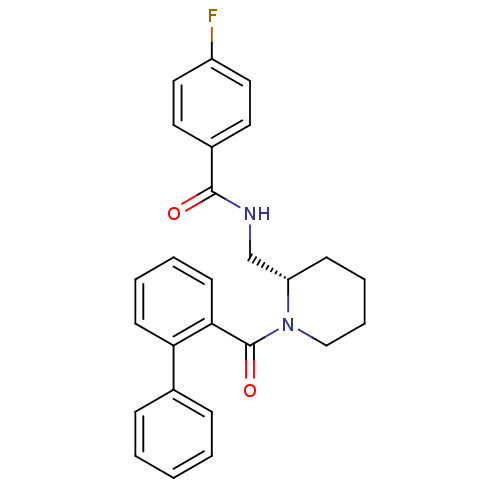
(CHEMBL1830963)Show SMILES Fc1ccc(cc1)C(=O)NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H25FN2O2/c27-21-15-13-20(14-16-21)25(30)28-18-22-10-6-7-17-29(22)26(31)24-12-5-4-11-23(24)19-8-2-1-3-9-19/h1-5,8-9,11-16,22H,6-7,10,17-18H2,(H,28,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419136
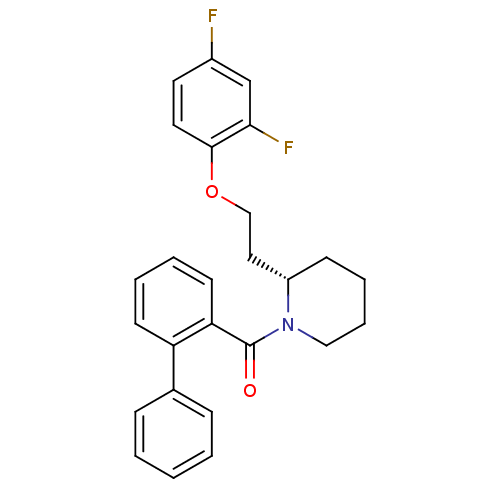
(CHEMBL1830961)Show SMILES Fc1ccc(OCC[C@@H]2CCCCN2C(=O)c2ccccc2-c2ccccc2)c(F)c1 |r| Show InChI InChI=1S/C26H25F2NO2/c27-20-13-14-25(24(28)18-20)31-17-15-21-10-6-7-16-29(21)26(30)23-12-5-4-11-22(23)19-8-2-1-3-9-19/h1-5,8-9,11-14,18,21H,6-7,10,15-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413698
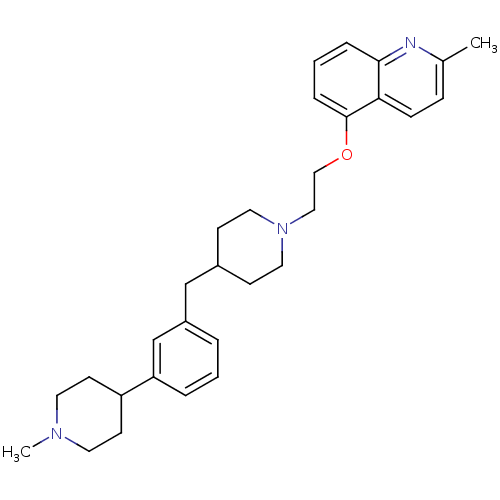
(CHEMBL459282)Show SMILES CN1CCC(CC1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C30H39N3O/c1-23-9-10-28-29(31-23)7-4-8-30(28)34-20-19-33-17-11-24(12-18-33)21-25-5-3-6-27(22-25)26-13-15-32(2)16-14-26/h3-10,22,24,26H,11-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419142

(CHEMBL1830963)Show SMILES Fc1ccc(cc1)C(=O)NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H25FN2O2/c27-21-15-13-20(14-16-21)25(30)28-18-22-10-6-7-17-29(22)26(31)24-12-5-4-11-23(24)19-8-2-1-3-9-19/h1-5,8-9,11-16,22H,6-7,10,17-18H2,(H,28,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412126
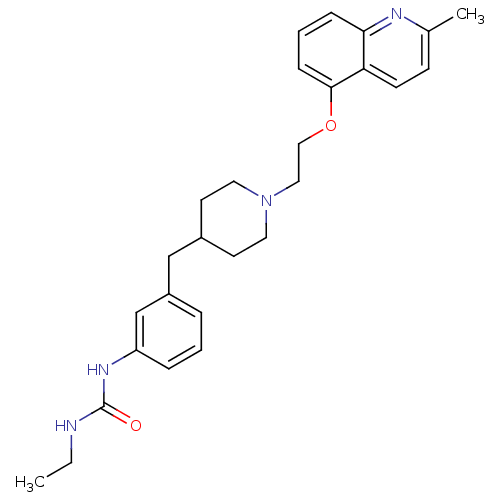
(CHEMBL525362)Show SMILES CCNC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C27H34N4O2/c1-3-28-27(32)30-23-7-4-6-22(19-23)18-21-12-14-31(15-13-21)16-17-33-26-9-5-8-25-24(26)11-10-20(2)29-25/h4-11,19,21H,3,12-18H2,1-2H3,(H2,28,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412121
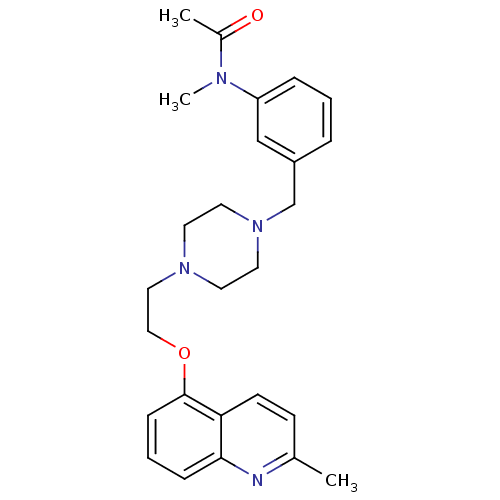
(CHEMBL506942)Show SMILES CN(C(C)=O)c1cccc(CN2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C26H32N4O2/c1-20-10-11-24-25(27-20)8-5-9-26(24)32-17-16-29-12-14-30(15-13-29)19-22-6-4-7-23(18-22)28(3)21(2)31/h4-11,18H,12-17,19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
