 Found 130 hits with Last Name = 'totaro' and Initial = 'ja'
Found 130 hits with Last Name = 'totaro' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81891
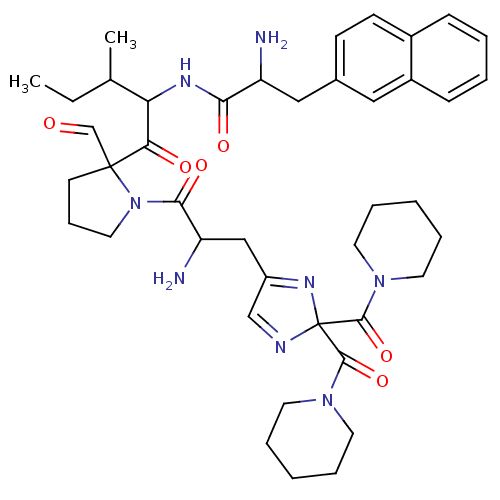
(CAS_188397 | L-366,948 | NSC_188397)Show SMILES CCC(C)C(NC(=O)C(N)Cc1ccc2ccccc2c1)C(=O)C1(CCCN1C(=O)C(N)CC1=NC(N=C1)(C(=O)N1CCCCC1)C(=O)N1CCCCC1)C=O |c:39,t:36| Show InChI InChI=1S/C42H56N8O6/c1-3-28(2)35(46-37(53)33(43)24-29-15-16-30-13-6-7-14-31(30)23-29)36(52)41(27-51)17-12-22-50(41)38(54)34(44)25-32-26-45-42(47-32,39(55)48-18-8-4-9-19-48)40(56)49-20-10-5-11-21-49/h6-7,13-16,23,26-28,33-35H,3-5,8-12,17-22,24-25,43-44H2,1-2H3,(H,46,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81891

(CAS_188397 | L-366,948 | NSC_188397)Show SMILES CCC(C)C(NC(=O)C(N)Cc1ccc2ccccc2c1)C(=O)C1(CCCN1C(=O)C(N)CC1=NC(N=C1)(C(=O)N1CCCCC1)C(=O)N1CCCCC1)C=O |c:39,t:36| Show InChI InChI=1S/C42H56N8O6/c1-3-28(2)35(46-37(53)33(43)24-29-15-16-30-13-6-7-14-31(30)23-29)36(52)41(27-51)17-12-22-50(41)38(54)34(44)25-32-26-45-42(47-32,39(55)48-18-8-4-9-19-48)40(56)49-20-10-5-11-21-49/h6-7,13-16,23,26-28,33-35H,3-5,8-12,17-22,24-25,43-44H2,1-2H3,(H,46,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM81894
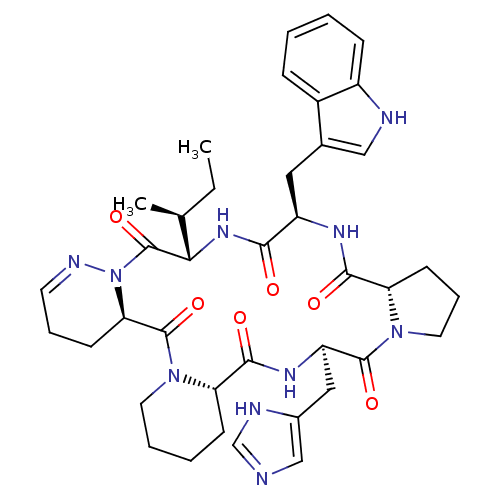
(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81894

(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Rhesus) | BDBM81894

(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81894

(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81893
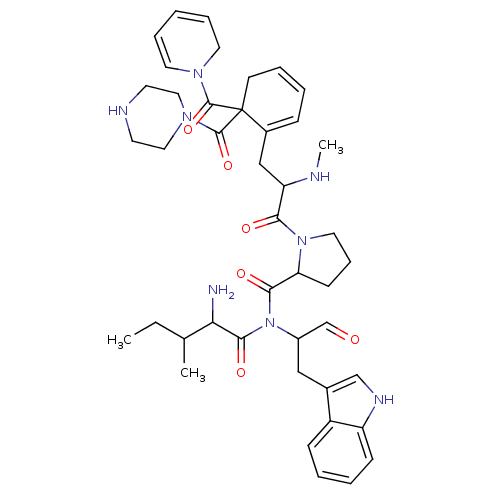
(CAS_3083084 | L-366,811 | NSC_3083084)Show SMILES CCC(C)C(N)C(=O)N(C(Cc1c[nH]c2ccccc12)C=O)C(=O)C1CCCN1C(=O)C(CC1=CC=CCC1(C(=O)N1CCNCC1)C(=O)N1CC=CC=C1)NC |c:38,56,58,t:36| Show InChI InChI=1S/C43H56N8O6/c1-4-29(2)37(44)40(55)51(32(28-52)25-30-27-47-34-15-7-6-14-33(30)34)39(54)36-16-12-22-50(36)38(53)35(45-3)26-31-13-8-9-17-43(31,41(56)48-20-10-5-11-21-48)42(57)49-23-18-46-19-24-49/h5-11,13-15,20,27-29,32,35-37,45-47H,4,12,16-19,21-26,44H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50017721

