 Found 379 hits with Last Name = 'trejo-martin' and Initial = 'a'
Found 379 hits with Last Name = 'trejo-martin' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310731
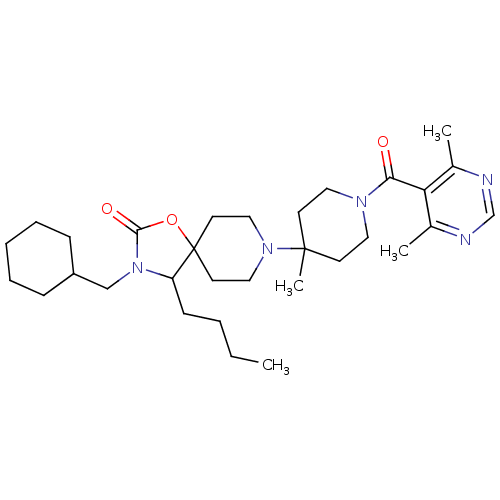
(4-butyl-3-(cyclohexylmethyl)-8-(1-(4,6-dimethylpyr...)Show SMILES CCCCC1N(CC2CCCCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C Show InChI InChI=1S/C31H49N5O3/c1-5-6-12-26-31(39-29(38)36(26)21-25-10-8-7-9-11-25)15-19-35(20-16-31)30(4)13-17-34(18-14-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50440019
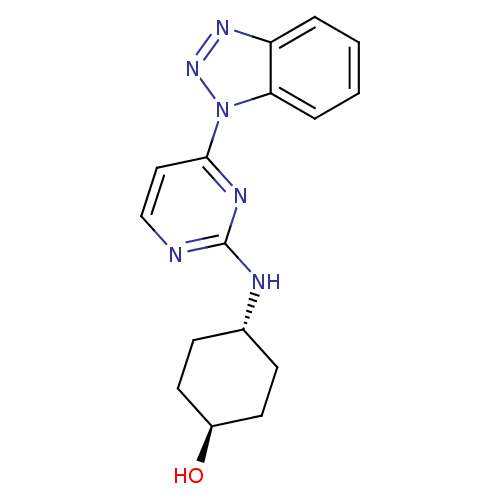
(CHEMBL2425654)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1nnc2ccccc12 |r,wU:4.7,wD:1.0,(2.77,-16.29,;4.12,-17.05,;4.13,-18.6,;5.48,-19.36,;6.8,-18.58,;6.79,-17.04,;5.47,-16.28,;8.15,-19.35,;9.49,-18.57,;9.49,-17.03,;10.83,-16.26,;12.15,-17.02,;12.16,-18.57,;10.83,-19.34,;13.49,-19.34,;14.91,-18.78,;15.89,-19.97,;15.06,-21.27,;15.46,-22.75,;14.39,-23.83,;12.88,-23.42,;12.49,-21.97,;13.58,-20.87,)| Show InChI InChI=1S/C16H18N6O/c23-12-7-5-11(6-8-12)18-16-17-10-9-15(19-16)22-14-4-2-1-3-13(14)20-21-22/h1-4,9-12,23H,5-8H2,(H,17,18,19)/t11-,12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310747
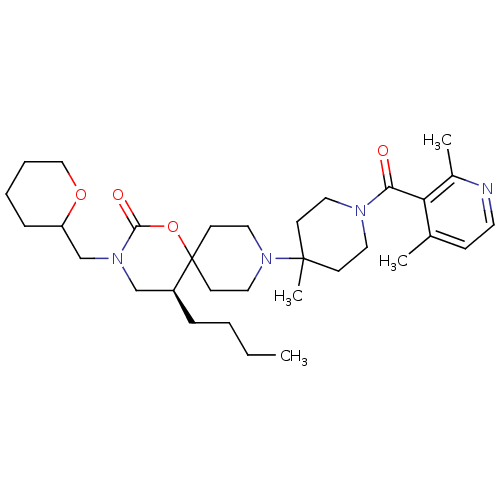
((5S)-5-butyl-9-(1-(2,4-dimethylnicotinoyl)-4-methy...)Show SMILES CCCC[C@H]1CN(CC2CCCCO2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ccnc1C |r| Show InChI InChI=1S/C32H50N4O4/c1-5-6-9-26-22-35(23-27-10-7-8-21-39-27)30(38)40-32(26)14-19-36(20-15-32)31(4)12-17-34(18-13-31)29(37)28-24(2)11-16-33-25(28)3/h11,16,26-27H,5-10,12-15,17-23H2,1-4H3/t26-,27?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310730
