

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032698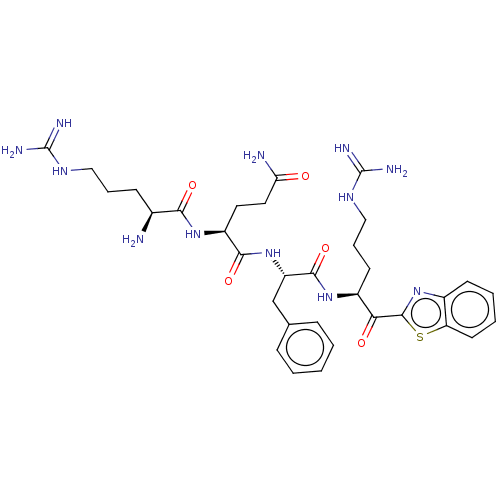 (CHEMBL3354676 | N-0130) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032698 (CHEMBL3354676 | N-0130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM525153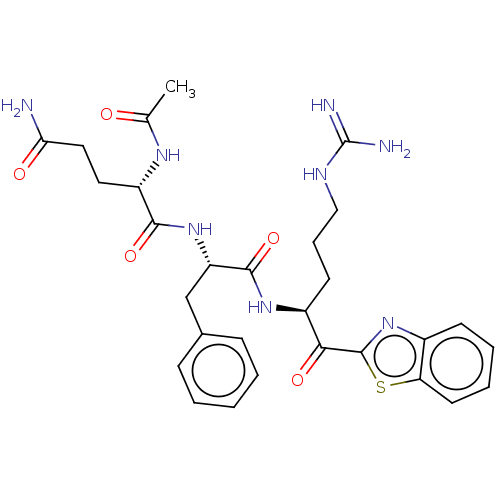 (Ac-QFR-kbt | N-0386 | US10988505, Example 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185304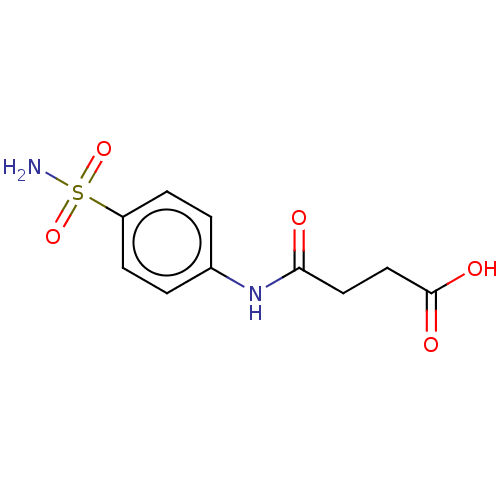 (Sulfasuccinamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM525256 ((H)Arg-Glu-Phe-Arg-kbt | N-0438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM525152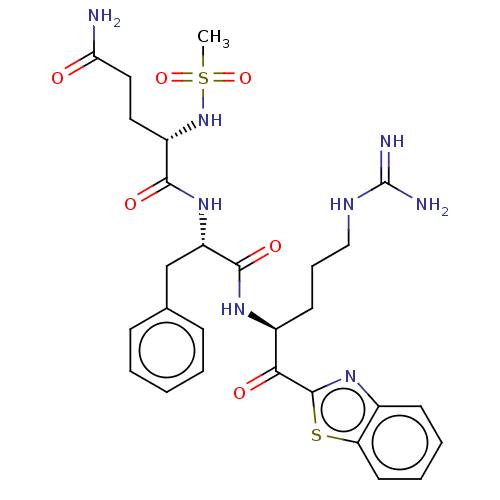 (Ms-QFR-kbt | N-0385 | US10988505, Example 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM525153 (Ac-QFR-kbt | N-0386 | US10988505, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM525256 ((H)Arg-Glu-Phe-Arg-kbt | N-0438) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50185295 (CHEMBL3822883) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50185297 (CHEMBL3822655) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM525152 (Ms-QFR-kbt | N-0385 | US10988505, Example 2) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185301 (CHEMBL3824097) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50185303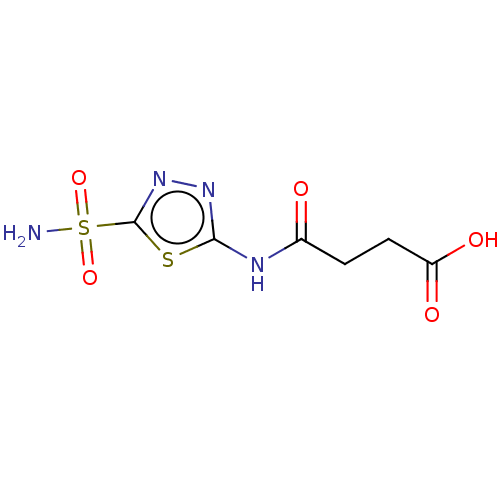 (CHEMBL88115) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185299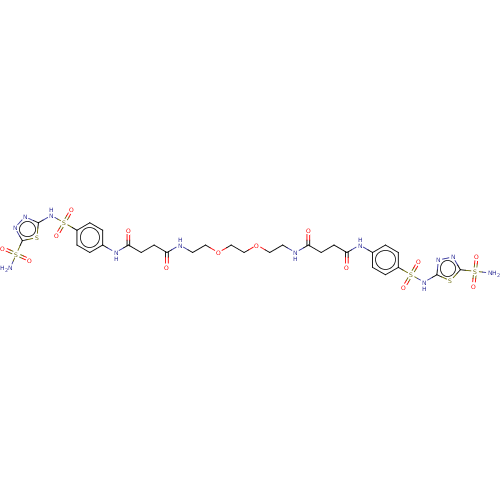 (CHEMBL3823200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50185301 (CHEMBL3824097) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50185302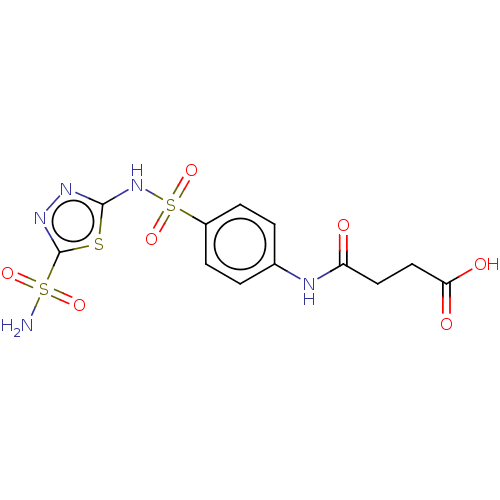 (CHEMBL3823618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185298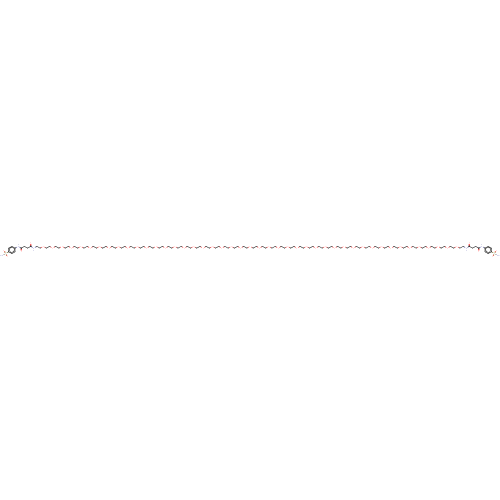 (CHEMBL3823514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185303 (CHEMBL88115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185295 (CHEMBL3822883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185302 (CHEMBL3823618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50185303 (CHEMBL88115) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50031706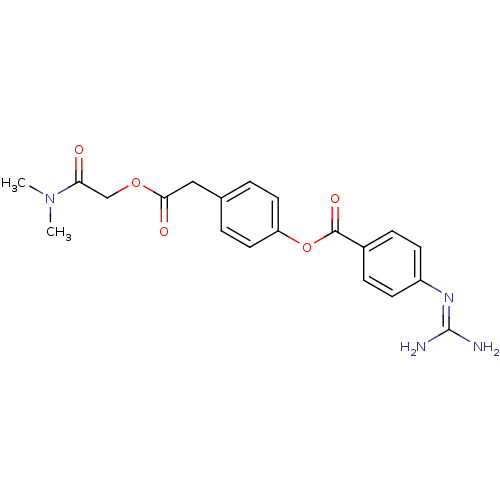 (4-Guanidino-benzoic acid 4-dimethylcarbamoylmethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50185295 (CHEMBL3822883) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50185302 (CHEMBL3823618) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50185295 (CHEMBL3822883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50185300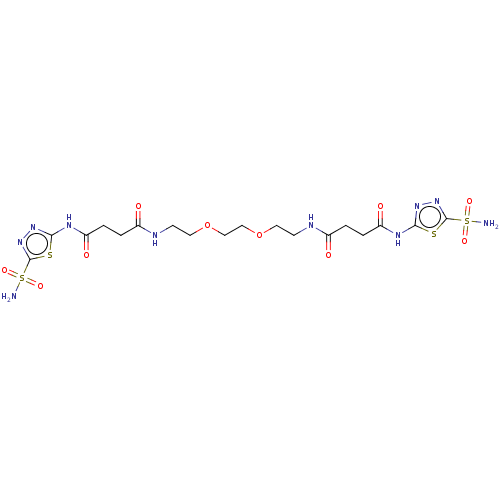 (CHEMBL3822486) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50185299 (CHEMBL3823200) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50185298 (CHEMBL3823514) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50185300 (CHEMBL3822486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185297 (CHEMBL3822655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50185302 (CHEMBL3823618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50031706 (4-Guanidino-benzoic acid 4-dimethylcarbamoylmethox...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50185297 (CHEMBL3822655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50031706 (4-Guanidino-benzoic acid 4-dimethylcarbamoylmethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50185300 (CHEMBL3822486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50185304 (Sulfasuccinamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50185301 (CHEMBL3824097) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50185299 (CHEMBL3823200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50185303 (CHEMBL88115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50185297 (CHEMBL3822655) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50185298 (CHEMBL3823514) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50185301 (CHEMBL3824097) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50185303 (CHEMBL88115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50185299 (CHEMBL3823200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50185300 (CHEMBL3822486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM525153 (Ac-QFR-kbt | N-0386 | US10988505, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50185302 (CHEMBL3823618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50185298 (CHEMBL3823514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50185300 (CHEMBL3822486) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | J Med Chem 59: 5077-88 (2016) Article DOI: 10.1021/acs.jmedchem.6b00492 BindingDB Entry DOI: 10.7270/Q2HT2R80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 146 total ) | Next | Last >> |