

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA6 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM50031472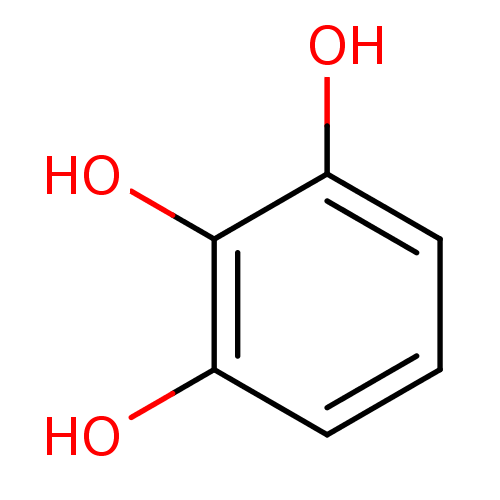 (1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA6 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50031472 (1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA2 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM50336490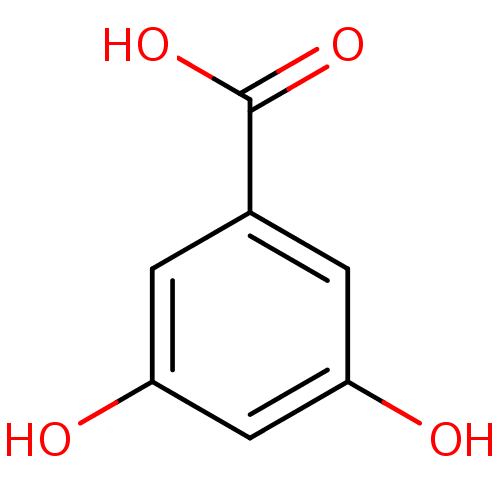 (3,5-DIHYDROXYBENZOATE | 3,5-Dihydroxy-benzoic acid...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019398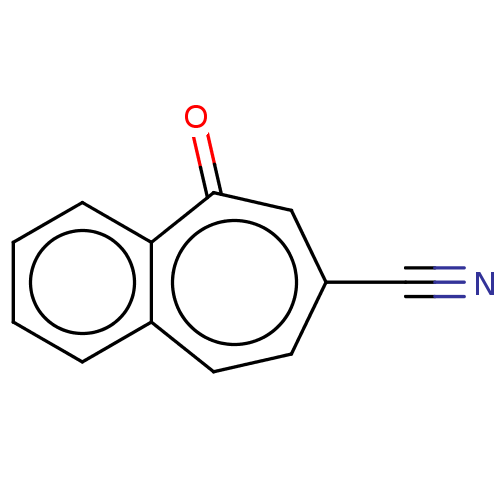 (CHEMBL3290059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019395 (CHEMBL3290057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019400 (CHEMBL3290055) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019400 (CHEMBL3290055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM50336491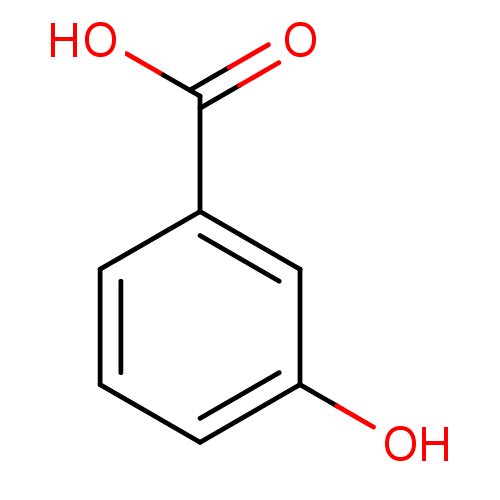 (3-Hydroxy-benzoic acid | 3-Hydroxybenzoate | 3-Hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA6 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM50100861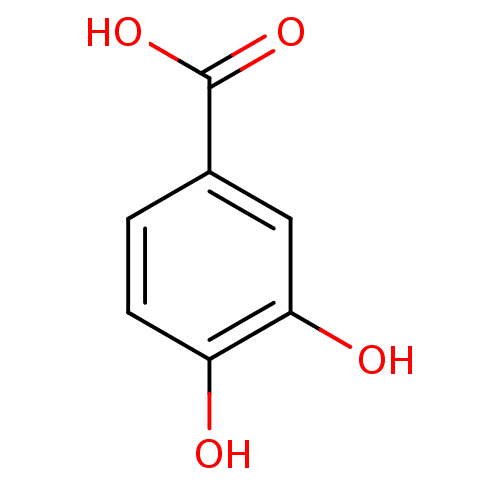 (3,4-Dihydroxybenzoate, VIII | 3,4-dihydroxybenzoic...