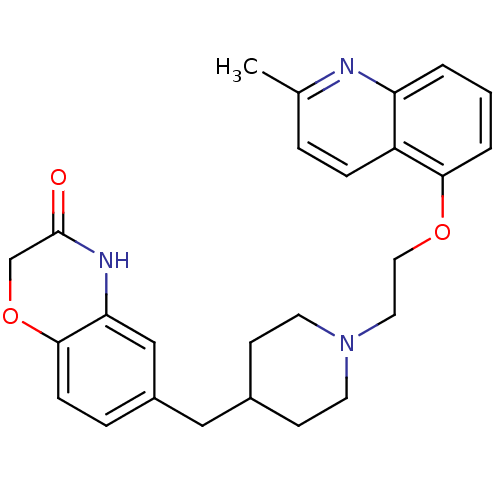
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114

(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50477395
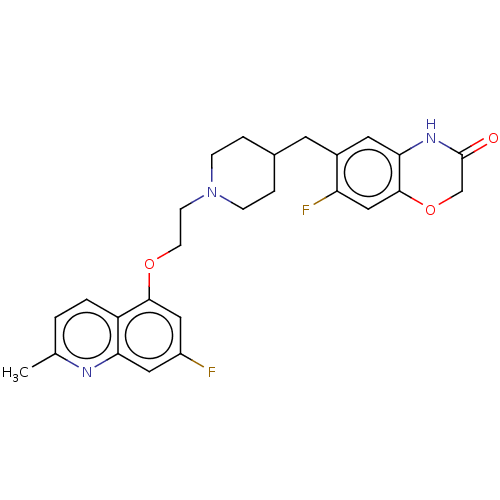
(CHEMBL238520)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cc5NC(=O)COc5cc4F)CC3)cc(F)cc2n1 Show InChI InChI=1S/C26H27F2N3O3/c1-16-2-3-20-22(29-16)12-19(27)13-24(20)33-9-8-31-6-4-17(5-7-31)10-18-11-23-25(14-21(18)28)34-15-26(32)30-23/h2-3,11-14,17H,4-10,15H2,1H3,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50477399
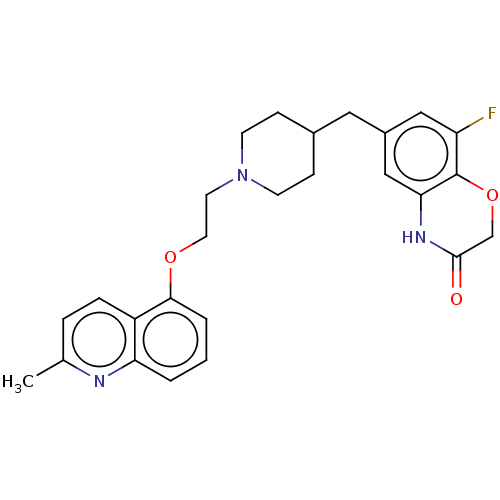
(CHEMBL241463)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cc(F)c5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H28FN3O3/c1-17-5-6-20-22(28-17)3-2-4-24(20)32-12-11-30-9-7-18(8-10-30)13-19-14-21(27)26-23(15-19)29-25(31)16-33-26/h2-6,14-15,18H,7-13,16H2,1H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human cloned 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412124
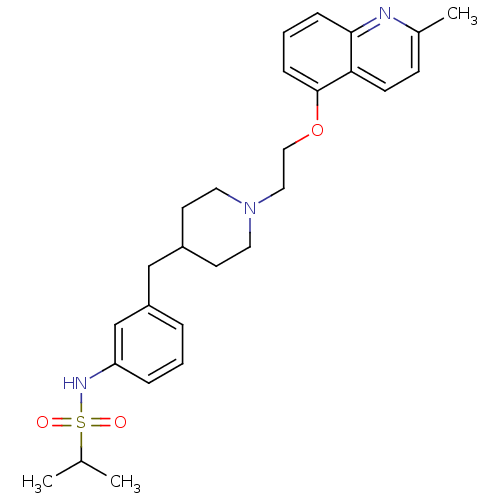
(CHEMBL495213)Show SMILES CC(C)S(=O)(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C27H35N3O3S/c1-20(2)34(31,32)29-24-7-4-6-23(19-24)18-22-12-14-30(15-13-22)16-17-33-27-9-5-8-26-25(27)11-10-21(3)28-26/h4-11,19-20,22,29H,12-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413691
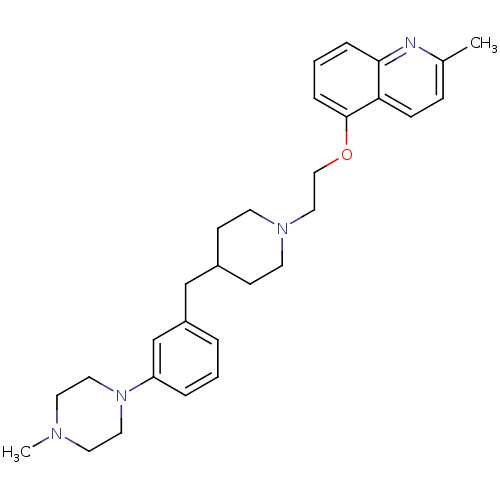
(CHEMBL458199)Show SMILES CN1CCN(CC1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C29H38N4O/c1-23-9-10-27-28(30-23)7-4-8-29(27)34-20-19-32-13-11-24(12-14-32)21-25-5-3-6-26(22-25)33-17-15-31(2)16-18-33/h3-10,22,24H,11-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412132
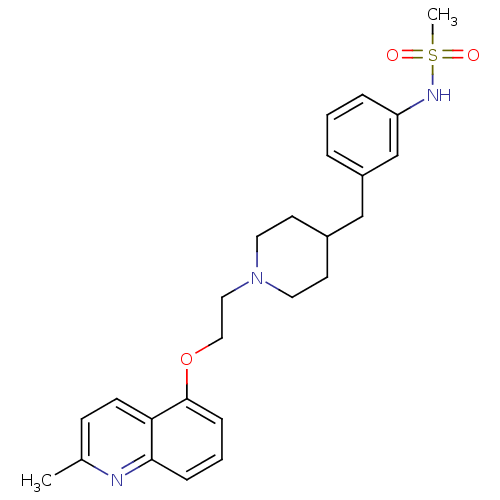
