

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin receptor type B (Sus scrofa) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287890 (CHEMBL427778 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287885 (CHEMBL405377 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263407 (CHEMBL4090599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287883 (CHEMBL412003 | Suc-Glu-Ala-Gly-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287882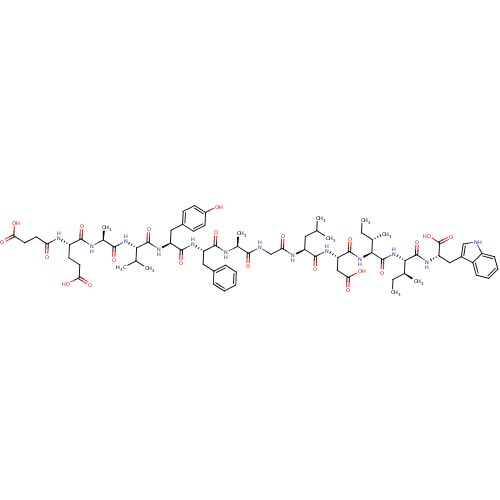 (CHEMBL412065 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-Gly-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50071433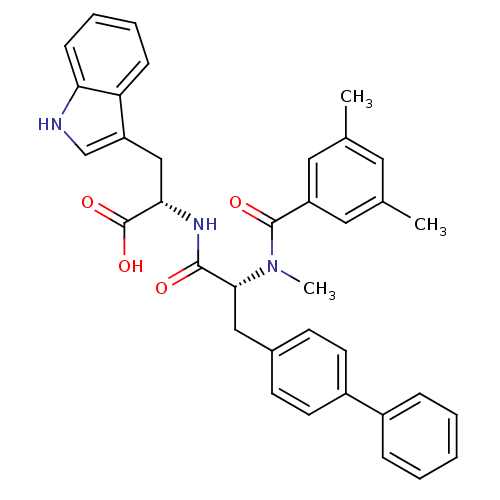 ((S)-2-{(R)-3-Biphenyl-4-yl-2-[(3,5-dimethyl-benzoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50263407 (CHEMBL4090599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190686 ((R)-3'-(2,3-dimethylbenzo[b]thiophen-5-yl)spiro[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190696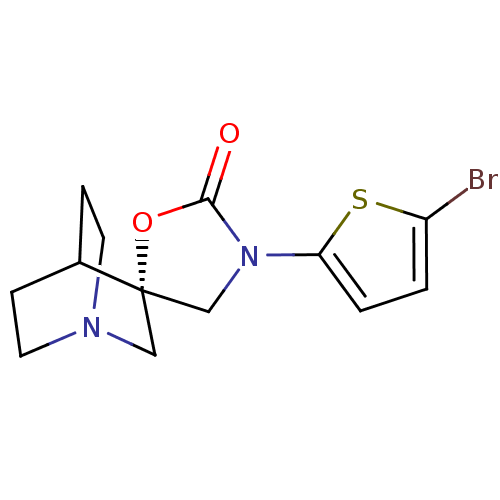 ((2S)-3'-(5-bromothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164606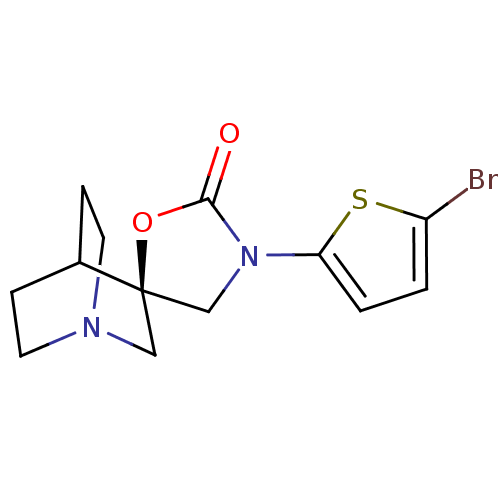 ((2R)-3'-(5-bromothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190677 ((R)-3'-(3-bromobenzo[b]thiophen-5-yl)spiro[1-azabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287878 (CGP-49941 | CHEMBL305615 | N-{(R)-1-[2-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287888 (CHEMBL407559 | Suc-Glu-Ala-Val-Tyr-Phe-Gly-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287886 (CHEMBL405796 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Gly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263435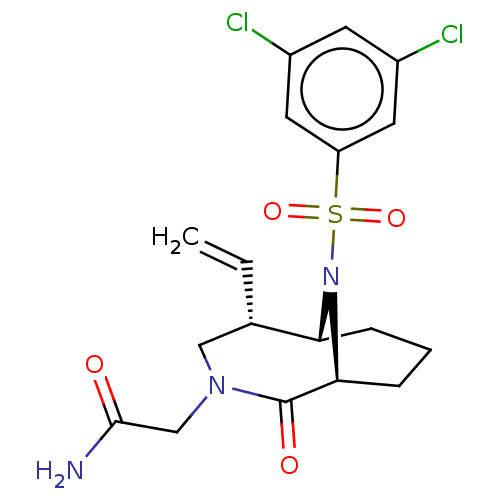 (CHEMBL4075704) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263422 (CHEMBL4072643) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263420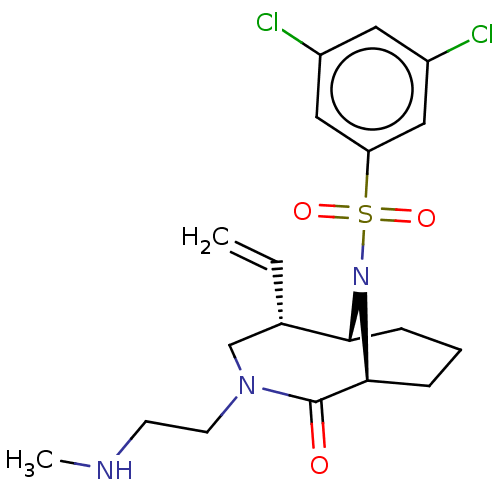 (CHEMBL4102121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263421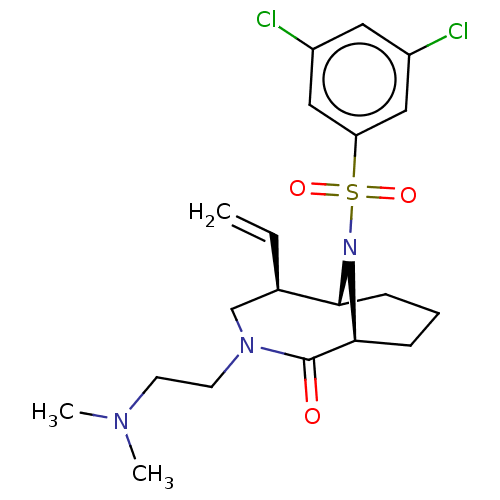 (CHEMBL4101268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263416 (CHEMBL4067970) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263408 (CHEMBL4063858) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164618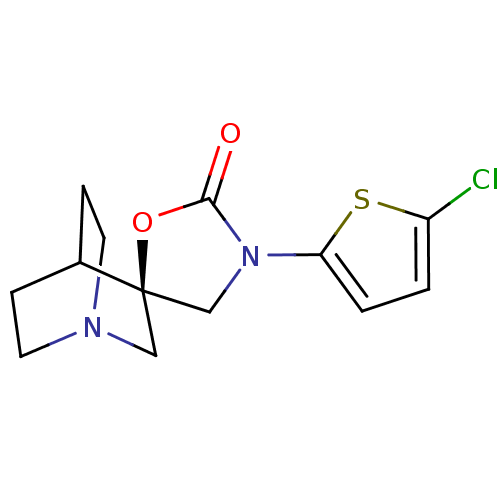 ((2R)-3'-(5-chlorothien-2-yl)-2'H-spiro[4-azabicycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287887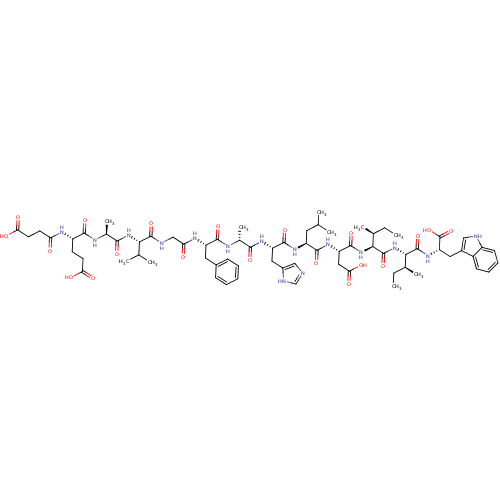 (CHEMBL413604 | Suc-Glu-Ala-Val-Gly-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164607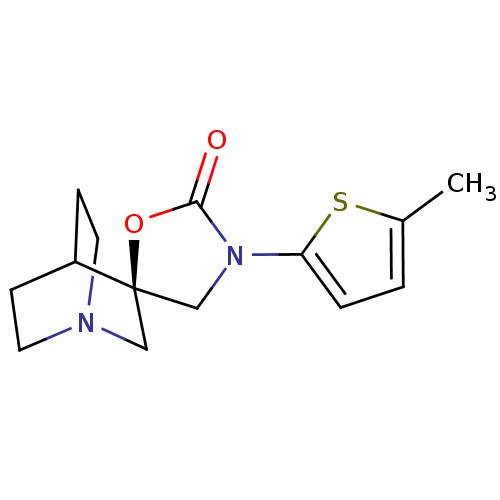 ((2R)-3'-(5-methylthien-2-yl)-2'H-spiro[4-azabicycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263417 (CHEMBL4068304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50149146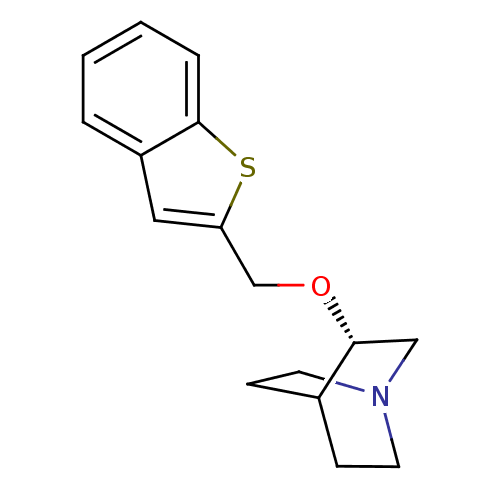 ((S)-3-(Benzo[b]thiophen-2-ylmethoxy)-1-aza-bicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287880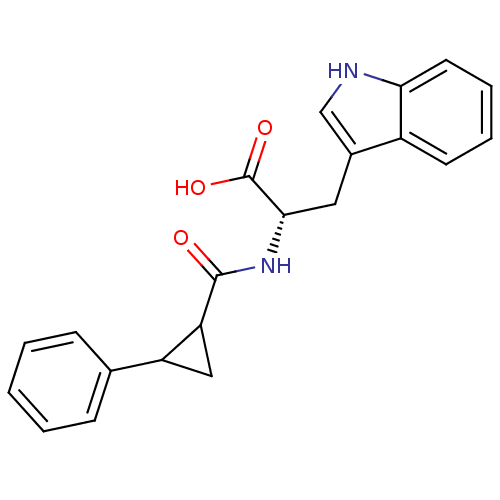 ((S)-3-(1H-Indol-3-yl)-2-[(2-phenyl-cyclopropanecar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50263407 (CHEMBL4090599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263424 (CHEMBL4077424) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164611 ((2R)-3'-(5-ethylthien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263437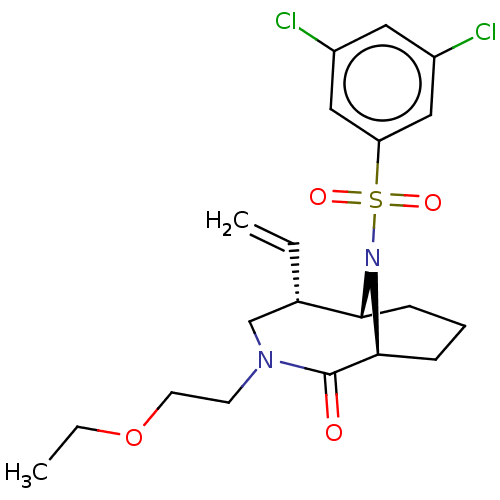 (CHEMBL4073944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50263407 (CHEMBL4090599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164621 ((2R)-3'-(2-naphthyl)-2'H-spiro[4-azabicyclo[2.2.2]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50071431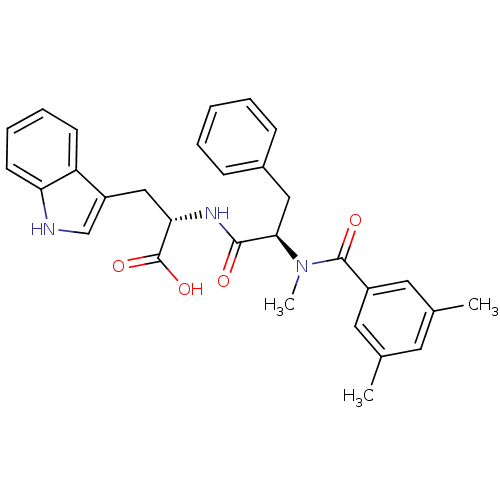 ((S)-2-{(R)-2-[(3,5-Dimethyl-benzoyl)-methyl-amino]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263409 (CHEMBL4086695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263423 (CHEMBL4077610) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263394 (CHEMBL4069155) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263436 (CHEMBL4075784) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263439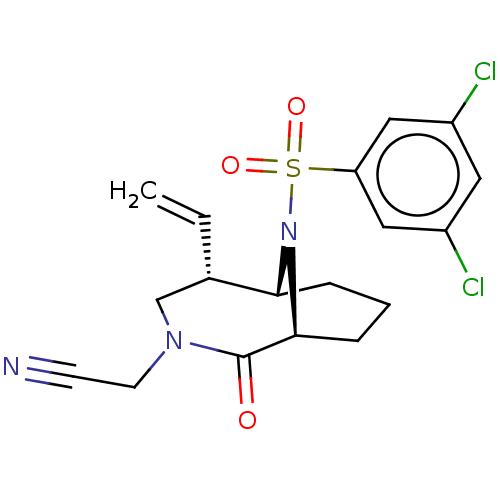 (CHEMBL4076387) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP2 (Homo sapiens) | BDBM50263407 (CHEMBL4090599) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50149147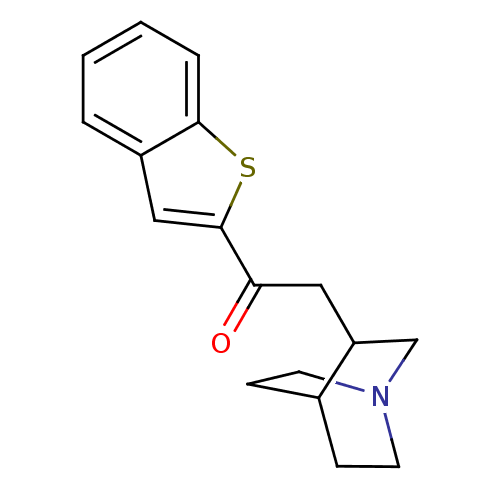 (2-(1-Aza-bicyclo[2.2.2]oct-3-yl)-1-benzo[b]thiophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164627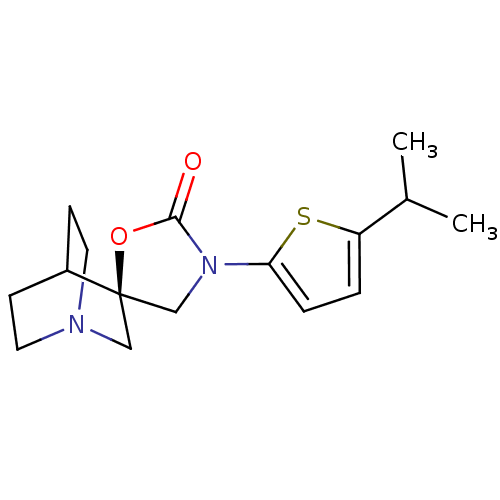 ((2R)-3'-(5-isopropylthien-2-yl)-2'H-spiro[4-azabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164622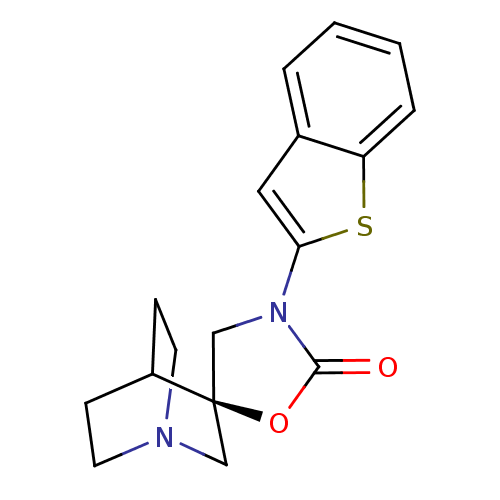 ((2R)-3'-(1-benzothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164612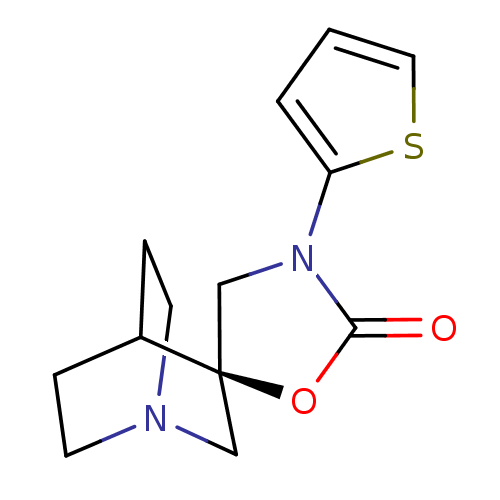 ((2R)-3'-thien-2-yl-2'H-spiro[4-azabicyclo[2.2.2]oc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164619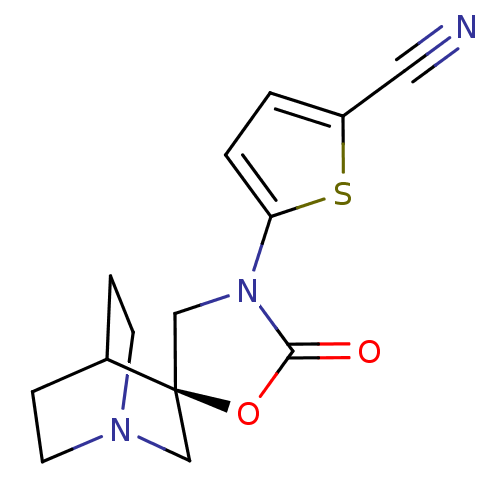 (5-[(2R)-2'-oxo-3'H-spiro[4-azabicyclo[2.2.2]octane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50287885 (CHEMBL405377 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263414 (CHEMBL4104603) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164628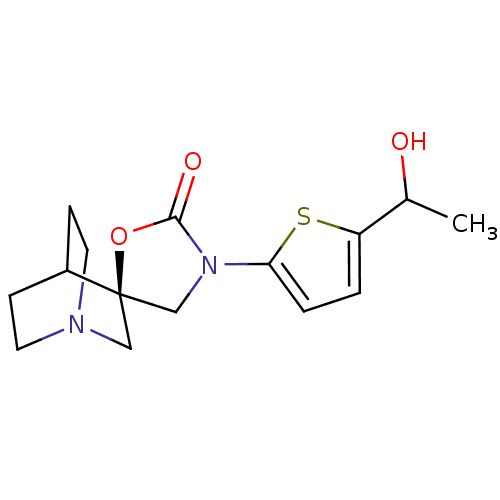 ((2R)-3'-[5-(1-hydroxyethyl)thien-2-yl]-2'H-spiro[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287884 (CHEMBL411399 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 152 total ) | Next | Last >> |