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019399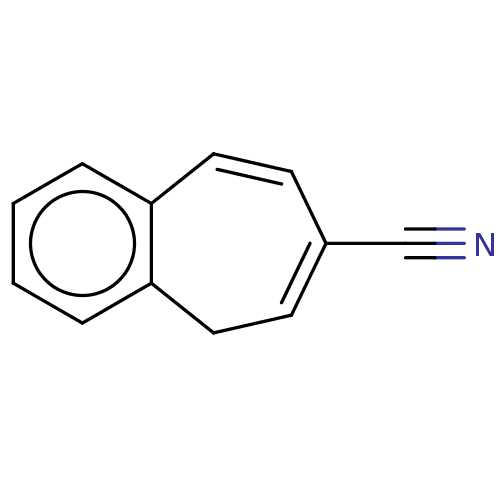 (CHEMBL3290054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019397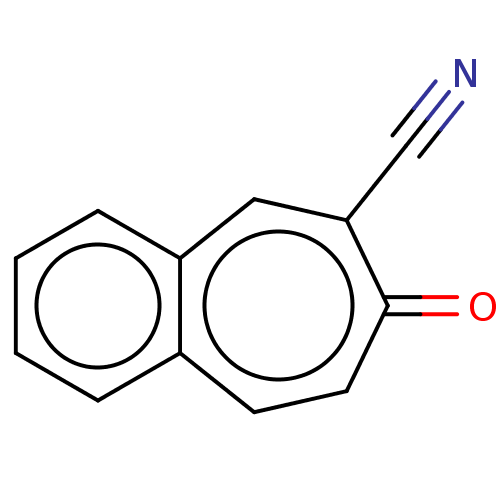 (CHEMBL3290058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019398 (CHEMBL3290059) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019397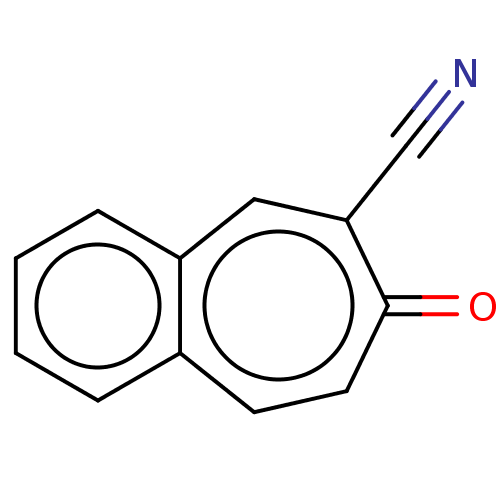 (CHEMBL3290058) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019394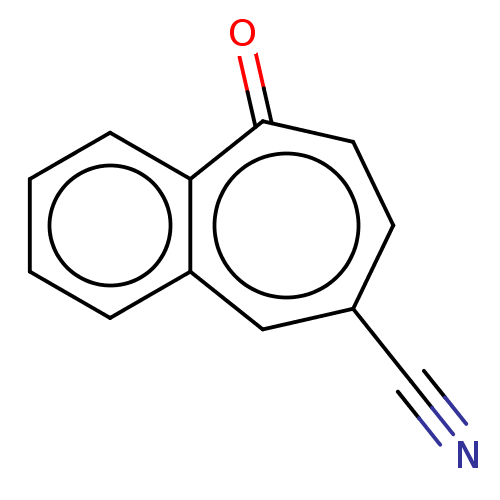 (CHEMBL3290056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019394 (CHEMBL3290056) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019395 (CHEMBL3290057) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019399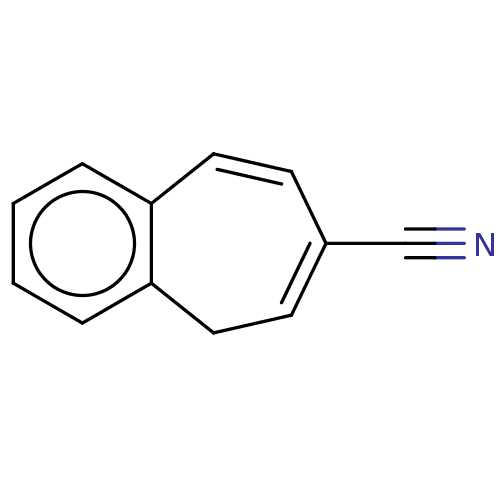 (CHEMBL3290054) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM50085536 (3,4,5-Trihydroxybenzoate, X | 3,4,5-trihydroxybenz...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM50031472 (1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM50100861 (3,4-Dihydroxybenzoate, VIII | 3,4-dihydroxybenzoic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA6 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM26190 (1,4-Dihydroxybenzene, XIII | 1,4-dihydroxybenzene ...