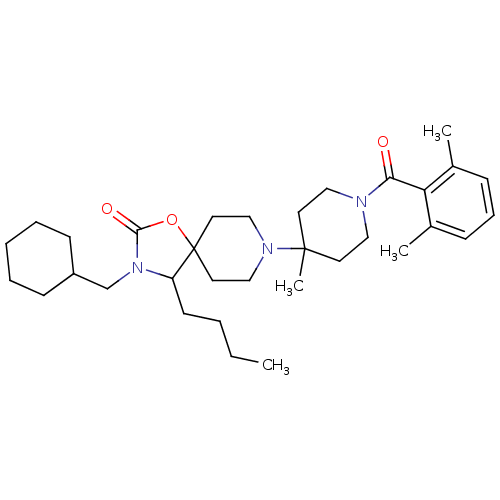
(4-butyl-3-(cyclohexylmethyl)-8-(1-(2,6-dimethylben...)Show SMILES CCCCC1N(CC2CCCCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C33H51N3O3/c1-5-6-15-28-33(39-31(38)36(28)24-27-13-8-7-9-14-27)18-22-35(23-19-33)32(4)16-20-34(21-17-32)30(37)29-25(2)11-10-12-26(29)3/h10-12,27-28H,5-9,13-24H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310744
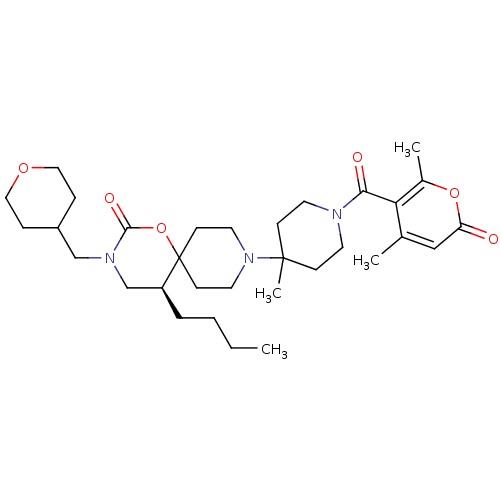
((S)-5-butyl-9-(1-(4,6-dimethyl-2-oxo-2H-pyran-5-ca...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)cc(=O)oc1C |r| Show InChI InChI=1S/C32H49N3O6/c1-5-6-7-26-22-34(21-25-8-18-39-19-9-25)30(38)41-32(26)12-16-35(17-13-32)31(4)10-14-33(15-11-31)29(37)28-23(2)20-27(36)40-24(28)3/h20,25-26H,5-19,21-22H2,1-4H3/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310726
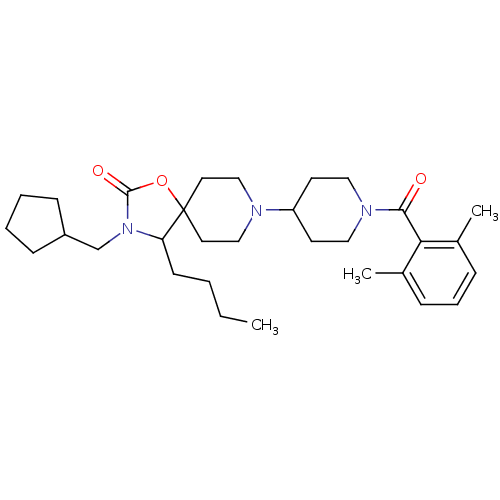
(4-butyl-3-(cyclopentylmethyl)-8-(1-(2,6-dimethylbe...)Show SMILES CCCCC1N(CC2CCCC2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C31H47N3O3/c1-4-5-13-27-31(37-30(36)34(27)22-25-11-6-7-12-25)16-20-32(21-17-31)26-14-18-33(19-15-26)29(35)28-23(2)9-8-10-24(28)3/h8-10,25-27H,4-7,11-22H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310743