(CHEMBL497963)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(NS(C)(=O)=O)c4)CC3)cccc2n1 Show InChI InChI=1S/C25H31N3O3S/c1-19-9-10-23-24(26-19)7-4-8-25(23)31-16-15-28-13-11-20(12-14-28)17-21-5-3-6-22(18-21)27-32(2,29)30/h3-10,18,20,27H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412134

(CHEMBL525712)Show SMILES CC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C26H31N3O2/c1-19-9-10-24-25(27-19)7-4-8-26(24)31-16-15-29-13-11-21(12-14-29)17-22-5-3-6-23(18-22)28-20(2)30/h3-10,18,21H,11-17H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412132

(CHEMBL497963)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(NS(C)(=O)=O)c4)CC3)cccc2n1 Show InChI InChI=1S/C25H31N3O3S/c1-19-9-10-23-24(26-19)7-4-8-25(23)31-16-15-28-13-11-20(12-14-28)17-21-5-3-6-22(18-21)27-32(2,29)30/h3-10,18,20,27H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412118
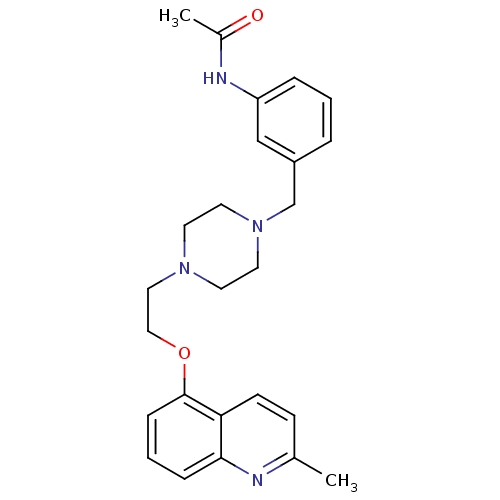
(CHEMBL494806)Show SMILES CC(=O)Nc1cccc(CN2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C25H30N4O2/c1-19-9-10-23-24(26-19)7-4-8-25(23)31-16-15-28-11-13-29(14-12-28)18-21-5-3-6-22(17-21)27-20(2)30/h3-10,17H,11-16,18H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50477400
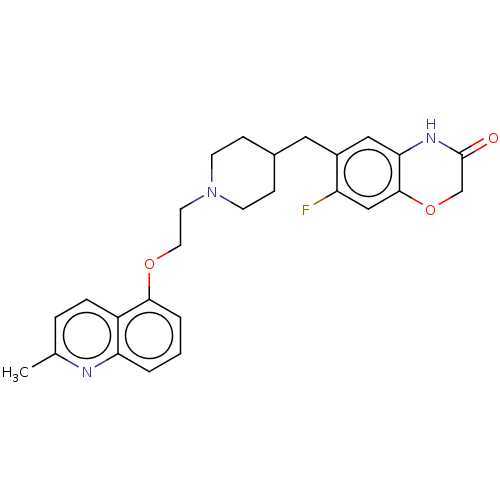
(CHEMBL239168)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cc5NC(=O)COc5cc4F)CC3)cccc2n1 Show InChI InChI=1S/C26H28FN3O3/c1-17-5-6-20-22(28-17)3-2-4-24(20)32-12-11-30-9-7-18(8-10-30)13-19-14-23-25(15-21(19)27)33-16-26(31)29-23/h2-6,14-15,18H,7-13,16H2,1H3,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412130

(CHEMBL498354)Show SMILES CCC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C27H33N3O2/c1-3-27(31)29-23-7-4-6-22(19-23)18-21-12-14-30(15-13-21)16-17-32-26-9-5-8-25-24(26)11-10-20(2)28-25/h4-11,19,21H,3,12-18H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413696
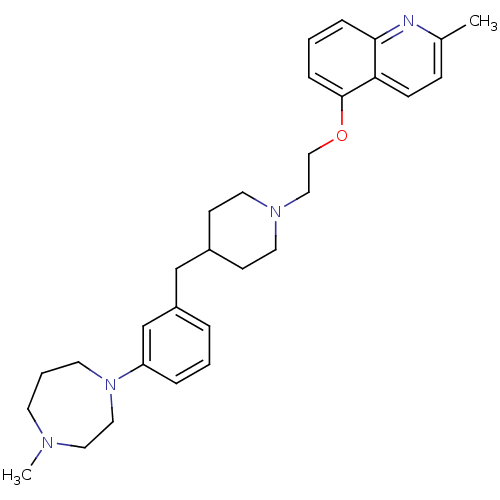