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM26189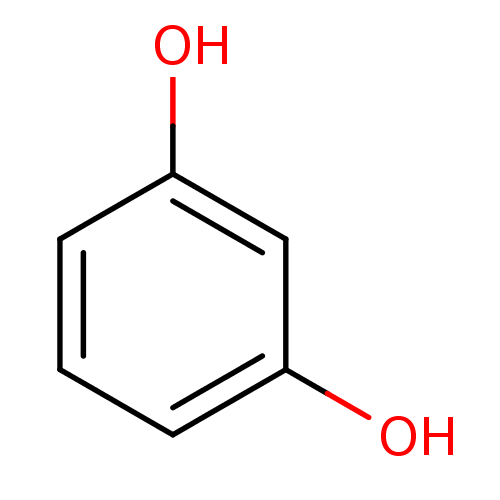 (α-CA inhibitor, 13 | 1,3-Dihydroxybenzene, XI...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50031472 (1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA1 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM26194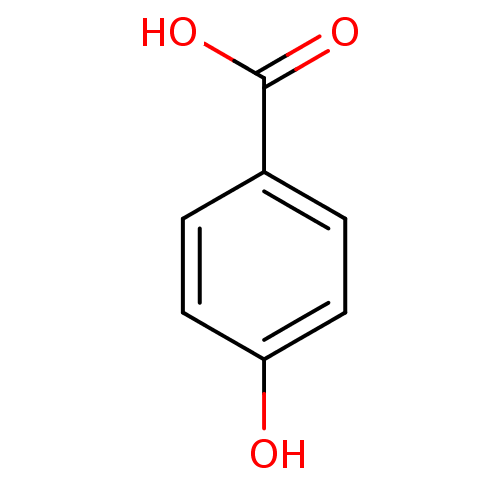 (4-Hydroxybenzoate, III | 4-hydroxybenzoic acid | C...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM60986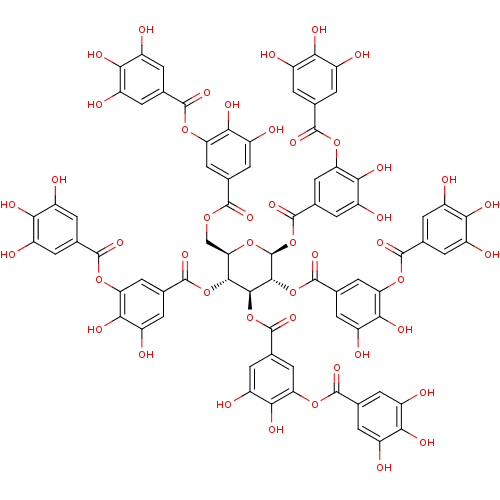 (MLS001335996 | SMR000857330 | TANNIC ACID | cid_16...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM26187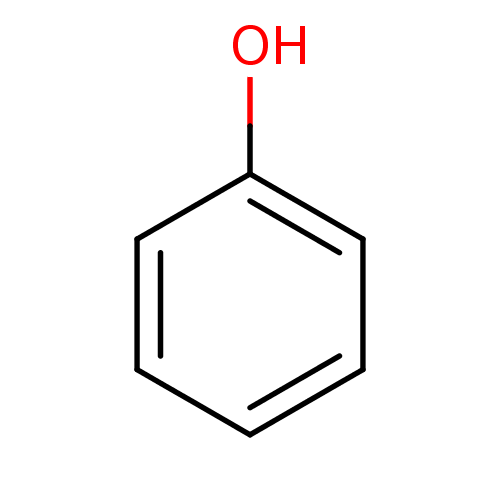 (α-CA inhibitor, 11 | CHEMBL14060 | US9688816,...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM26188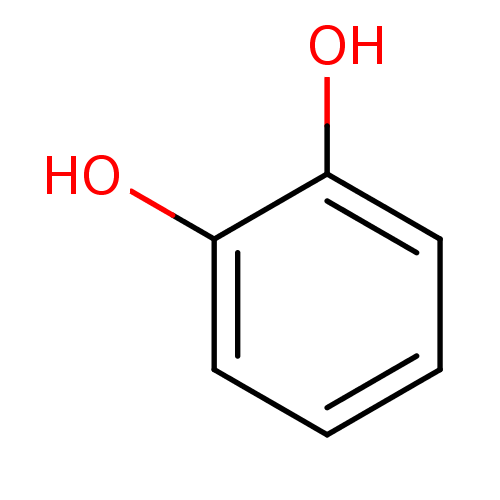 (α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM60986 (MLS001335996 | SMR000857330 | TANNIC ACID | cid_16...