(1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccccc2-[#6]=[#6]-c2ccccc-12 |c:16| Show InChI InChI=1S/C21H21N/c1-22-14-12-18(13-15-22)21-19-8-4-2-6-16(19)10-11-17-7-3-5-9-20(17)21/h2-11H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- QNB binding at the muscarinic-cholinergic binding site of rat brain S1 |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM81889

(Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-5-[(di...)Show SMILES CCC(C)C1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H]2CC(CN(C)C)CNN2C(=O)[C@H]2CCC=NN2C1=O |c:57| Show InChI InChI=1S/C43H59N9O6/c1-6-28(2)37-43(58)51-34(19-13-21-44-51)42(57)52-36(25-31(26-45-52)27-48(3)4)40(55)49(5)35(24-30-17-11-8-12-18-30)41(56)50-22-14-20-33(50)39(54)46-32(38(53)47-37)23-29-15-9-7-10-16-29/h7-12,15-18,21,28,31-37,45H,6,13-14,19-20,22-27H2,1-5H3,(H,46,54)(H,47,53)/t28?,31?,32-,33+,34+,35-,36+,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81893

(CAS_3083084 | L-366,811 | NSC_3083084)Show SMILES CCC(C)C(N)C(=O)N(C(Cc1c[nH]c2ccccc12)C=O)C(=O)C1CCCN1C(=O)C(CC1=CC=CCC1(C(=O)N1CCNCC1)C(=O)N1CC=CC=C1)NC |c:38,56,58,t:36| Show InChI InChI=1S/C43H56N8O6/c1-4-29(2)37(44)40(55)51(32(28-52)25-30-27-47-34-15-7-6-14-33(30)34)39(54)36-16-12-22-50(36)38(53)35(45-3)26-31-13-8-9-17-43(31,41(56)48-20-10-5-11-21-48)42(57)49-23-18-46-19-24-49/h5-11,13-15,20,27-29,32,35-37,45-47H,4,12,16-19,21-26,44H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81889

(Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-5-[(di...)Show SMILES CCC(C)C1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H]2CC(CN(C)C)CNN2C(=O)[C@H]2CCC=NN2C1=O |c:57| Show InChI InChI=1S/C43H59N9O6/c1-6-28(2)37-43(58)51-34(19-13-21-44-51)42(57)52-36(25-31(26-45-52)27-48(3)4)40(55)49(5)35(24-30-17-11-8-12-18-30)41(56)50-22-14-20-33(50)39(54)46-32(38(53)47-37)23-29-15-9-7-10-16-29/h7-12,15-18,21,28,31-37,45H,6,13-14,19-20,22-27H2,1-5H3,(H,46,54)(H,47,53)/t28?,31?,32-,33+,34+,35-,36+,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM81893

(CAS_3083084 | L-366,811 | NSC_3083084)Show SMILES CCC(C)C(N)C(=O)N(C(Cc1c[nH]c2ccccc12)C=O)C(=O)C1CCCN1C(=O)C(CC1=CC=CCC1(C(=O)N1CCNCC1)C(=O)N1CC=CC=C1)NC |c:38,56,58,t:36| Show InChI InChI=1S/C43H56N8O6/c1-4-29(2)37(44)40(55)51(32(28-52)25-30-27-47-34-15-7-6-14-33(30)34)39(54)36-16-12-22-50(36)38(53)35(45-3)26-31-13-8-9-17-43(31,41(56)48-20-10-5-11-21-48)42(57)49-23-18-46-19-24-49/h5-11,13-15,20,27-29,32,35-37,45-47H,4,12,16-19,21-26,44H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Rhesus) | BDBM81893

(CAS_3083084 | L-366,811 | NSC_3083084)Show SMILES CCC(C)C(N)C(=O)N(C(Cc1c[nH]c2ccccc12)C=O)C(=O)C1CCCN1C(=O)C(CC1=CC=CCC1(C(=O)N1CCNCC1)C(=O)N1CC=CC=C1)NC |c:38,56,58,t:36| Show InChI InChI=1S/C43H56N8O6/c1-4-29(2)37(44)40(55)51(32(28-52)25-30-27-47-34-15-7-6-14-33(30)34)39(54)36-16-12-22-50(36)38(53)35(45-3)26-31-13-8-9-17-43(31,41(56)48-20-10-5-11-21-48)42(57)49-23-18-46-19-24-49/h5-11,13-15,20,27-29,32,35-37,45-47H,4,12,16-19,21-26,44H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM81891