((S)-5-(4-(5-butyl-3-methyl-2-oxo-1-oxa-3,9-diazasp...)Show SMILES CCCC[C@H]1CN(C)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)cc(nc1C)C#N |r| Show InChI InChI=1S/C28H41N5O3/c1-6-7-8-22-19-31(5)26(35)36-28(22)11-15-33(16-12-28)27(4)9-13-32(14-10-27)25(34)24-20(2)17-23(18-29)30-21(24)3/h17,22H,6-16,19H2,1-5H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310727
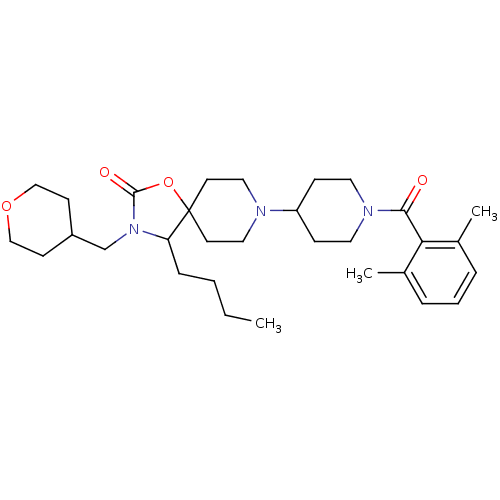
(4-butyl-8-(1-(2,6-dimethylbenzoyl)piperidin-4-yl)-...)Show SMILES CCCCC1N(CC2CCOCC2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C31H47N3O4/c1-4-5-9-27-31(38-30(36)34(27)22-25-12-20-37-21-13-25)14-18-32(19-15-31)26-10-16-33(17-11-26)29(35)28-23(2)7-6-8-24(28)3/h6-8,25-27H,4-5,9-22H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310742
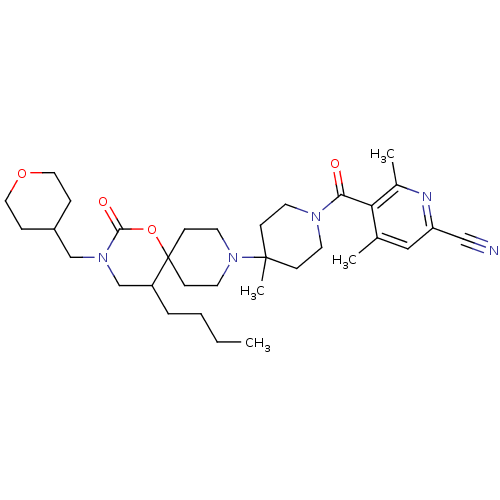
((+/-)-5-(4-(5-butyl-2-oxo-3-((tetrahydro-2H-pyran-...)Show SMILES CCCCC1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)cc(nc1C)C#N Show InChI InChI=1S/C33H49N5O4/c1-5-6-7-27-23-37(22-26-8-18-41-19-9-26)31(40)42-33(27)12-16-38(17-13-33)32(4)10-14-36(15-11-32)30(39)29-24(2)20-28(21-34)35-25(29)3/h20,26-27H,5-19,22-23H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310739
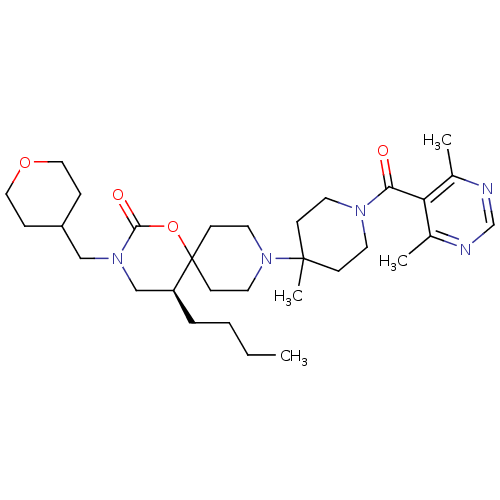
((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310737
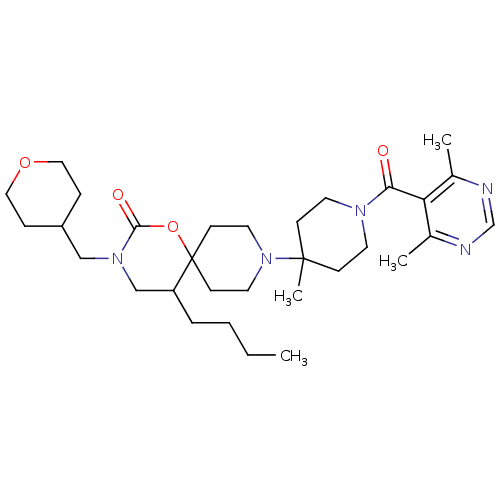
(5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbonyl)-4...)Show SMILES CCCCC1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310746