(CHEMBL514429)Show SMILES CN1CCCN(CC1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C30H40N4O/c1-24-10-11-28-29(31-24)8-4-9-30(28)35-21-20-33-16-12-25(13-17-33)22-26-6-3-7-27(23-26)34-15-5-14-32(2)18-19-34/h3-4,6-11,23,25H,5,12-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413688
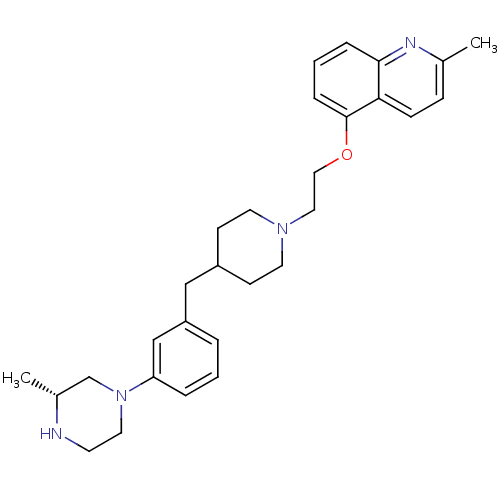
(CHEMBL517170)Show SMILES C[C@@H]1CN(CCN1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 |r| Show InChI InChI=1S/C29H38N4O/c1-22-9-10-27-28(31-22)7-4-8-29(27)34-18-17-32-14-11-24(12-15-32)19-25-5-3-6-26(20-25)33-16-13-30-23(2)21-33/h3-10,20,23-24,30H,11-19,21H2,1-2H3/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]almorexant from recombinant human OX1R expressed in CHO cells |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419136

(CHEMBL1830961)Show SMILES Fc1ccc(OCC[C@@H]2CCCCN2C(=O)c2ccccc2-c2ccccc2)c(F)c1 |r| Show InChI InChI=1S/C26H25F2NO2/c27-20-13-14-25(24(28)18-20)31-17-15-21-10-6-7-16-29(21)26(30)23-12-5-4-11-22(23)19-8-2-1-3-9-19/h1-5,8-9,11-14,18,21H,6-7,10,15-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413687
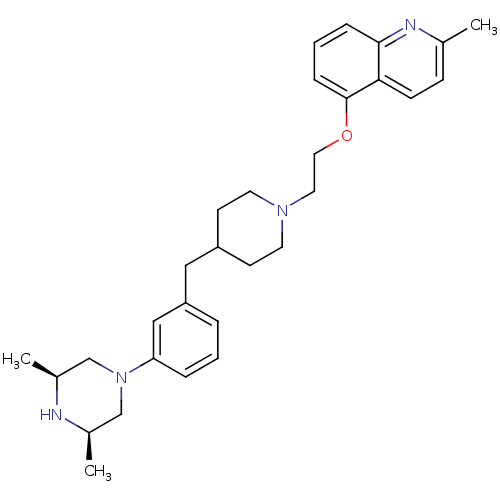
(CHEMBL461670)Show SMILES C[C@H]1CN(C[C@@H](C)N1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 |r| Show InChI InChI=1S/C30H40N4O/c1-22-10-11-28-29(32-22)8-5-9-30(28)35-17-16-33-14-12-25(13-15-33)18-26-6-4-7-27(19-26)34-20-23(2)31-24(3)21-34/h4-11,19,23-25,31H,12-18,20-21H2,1-3H3/t23-,24+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474384

(CHEMBL2113364)Show SMILES Cl.CS(=O)(=O)Oc1ccc2CCN(CC[C@H]3CC[C@@H](CC3)NC(=O)\C=C\c3ccc(F)cc3)CCc2c1 |r,wU:15.13,wD:18.20,(23.3,.02,;-6.64,-.16,;-5.58,-.78,;-6.64,-1.39,;-5.57,.45,;-4.24,-1.55,;-2.91,-.78,;-2.91,.78,;-1.53,1.57,;,.78,;.8,1.73,;2.31,1.43,;3.02,,;4.56,.01,;5.33,1.34,;6.87,1.35,;7.63,2.69,;9.17,2.69,;9.95,1.36,;9.18,.02,;7.64,.02,;11.49,1.36,;12.26,.02,;11.64,-1.04,;13.8,.03,;14.57,-1.31,;16.11,-1.31,;16.88,.03,;18.42,.03,;19.19,-1.3,;20.42,-1.3,;18.43,-2.64,;16.89,-2.64,;2.29,-1.45,;.76,-1.7,;,-.78,;-1.53,-1.57,)| Show