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA6 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM60986 (MLS001335996 | SMR000857330 | TANNIC ACID | cid_16...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA2 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM60986 (MLS001335996 | SMR000857330 | TANNIC ACID | cid_16...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA1 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM50336491 (3-Hydroxy-benzoic acid | 3-Hydroxybenzoate | 3-Hyd...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Dicentrarchus labrax) | BDBM26193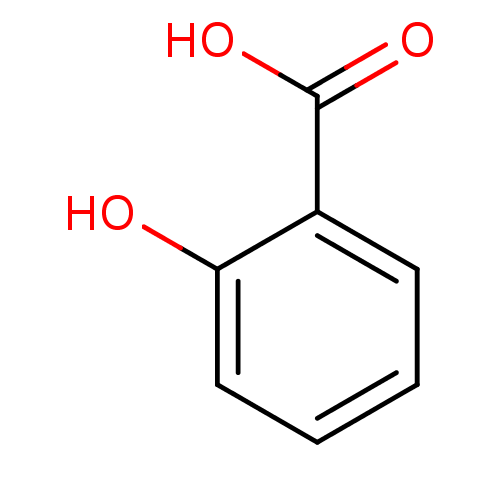 (2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM26193 (2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.32E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University Curated by ChEMBL | Assay Description Inhibition of human CA6 using 4-nitrophenylacetate substrate by esterase assay | Bioorg Med Chem Lett 21: 4259-62 (2011) Article DOI: 10.1016/j.bmcl.2011.05.071 BindingDB Entry DOI: 10.7270/Q2PC32Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019397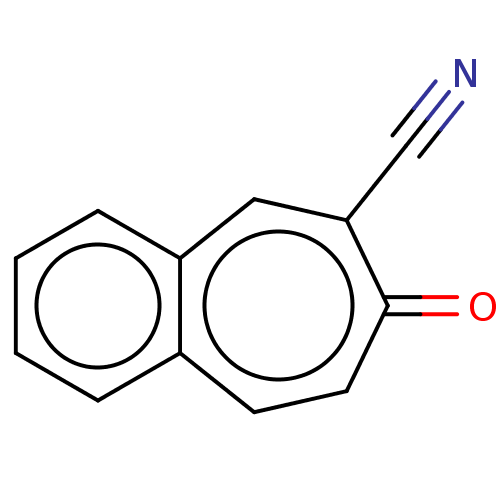 (CHEMBL3290058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019395 (CHEMBL3290057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019398 (CHEMBL3290059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019394 (CHEMBL3290056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019400 (CHEMBL3290055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50019399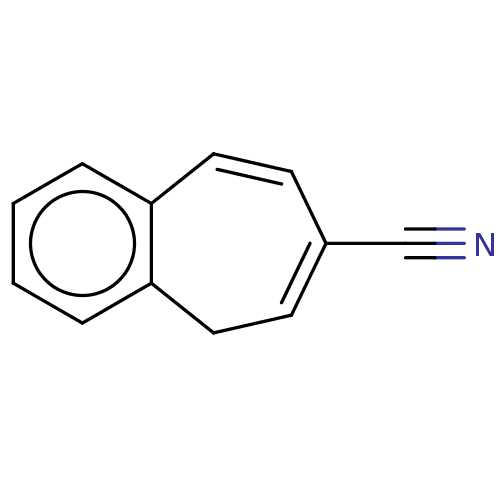 (CHEMBL3290054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019399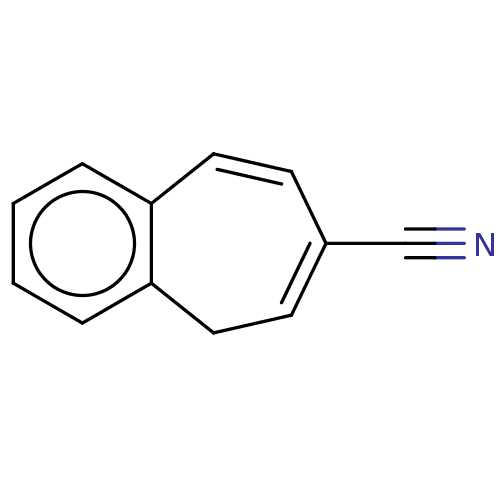 (CHEMBL3290054) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019398 (CHEMBL3290059) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019397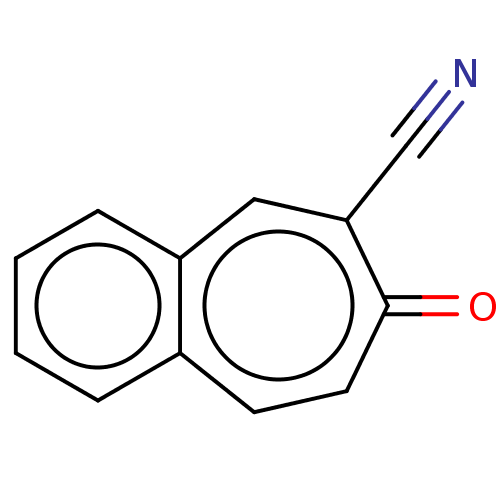 (CHEMBL3290058) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019400 (CHEMBL3290055) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019394 (CHEMBL3290056) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50019395 (CHEMBL3290057) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer | Bioorg Med Chem 22: 3537-43 (2014) Article DOI: 10.1016/j.bmc.2014.04.007 BindingDB Entry DOI: 10.7270/Q2Z60QM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||