(CAS_188397 | L-366,948 | NSC_188397)Show SMILES CCC(C)C(NC(=O)C(N)Cc1ccc2ccccc2c1)C(=O)C1(CCCN1C(=O)C(N)CC1=NC(N=C1)(C(=O)N1CCCCC1)C(=O)N1CCCCC1)C=O |c:39,t:36| Show InChI InChI=1S/C42H56N8O6/c1-3-28(2)35(46-37(53)33(43)24-29-15-16-30-13-6-7-14-31(30)23-29)36(52)41(27-51)17-12-22-50(41)38(54)34(44)25-32-26-45-42(47-32,39(55)48-18-8-4-9-19-48)40(56)49-20-10-5-11-21-49/h6-7,13-16,23,26-28,33-35H,3-5,8-12,17-22,24-25,43-44H2,1-2H3,(H,46,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81890
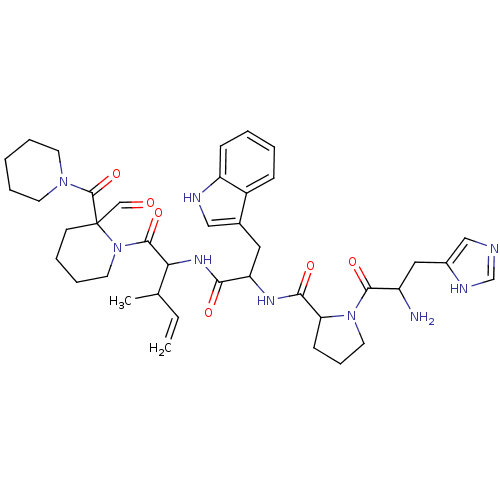
(CAS_196819 | L-366,682 | NSC_196819)Show SMILES CC(C=C)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C1CCCN1C(=O)C(N)Cc1cnc[nH]1)C(=O)N1CCCCC1(C=O)C(=O)N1CCCCC1 Show InChI InChI=1S/C40H53N9O6/c1-3-26(2)34(38(54)49-19-10-7-15-40(49,24-50)39(55)47-16-8-4-9-17-47)46-35(51)32(20-27-22-43-31-13-6-5-12-29(27)31)45-36(52)33-14-11-18-48(33)37(53)30(41)21-28-23-42-25-44-28/h3,5-6,12-13,22-26,30,32-34,43H,1,4,7-11,14-21,41H2,2H3,(H,42,44)(H,45,52)(H,46,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81892
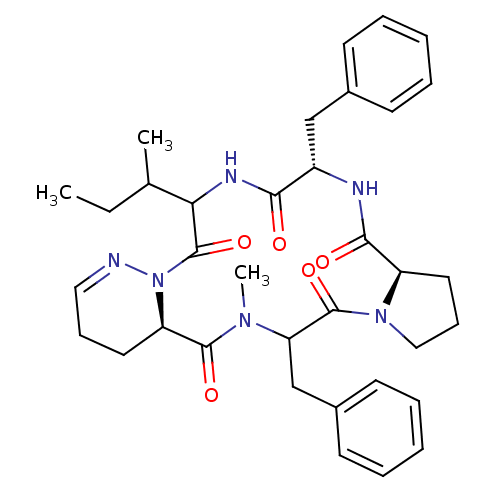
(Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-N-meth...)Show SMILES CCC(C)C1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)C(Cc2ccccc2)N(C)C(=O)[C@H]2CCC=NN2C1=O |c:44| Show InChI InChI=1S/C35H44N6O5/c1-4-23(2)30-35(46)41-28(17-11-19-36-41)33(44)39(3)29(22-25-15-9-6-10-16-25)34(45)40-20-12-18-27(40)32(43)37-26(31(42)38-30)21-24-13-7-5-8-14-24/h5-10,13-16,19,23,26-30H,4,11-12,17-18,20-22H2,1-3H3,(H,37,43)(H,38,42)/t23?,26-,27+,28+,29?,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81889

(Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-5-[(di...)Show SMILES CCC(C)C1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H]2CC(CN(C)C)CNN2C(=O)[C@H]2CCC=NN2C1=O |c:57| Show InChI InChI=1S/C43H59N9O6/c1-6-28(2)37-43(58)51-34(19-13-21-44-51)42(57)52-36(25-31(26-45-52)27-48(3)4)40(55)49(5)35(24-30-17-11-8-12-18-30)41(56)50-22-14-20-33(50)39(54)46-32(38(53)47-37)23-29-15-9-7-10-16-29/h7-12,15-18,21,28,31-37,45H,6,13-14,19-20,22-27H2,1-5H3,(H,46,54)(H,47,53)/t28?,31?,32-,33+,34+,35-,36+,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Rhesus) | BDBM81891