InChI InChI=1S/C28H35FN2O4S.ClH/c1-36(33,34)35-27-12-7-23-15-18-31(19-16-24(23)20-27)17-14-22-4-10-26(11-5-22)30-28(32)13-6-21-2-8-25(29)9-3-21;/h2-3,6-9,12-13,20,22,26H,4-5,10-11,14-19H2,1H3,(H,30,32);1H/b13-6+;/t22-,26-; | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand |
J Med Chem 46: 4952-64 (2003)
Article DOI: 10.1021/jm030817d
BindingDB Entry DOI: 10.7270/Q2TT4TPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412120
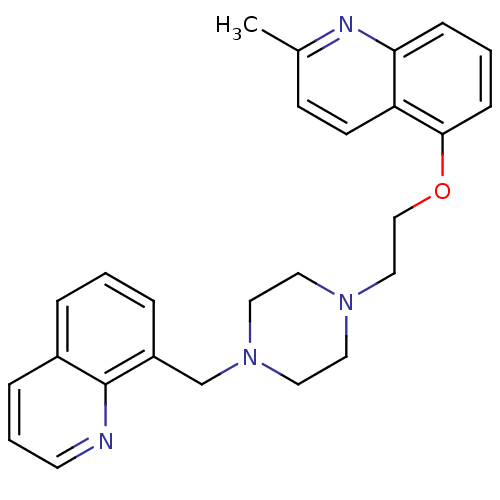
(CHEMBL425190 | SB-714786)Show SMILES Cc1ccc2c(OCCN3CCN(Cc4cccc5cccnc45)CC3)cccc2n1 Show InChI InChI=1S/C26H28N4O/c1-20-10-11-23-24(28-20)8-3-9-25(23)31-18-17-29-13-15-30(16-14-29)19-22-6-2-5-21-7-4-12-27-26(21)22/h2-12H,13-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY100635 from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50477404
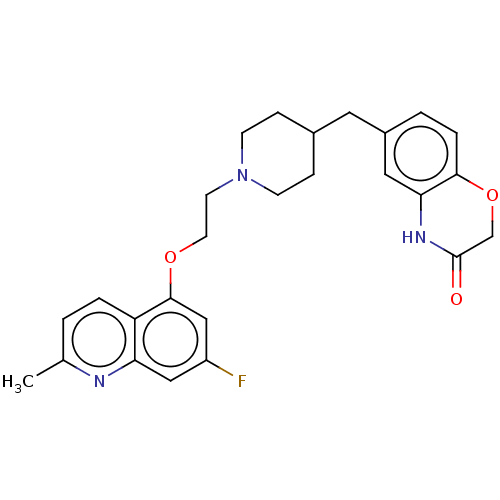
(CHEMBL442087)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cc(F)cc2n1 Show InChI InChI=1S/C26H28FN3O3/c1-17-2-4-21-22(28-17)14-20(27)15-25(21)32-11-10-30-8-6-18(7-9-30)12-19-3-5-24-23(13-19)29-26(31)16-33-24/h2-5,13-15,18H,6-12,16H2,1H3,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50477402
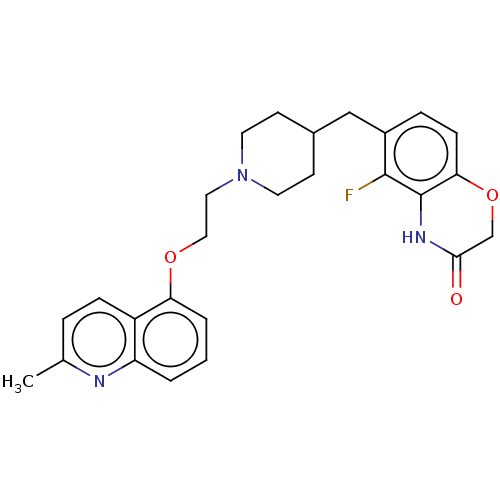
(CHEMBL241503)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4F)CC3)cccc2n1 Show InChI InChI=1S/C26H28FN3O3/c1-17-5-7-20-21(28-17)3-2-4-22(20)32-14-13-30-11-9-18(10-12-30)15-19-6-8-23-26(25(19)27)29-24(31)16-33-23/h2-8,18H,9-16H2,1H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human cloned 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50477399