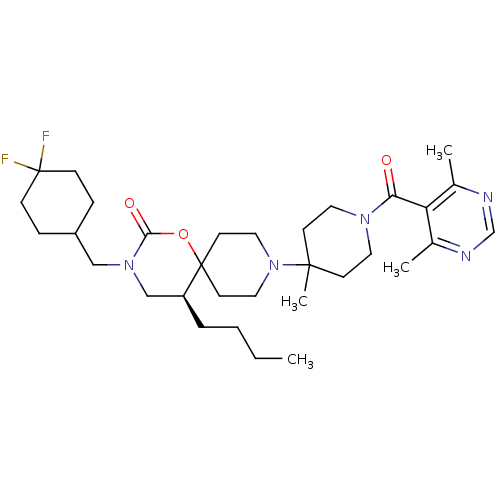
((5S)-5-butyl-3-((4,4-difluorocyclohexyl)methyl)-9-...)Show SMILES CCCC[C@H]1CN(CC2CCC(F)(F)CC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C32H49F2N5O3/c1-5-6-7-26-21-38(20-25-8-10-32(33,34)11-9-25)29(41)42-31(26)14-18-39(19-15-31)30(4)12-16-37(17-13-30)28(40)27-23(2)35-22-36-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310733
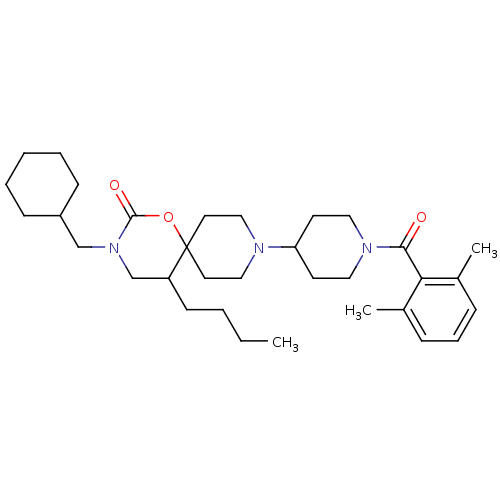
(5-butyl-3-(cyclohexylmethyl)-9-(1-(2,6-dimethylben...)Show SMILES CCCCC1CN(CC2CCCCC2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C33H51N3O3/c1-4-5-14-28-24-36(23-27-12-7-6-8-13-27)32(38)39-33(28)17-21-34(22-18-33)29-15-19-35(20-16-29)31(37)30-25(2)10-9-11-26(30)3/h9-11,27-29H,4-8,12-24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50440017
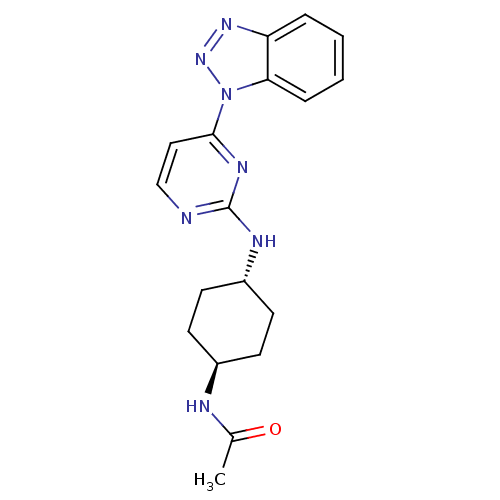
(CHEMBL2425651)Show SMILES CC(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1nnc2ccccc12 |r,wU:7.10,wD:4.3,(22.14,-19.72,;22.13,-18.18,;20.79,-17.42,;23.46,-17.4,;24.81,-18.17,;24.83,-19.72,;26.17,-20.47,;27.5,-19.7,;27.49,-18.16,;26.16,-17.39,;28.85,-20.47,;30.19,-19.68,;30.19,-18.14,;31.53,-17.37,;32.86,-18.13,;32.86,-19.68,;31.53,-20.45,;34.2,-20.45,;35.62,-19.9,;36.6,-21.09,;35.77,-22.38,;36.17,-23.87,;35.1,-24.95,;33.59,-24.54,;33.19,-23.09,;34.28,-21.99,)| Show InChI InChI=1S/C18H21N7O/c1-12(26)20-13-6-8-14(9-7-13)21-18-19-11-10-17(22-18)25-16-5-3-2-4-15(16)23-24-25/h2-5,10-11,13-14H,6-9H2,1H3,(H,20,26)(H,19,21,22)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310724