(CAS_188397 | L-366,948 | NSC_188397)Show SMILES CCC(C)C(NC(=O)C(N)Cc1ccc2ccccc2c1)C(=O)C1(CCCN1C(=O)C(N)CC1=NC(N=C1)(C(=O)N1CCCCC1)C(=O)N1CCCCC1)C=O |c:39,t:36| Show InChI InChI=1S/C42H56N8O6/c1-3-28(2)35(46-37(53)33(43)24-29-15-16-30-13-6-7-14-31(30)23-29)36(52)41(27-51)17-12-22-50(41)38(54)34(44)25-32-26-45-42(47-32,39(55)48-18-8-4-9-19-48)40(56)49-20-10-5-11-21-49/h6-7,13-16,23,26-28,33-35H,3-5,8-12,17-22,24-25,43-44H2,1-2H3,(H,46,53) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026964
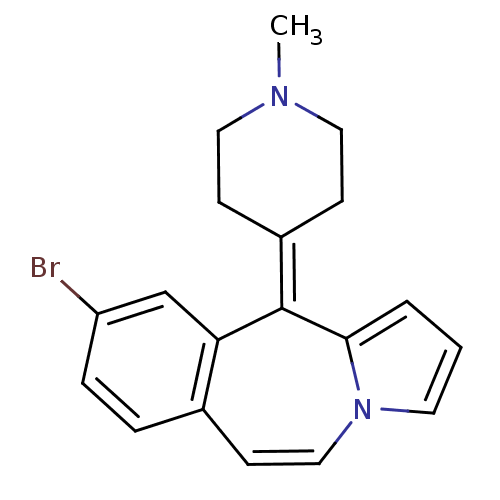
(9-Bromo-11-(1-methyl-piperidin-4-ylidene)-11H-benz...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(Br)cc-12 |c:15| Show InChI InChI=1S/C19H19BrN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- apomorphine radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026968
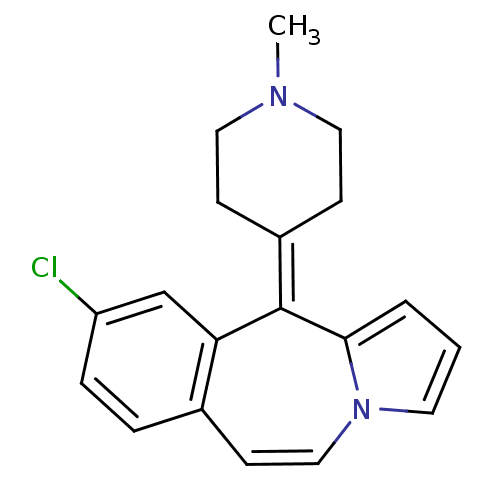
(9-Chloro-11-(1-methyl-piperidin-4-ylidene)-11H-ben...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(Cl)cc-12 |c:15| Show InChI InChI=1S/C19H19ClN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- apomorphine radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026964

(9-Bromo-11-(1-methyl-piperidin-4-ylidene)-11H-benz...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(Br)cc-12 |c:15| Show InChI InChI=1S/C19H19BrN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026974

(9-Bromo-11-(1-methyl-piperidin-4-ylidene)-6,11-dih...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]-[#6]-c2ccc(Br)cc-12 Show InChI InChI=1S/C19H21BrN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,9,13H,6-8,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- apomorphine radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81895
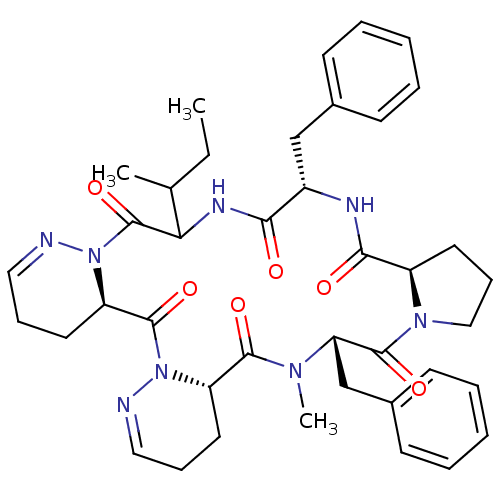
(Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-1,6-di...)Show SMILES CCC(C)C1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@@H]2CCC=NN2C(=O)[C@H]2CCC=NN2C1=O |wU:8.8,46.55,wD:26.28,19.24,38.46,c:44,53,(3.12,-16.88,;2.34,-15.35,;.78,-14.1,;.78,-15.49,;.62,-12.72,;.52,-11.33,;.52,-9.95,;1.56,-11.19,;.78,-8.56,;1.56,-9.81,;2.6,-10.95,;1.87,-12.58,;3.9,-13.72,;5.46,-15.25,;5.46,-13.86,;3.12,-12.34,;.52,-7.17,;1.04,-5.65,;1.56,-7.03,;1.56,-4.26,;1.73,-5.41,;.35,-5.79,;.52,-4.4,;.62,-3.01,;.78,-1.63,;1.87,-2.88,;3.12,,;3.9,-1.25,;5.61,-3.01,;4.68,-4.4,;5.2,-5.54,;5.72,-7.03,;5.89,-5.79,;5.72,-4.26,;2.34,-1.49,;4.37,-2.77,;3.12,-2.63,;3.64,-4.16,;2.6,-4.02,;3.81,-5.65,;4.68,-7.17,;5.46,-8.32,;3.64,-6.93,;3.12,-5.79,;2.6,-6.79,;3.9,-8.18,;2.34,-8.42,;4.68,-9.95,;5.72,-11.19,;5.61,-12.72,;4.68,-11.33,;3.64,-9.7,;3.64,-11.09,;4.37,-12.48,)| Show InChI InChI=1S/C40H50N8O6/c1-4-26(2)34-40(54)48-32(19-12-22-42-48)39(53)47-31(18-11-21-41-47)37(51)45(3)33(25-28-16-9-6-10-17-28)38(52)46-23-13-20-30(46)36(50)43-29(35(49)44-34)24-27-14-7-5-8-15-27/h5-10,14-17,21-22,26,29-34H,4,11-13,18-20,23-25H2,1-3H3,(H,43,50)(H,44,49)/t26?,29-,30+,31-,32+,33-,34?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026968