(CHEMBL241463)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cc(F)c5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H28FN3O3/c1-17-5-6-20-22(28-17)3-2-4-24(20)32-12-11-30-9-7-18(8-10-30)13-19-14-21(27)26-23(15-19)29-25(31)16-33-26/h2-6,14-15,18H,7-13,16H2,1H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human cloned 5HT1B receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413694
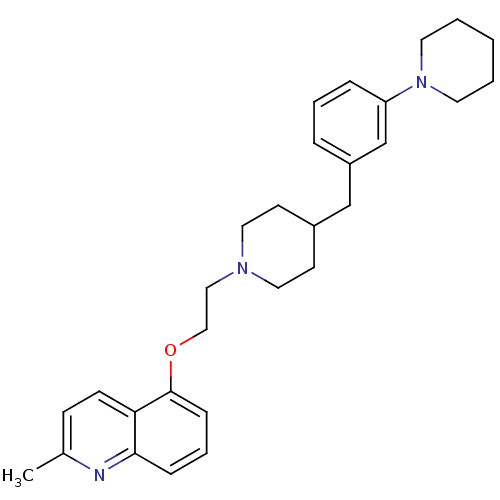
(CHEMBL459061)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(c4)N4CCCCC4)CC3)cccc2n1 Show InChI InChI=1S/C29H37N3O/c1-23-11-12-27-28(30-23)9-6-10-29(27)33-20-19-31-17-13-24(14-18-31)21-25-7-5-8-26(22-25)32-15-3-2-4-16-32/h5-12,22,24H,2-4,13-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50477395

(CHEMBL238520)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cc5NC(=O)COc5cc4F)CC3)cc(F)cc2n1 Show InChI InChI=1S/C26H27F2N3O3/c1-16-2-3-20-22(29-16)12-19(27)13-24(20)33-9-8-31-6-4-17(5-7-31)10-18-11-23-25(14-21(18)28)34-15-26(32)30-23/h2-3,11-14,17H,4-10,15H2,1H3,(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at rat 5HT1A receptor |
Bioorg Med Chem Lett 17: 1033-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.031
BindingDB Entry DOI: 10.7270/Q2348P5G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412119
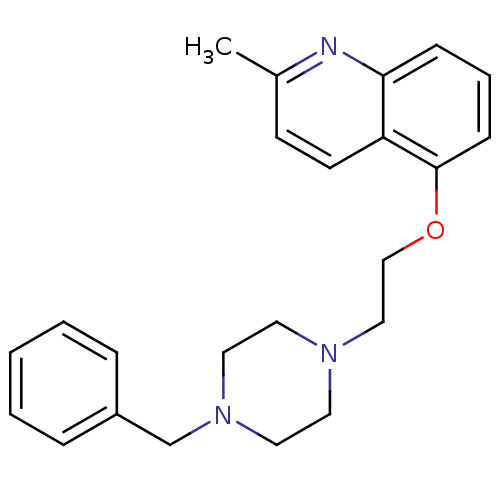
(CHEMBL494631)Show InChI InChI=1S/C23H27N3O/c1-19-10-11-21-22(24-19)8-5-9-23(21)27-17-16-25-12-14-26(15-13-25)18-20-6-3-2-4-7-20/h2-11H,12-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50132022
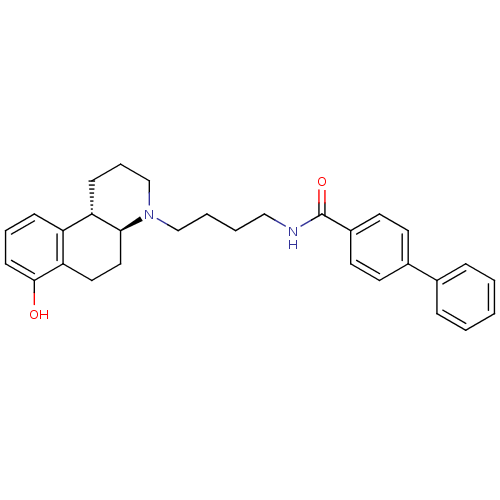
(Biphenyl-4-carboxylic acid [4-((4aS,10bS)-7-hydrox...)Show SMILES Oc1cccc2[C@@H]3CCCN(CCCCNC(=O)c4ccc(cc4)-c4ccccc4)[C@H]3CCc12 Show InChI InChI=1S/C30H34N2O2/c33-29-12-6-10-25-26-11-7-21-32(28(26)18-17-27(25)29)20-5-4-19-31-30(34)24-15-13-23(14-16-24)22-8-2-1-3-9-22/h1-3,6,8-10,12-16,26,28,33H,4-5,7,11,17-21H2,(H,31,34)/t26-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. |