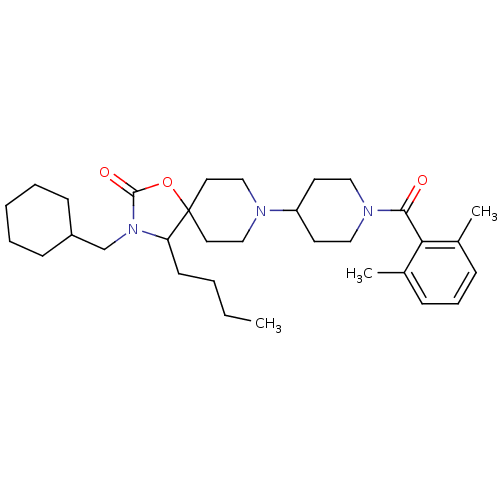
(4-butyl-3-(cyclohexylmethyl)-8-(1-(2,6-dimethylben...)Show SMILES CCCCC1N(CC2CCCCC2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C32H49N3O3/c1-4-5-14-28-32(38-31(37)35(28)23-26-12-7-6-8-13-26)17-21-33(22-18-32)27-15-19-34(20-16-27)30(36)29-24(2)10-9-11-25(29)3/h9-11,26-28H,4-8,12-23H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310732
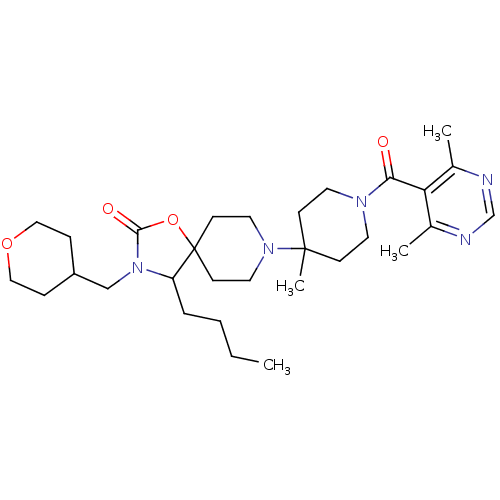
(4-butyl-8-(1-(4,6-dimethylpyrimidine-5-carbonyl)-4...)Show SMILES CCCCC1N(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C Show InChI InChI=1S/C30H47N5O4/c1-5-6-7-25-30(39-28(37)35(25)20-24-8-18-38-19-9-24)12-16-34(17-13-30)29(4)10-14-33(15-11-29)27(36)26-22(2)31-21-32-23(26)3/h21,24-25H,5-20H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310740
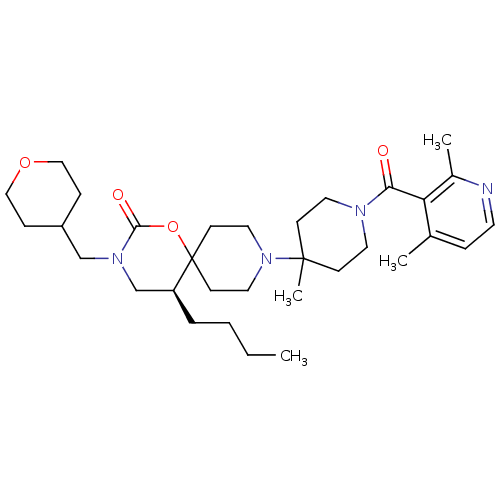
((S)-5-butyl-9-(1-(2,4-dimethylnicotinoyl)-4-methyl...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ccnc1C |r| Show InChI InChI=1S/C32H50N4O4/c1-5-6-7-27-23-35(22-26-9-20-39-21-10-26)30(38)40-32(27)13-18-36(19-14-32)31(4)11-16-34(17-12-31)29(37)28-24(2)8-15-33-25(28)3/h8,15,26-27H,5-7,9-14,16-23H2,1-4H3/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310734
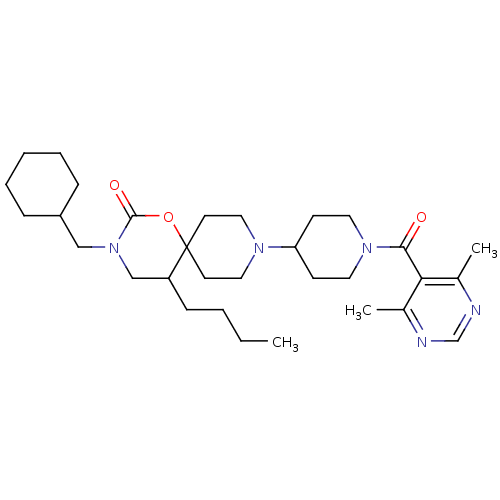