(9-Chloro-11-(1-methyl-piperidin-4-ylidene)-11H-ben...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(Cl)cc-12 |c:15| Show InChI InChI=1S/C19H19ClN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026974

(9-Bromo-11-(1-methyl-piperidin-4-ylidene)-6,11-dih...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]-[#6]-c2ccc(Br)cc-12 Show InChI InChI=1S/C19H21BrN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,9,13H,6-8,10-12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Rhesus) | BDBM81889

(Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-5-[(di...)Show SMILES CCC(C)C1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H]2CC(CN(C)C)CNN2C(=O)[C@H]2CCC=NN2C1=O |c:57| Show InChI InChI=1S/C43H59N9O6/c1-6-28(2)37-43(58)51-34(19-13-21-44-51)42(57)52-36(25-31(26-45-52)27-48(3)4)40(55)49(5)35(24-30-17-11-8-12-18-30)41(56)50-22-14-20-33(50)39(54)46-32(38(53)47-37)23-29-15-9-7-10-16-29/h7-12,15-18,21,28,31-37,45H,6,13-14,19-20,22-27H2,1-5H3,(H,46,54)(H,47,53)/t28?,31?,32-,33+,34+,35-,36+,37?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026970
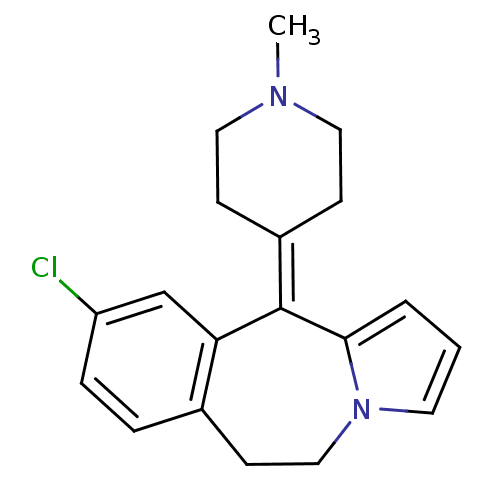
(9-Chloro-11-(1-methyl-piperidin-4-ylidene)-6,11-di...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]-[#6]-c2ccc(Cl)cc-12 Show InChI InChI=1S/C19H21ClN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,9,13H,6-8,10-12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026964

(9-Bromo-11-(1-methyl-piperidin-4-ylidene)-11H-benz...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(Br)cc-12 |c:15| Show InChI InChI=1S/C19H19BrN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM81890

(CAS_196819 | L-366,682 | NSC_196819)Show SMILES CC(C=C)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C1CCCN1C(=O)C(N)Cc1cnc[nH]1)C(=O)N1CCCCC1(C=O)C(=O)N1CCCCC1 Show InChI InChI=1S/C40H53N9O6/c1-3-26(2)34(38(54)49-19-10-7-15-40(49,24-50)39(55)47-16-8-4-9-17-47)46-35(51)32(20-27-22-43-31-13-6-5-12-29(27)31)45-36(52)33-14-11-18-48(33)37(53)30(41)21-28-23-42-25-44-28/h3,5-6,12-13,22-26,30,32-34,43H,1,4,7-11,14-21,41H2,2H3,(H,42,44)(H,45,52)(H,46,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81890

(CAS_196819 | L-366,682 | NSC_196819)Show SMILES CC(C=C)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C1CCCN1C(=O)C(N)Cc1cnc[nH]1)C(=O)N1CCCCC1(C=O)C(=O)N1CCCCC1 Show InChI InChI=1S/C40H53N9O6/c1-3-26(2)34(38(54)49-19-10-7-15-40(49,24-50)39(55)47-16-8-4-9-17-47)46-35(51)32(20-27-22-43-31-13-6-5-12-29(27)31)45-36(52)33-14-11-18-48(33)37(53)30(41)21-28-23-42-25-44-28/h3,5-6,12-13,22-26,30,32-34,43H,1,4,7-11,14-21,41H2,2H3,(H,42,44)(H,45,52)(H,46,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026968

(9-Chloro-11-(1-methyl-piperidin-4-ylidene)-11H-ben...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(Cl)cc-12 |c:15| Show InChI InChI=1S/C19H19ClN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026970