Bioorg Med Chem Lett 8: 2859-64 (1998)
BindingDB Entry DOI: 10.7270/Q2319Z1B |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419131
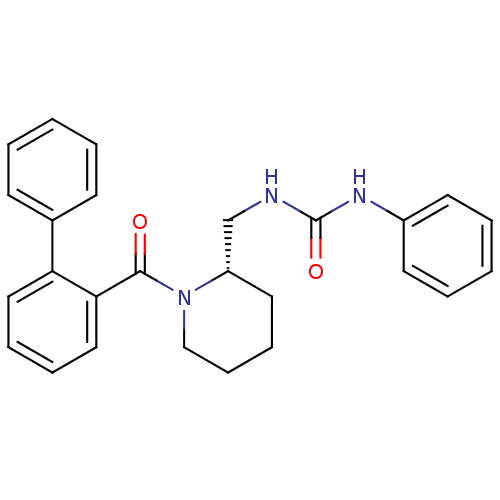
(CHEMBL1830968)Show SMILES O=C(NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)Nc1ccccc1 |r| Show InChI InChI=1S/C26H27N3O2/c30-25(24-17-8-7-16-23(24)20-11-3-1-4-12-20)29-18-10-9-15-22(29)19-27-26(31)28-21-13-5-2-6-14-21/h1-8,11-14,16-17,22H,9-10,15,18-19H2,(H2,27,28,31)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413689
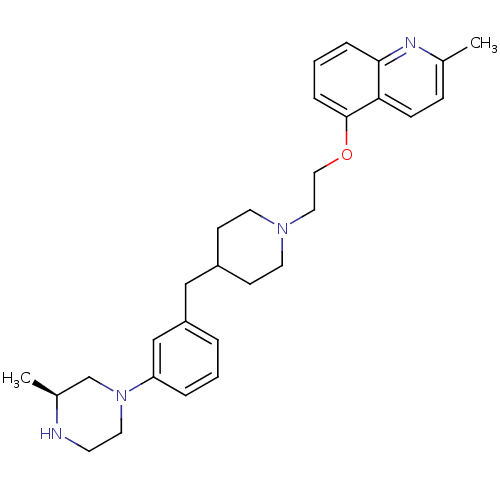
(CHEMBL518545)Show SMILES C[C@H]1CN(CCN1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 |r| Show InChI InChI=1S/C29H38N4O/c1-22-9-10-27-28(31-22)7-4-8-29(27)34-18-17-32-14-11-24(12-15-32)19-25-5-3-6-26(20-25)33-16-13-30-23(2)21-33/h3-10,20,23-24,30H,11-19,21H2,1-2H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474396

(CHEMBL2113356)Show SMILES Cl.COc1ccc(\C=C\C(=O)N[C@H]2CC[C@H](CCN3CCc4ccc(OS(C)(=O)=O)cc4CC3)CC2)cc1 |r,wU:12.10,wD:15.14,(24.07,.02,;21.35,-2.37,;20.73,-1.3,;19.19,-1.3,;18.42,.03,;16.88,.03,;16.11,-1.31,;14.57,-1.31,;13.8,.03,;12.26,.02,;11.64,-1.04,;11.49,1.36,;9.95,1.36,;9.18,.02,;7.64,.02,;6.87,1.35,;5.33,1.34,;4.56,.01,;3.02,,;2.31,1.43,;.8,1.73,;,.78,;-1.53,1.57,;-2.91,.78,;-2.91,-.78,;-4.24,-1.55,;-5.58,-.78,;-6.64,-.16,;-6.64,-1.39,;-5.57,.45,;-1.53,-1.57,;,-.78,;.76,-1.7,;2.29,-1.45,;7.63,2.69,;9.17,2.69,;16.89,-2.64,;18.43,-2.64,)| Show InChI InChI=1S/C29H38N2O5S.ClH/c1-35-27-11-5-22(6-12-27)7-14-29(32)30-26-9-3-23(4-10-26)15-18-31-19-16-24-8-13-28(36-37(2,33)34)21-25(24)17-20-31;/h5-8,11-14,21,23,26H,3-4,9-10,15-20H2,1-2H3,(H,30,32);1H/b14-7+;/t23-,26-; | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand |
J Med Chem 46: 4952-64 (2003)
Article DOI: 10.1021/jm030817d