(9-Chloro-11-(1-methyl-piperidin-4-ylidene)-6,11-di...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]-[#6]-c2ccc(Cl)cc-12 Show InChI InChI=1S/C19H21ClN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,9,13H,6-8,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- apomorphine radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026974

(9-Bromo-11-(1-methyl-piperidin-4-ylidene)-6,11-dih...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]-[#6]-c2ccc(Br)cc-12 Show InChI InChI=1S/C19H21BrN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,9,13H,6-8,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81895

(Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-1,6-di...)Show SMILES CCC(C)C1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@@H]2CCC=NN2C(=O)[C@H]2CCC=NN2C1=O |wU:8.8,46.55,wD:26.28,19.24,38.46,c:44,53,(3.12,-16.88,;2.34,-15.35,;.78,-14.1,;.78,-15.49,;.62,-12.72,;.52,-11.33,;.52,-9.95,;1.56,-11.19,;.78,-8.56,;1.56,-9.81,;2.6,-10.95,;1.87,-12.58,;3.9,-13.72,;5.46,-15.25,;5.46,-13.86,;3.12,-12.34,;.52,-7.17,;1.04,-5.65,;1.56,-7.03,;1.56,-4.26,;1.73,-5.41,;.35,-5.79,;.52,-4.4,;.62,-3.01,;.78,-1.63,;1.87,-2.88,;3.12,,;3.9,-1.25,;5.61,-3.01,;4.68,-4.4,;5.2,-5.54,;5.72,-7.03,;5.89,-5.79,;5.72,-4.26,;2.34,-1.49,;4.37,-2.77,;3.12,-2.63,;3.64,-4.16,;2.6,-4.02,;3.81,-5.65,;4.68,-7.17,;5.46,-8.32,;3.64,-6.93,;3.12,-5.79,;2.6,-6.79,;3.9,-8.18,;2.34,-8.42,;4.68,-9.95,;5.72,-11.19,;5.61,-12.72,;4.68,-11.33,;3.64,-9.7,;3.64,-11.09,;4.37,-12.48,)| Show InChI InChI=1S/C40H50N8O6/c1-4-26(2)34-40(54)48-32(19-12-22-42-48)39(53)47-31(18-11-21-41-47)37(51)45(3)33(25-28-16-9-6-10-17-28)38(52)46-23-13-20-30(46)36(50)43-29(35(49)44-34)24-27-14-7-5-8-15-27/h5-10,14-17,21-22,26,29-34H,4,11-13,18-20,23-25H2,1-3H3,(H,43,50)(H,44,49)/t26?,29-,30+,31-,32+,33-,34?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026973
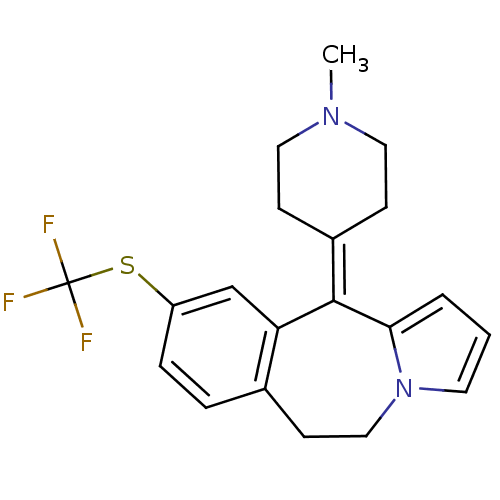
(11-(1-Methyl-piperidin-4-ylidene)-9-trifluoromethy...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]-[#6]-c2ccc(-[#16]C(F)(F)F)cc-12 Show InChI InChI=1S/C20H21F3N2S/c1-24-10-6-15(7-11-24)19-17-13-16(26-20(21,22)23)5-4-14(17)8-12-25-9-2-3-18(19)25/h2-5,9,13H,6-8,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Rhesus) | BDBM81895

(Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-1,6-di...)Show SMILES CCC(C)C1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@@H]2CCC=NN2C(=O)[C@H]2CCC=NN2C1=O |wU:8.8,46.55,wD:26.28,19.24,38.46,c:44,53,(3.12,-16.88,;2.34,-15.35,;.78,-14.1,;.78,-15.49,;.62,-12.72,;.52,-11.33,;.52,-9.95,;1.56,-11.19,;.78,-8.56,;1.56,-9.81,;2.6,-10.95,;1.87,-12.58,;3.9,-13.72,;5.46,-15.25,;5.46,-13.86,;3.12,-12.34,;.52,-7.17,;1.04,-5.65,;1.56,-7.03,;1.56,-4.26,;1.73,-5.41,;.35,-5.79,;.52,-4.4,;.62,-3.01,;.78,-1.63,;1.87,-2.88,;3.12,,;3.9,-1.25,;5.61,-3.01,;4.68,-4.4,;5.2,-5.54,;5.72,-7.03,;5.89,-5.79,;5.72,-4.26,;2.34,-1.49,;4.37,-2.77,;3.12,-2.63,;3.64,-4.16,;2.6,-4.02,;3.81,-5.65,;4.68,-7.17,;5.46,-8.32,;3.64,-6.93,;3.12,-5.79,;2.6,-6.79,;3.9,-8.18,;2.34,-8.42,;4.68,-9.95,;5.72,-11.19,;5.61,-12.72,;4.68,-11.33,;3.64,-9.7,;3.64,-11.09,;4.37,-12.48,)| Show InChI InChI=1S/C40H50N8O6/c1-4-26(2)34-40(54)48-32(19-12-22-42-48)39(53)47-31(18-11-21-41-47)37(51)45(3)33(25-28-16-9-6-10-17-28)38(52)46-23-13-20-30(46)36(50)43-29(35(49)44-34)24-27-14-7-5-8-15-27/h5-10,14-17,21-22,26,29-34H,4,11-13,18-20,23-25H2,1-3H3,(H,43,50)(H,44,49)/t26?,29-,30+,31-,32+,33-,34?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026971
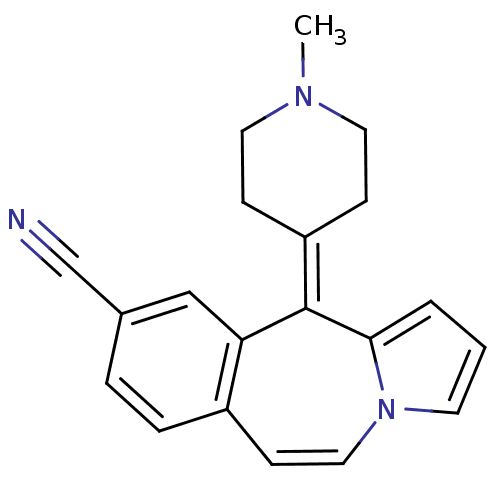
(11-(1-Methyl-piperidin-4-ylidene)-11H-benzo[d]pyrr...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2cccn2-[#6]=[#6]-c2ccc(cc-12)C#N |c:15| Show InChI InChI=1S/C20H19N3/c1-22-10-6-17(7-11-22)20-18-13-15(14-21)4-5-16(18)8-12-23-9-2-3-19(20)23/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026972
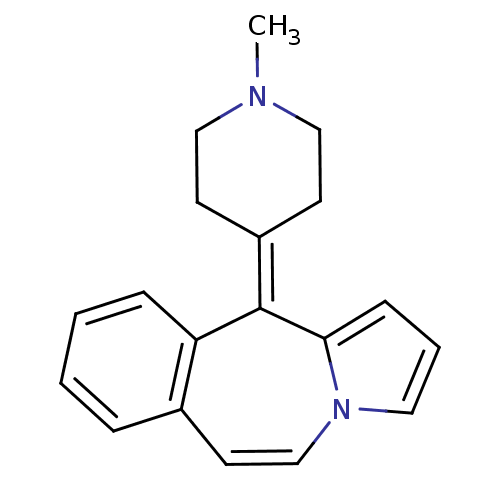
(11-(1-Methyl-piperidin-4-ylidene)-11H-benzo[d]pyrr...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccccc-12 |c:15| Show InChI InChI=1S/C19H20N2/c1-20-12-8-16(9-13-20)19-17-6-3-2-5-15(17)10-14-21-11-4-7-18(19)21/h2-7,10-11,14H,8-9,12-13H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50026967
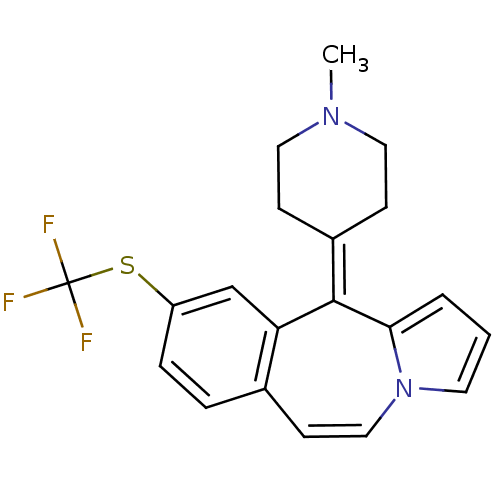
(11-(1-Methyl-piperidin-4-ylidene)-9-trifluoromethy...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(-[#16]C(F)(F)F)cc-12 |c:15| Show InChI InChI=1S/C20H19F3N2S/c1-24-10-6-15(7-11-24)19-17-13-16(26-20(21,22)23)5-4-14(17)8-12-25-9-2-3-18(19)25/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- prazosin radioligand binding at the alpha 1-adrenergic binding site of calf cerebral cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81892