BindingDB Entry DOI: 10.7270/Q2TT4TPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413702
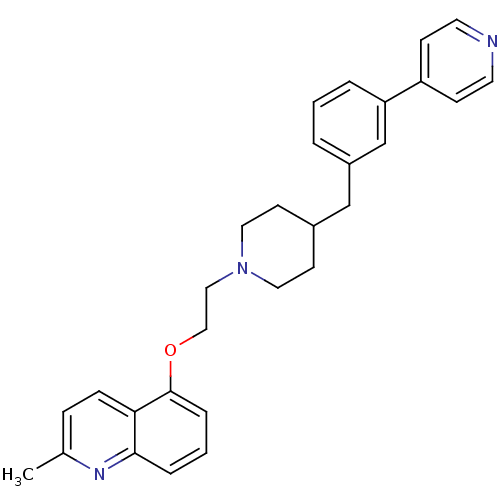
(CHEMBL462916)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(c4)-c4ccncc4)CC3)cccc2n1 Show InChI InChI=1S/C29H31N3O/c1-22-8-9-27-28(31-22)6-3-7-29(27)33-19-18-32-16-12-23(13-17-32)20-24-4-2-5-26(21-24)25-10-14-30-15-11-25/h2-11,14-15,21,23H,12-13,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413690
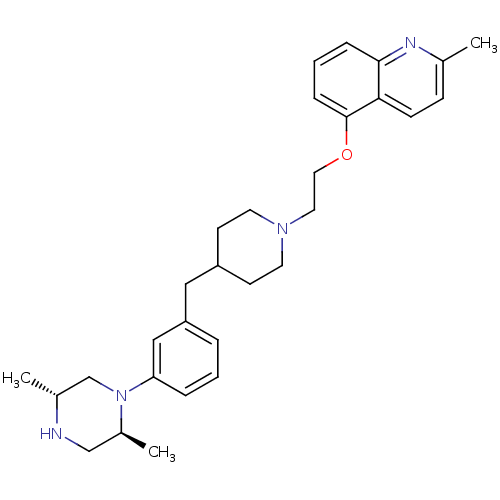
(CHEMBL457982)Show SMILES C[C@@H]1CN([C@@H](C)CN1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 |r| Show InChI InChI=1S/C30H40N4O/c1-22-10-11-28-29(32-22)8-5-9-30(28)35-17-16-33-14-12-25(13-15-33)18-26-6-4-7-27(19-26)34-21-23(2)31-20-24(34)3/h4-11,19,23-25,31H,12-18,20-21H2,1-3H3/t23-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474398
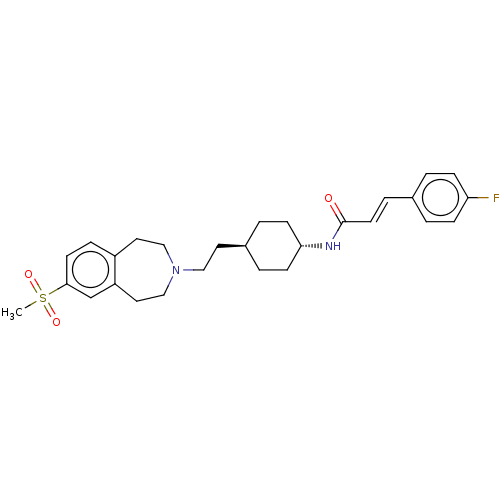
(CHEMBL2368629)Show SMILES Cl.CS(=O)(=O)c1ccc2CCN(CC[C@H]3CC[C@@H](CC3)NC(=O)\C=C\c3ccc(F)cc3)CCc2c1 |r,wU:17.19,wD:14.12,(18.31,,;-11.73,1.48,;-10.96,.14,;-12.29,-.63,;-10.19,-1.19,;-9.62,.91,;-9.62,2.45,;-8.29,3.22,;-6.96,2.45,;-5.75,3.41,;-4.25,3.07,;-3.58,1.68,;-2.04,1.68,;-1.27,.35,;.27,.35,;1.04,-.98,;2.58,-.98,;3.35,.35,;2.58,1.68,;1.04,1.68,;4.89,.35,;5.66,-.98,;4.89,-2.32,;7.2,-.98,;7.97,-2.32,;9.51,-2.32,;10.28,-3.65,;11.82,-3.65,;12.59,-2.32,;14.13,-2.32,;11.82,-.98,;10.28,-.98,;-4.25,.3,;-5.75,-.05,;-6.96,.91,;-8.29,.14,)| Show InChI InChI=1S/C28H35FN2O3S.ClH/c1-35(33,34)27-12-7-23-15-18-31(19-16-24(23)20-27)17-14-22-4-10-26(11-5-22)30-28(32)13-6-21-2-8-25(29)9-3-21;/h2-3,6-9,12-13,20,22,26H,4-5,10-11,14-19H2,1H3,(H,30,32);1H/b13-6+;/t22-,26-; | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand |
J Med Chem 46: 4952-64 (2003)
Article DOI: 10.1021/jm030817d
BindingDB Entry DOI: 10.7270/Q2TT4TPR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data