(Cyclo[L-Pro-D-Phe-L-Ile-1,6-didehydro-D-Pyz-N-meth...)Show SMILES CCC(C)C1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)C(Cc2ccccc2)N(C)C(=O)[C@H]2CCC=NN2C1=O |c:44| Show InChI InChI=1S/C35H44N6O5/c1-4-23(2)30-35(46)41-28(17-11-19-36-41)33(44)39(3)29(22-25-15-9-6-10-16-25)34(45)40-20-12-18-27(40)32(43)37-26(31(42)38-30)21-24-13-7-5-8-14-24/h5-10,13-16,19,23,26-30H,4,11-12,17-18,20-22H2,1-3H3,(H,37,43)(H,38,42)/t23?,26-,27+,28+,29?,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026963
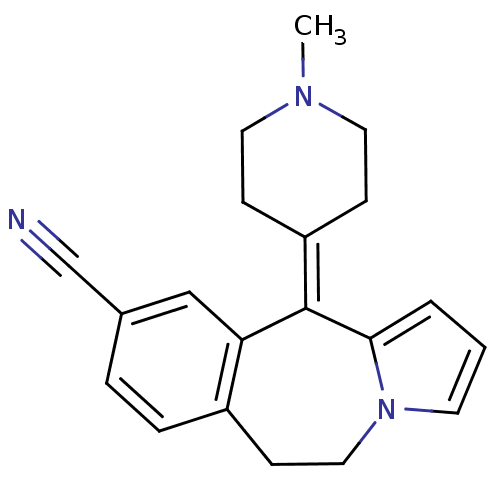
(11-(1-Methyl-piperidin-4-ylidene)-6,11-dihydro-5H-...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2cccn2-[#6]-[#6]-c2ccc(cc-12)C#N Show InChI InChI=1S/C20H21N3/c1-22-10-6-17(7-11-22)20-18-13-15(14-21)4-5-16(18)8-12-23-9-2-3-19(20)23/h2-5,9,13H,6-8,10-12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50026972

(11-(1-Methyl-piperidin-4-ylidene)-11H-benzo[d]pyrr...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccccc-12 |c:15| Show InChI InChI=1S/C19H20N2/c1-20-12-8-16(9-13-20)19-17-6-3-2-5-15(17)10-14-21-11-4-7-18(19)21/h2-7,10-11,14H,8-9,12-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- QNB binding at the muscarinic-cholinergic binding site of rat brain S1 |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026963

(11-(1-Methyl-piperidin-4-ylidene)-6,11-dihydro-5H-...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2cccn2-[#6]-[#6]-c2ccc(cc-12)C#N Show InChI InChI=1S/C20H21N3/c1-22-10-6-17(7-11-22)20-18-13-15(14-21)4-5-16(18)8-12-23-9-2-3-19(20)23/h2-5,9,13H,6-8,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- apomorphine radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026967

(11-(1-Methyl-piperidin-4-ylidene)-9-trifluoromethy...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(-[#16]C(F)(F)F)cc-12 |c:15| Show InChI InChI=1S/C20H19F3N2S/c1-24-10-6-15(7-11-24)19-17-13-16(26-20(21,22)23)5-4-14(17)8-12-25-9-2-3-18(19)25/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Rhesus) | BDBM81890

(CAS_196819 | L-366,682 | NSC_196819)Show SMILES CC(C=C)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C1CCCN1C(=O)C(N)Cc1cnc[nH]1)C(=O)N1CCCCC1(C=O)C(=O)N1CCCCC1 Show InChI InChI=1S/C40H53N9O6/c1-3-26(2)34(38(54)49-19-10-7-15-40(49,24-50)39(55)47-16-8-4-9-17-47)46-35(51)32(20-27-22-43-31-13-6-5-12-29(27)31)45-36(52)33-14-11-18-48(33)37(53)30(41)21-28-23-42-25-44-28/h3,5-6,12-13,22-26,30,32-34,43H,1,4,7-11,14-21,41H2,2H3,(H,42,44)(H,45,52)(H,46,51) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026973

(11-(1-Methyl-piperidin-4-ylidene)-9-trifluoromethy...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]-[#6]-c2ccc(-[#16]C(F)(F)F)cc-12 Show InChI InChI=1S/C20H21F3N2S/c1-24-10-6-15(7-11-24)19-17-13-16(26-20(21,22)23)5-4-14(17)8-12-25-9-2-3-18(19)25/h2-5,9,13H,6-8,10-12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50026964

(9-Bromo-11-(1-methyl-piperidin-4-ylidene)-11H-benz...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(Br)cc-12 |c:15| Show InChI InChI=1S/C19H19BrN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- QNB binding at the muscarinic-cholinergic binding site of rat brain S1 |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50026964

(9-Bromo-11-(1-methyl-piperidin-4-ylidene)-11H-benz...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2cccn2-[#6]=[#6]-c2ccc(Br)cc-12 |c:15| Show InChI InChI=1S/C19H19BrN2/c1-21-10-6-15(7-11-21)19-17-13-16(20)5-4-14(17)8-12-22-9-2-3-18(19)22/h2-5,8-9,12-13H,6-7,10-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]- prazosin radioligand binding at the alpha 1-adrenergic binding site of calf cerebral cortex |
J Med Chem 26: 974-80 (1983)
BindingDB Entry DOI: 10.7270/Q2DR2W2B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data