(5-butyl-3-(cyclohexylmethyl)-9-(1-(4,6-dimethylpyr...)Show SMILES CCCCC1CN(CC2CCCCC2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1c(C)ncnc1C Show InChI InChI=1S/C31H49N5O3/c1-4-5-11-26-21-36(20-25-9-7-6-8-10-25)30(38)39-31(26)14-18-34(19-15-31)27-12-16-35(17-13-27)29(37)28-23(2)32-22-33-24(28)3/h22,25-27H,4-21H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310736
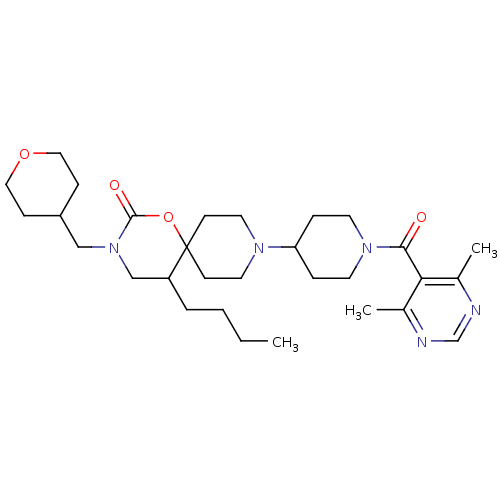
(5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbonyl)pi...)Show SMILES CCCCC1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1c(C)ncnc1C Show InChI InChI=1S/C30H47N5O4/c1-4-5-6-25-20-35(19-24-9-17-38-18-10-24)29(37)39-30(25)11-15-33(16-12-30)26-7-13-34(14-8-26)28(36)27-22(2)31-21-32-23(27)3/h21,24-26H,4-20H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310741

((S)-3-(4-(5-butyl-2-oxo-3-((tetrahydro-2H-pyran-4-...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)cc[n+]([O-])c1C |r| Show InChI InChI=1S/C32H50N4O5/c1-5-6-7-27-23-34(22-26-9-20-40-21-10-26)30(38)41-32(27)13-18-35(19-14-32)31(4)11-16-33(17-12-31)29(37)28-24(2)8-15-36(39)25(28)3/h8,15,26-27H,5-7,9-14,16-23H2,1-4H3/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310735
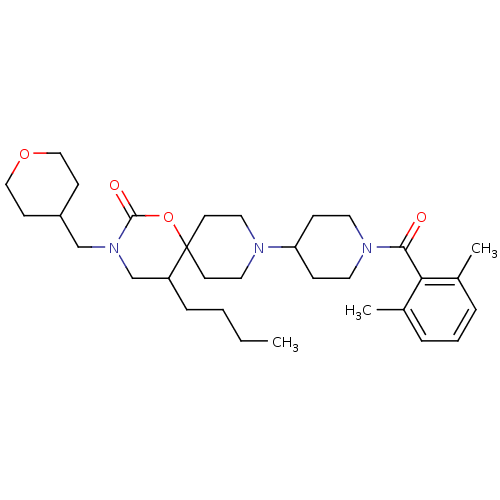
(5-butyl-9-(1-(2,6-dimethylbenzoyl)piperidin-4-yl)-...)Show SMILES CCCCC1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C32H49N3O4/c1-4-5-9-27-23-35(22-26-12-20-38-21-13-26)31(37)39-32(27)14-18-33(19-15-32)28-10-16-34(17-11-28)30(36)29-24(2)7-6-8-25(29)3/h6-8,26-28H,4-5,9-23H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310745

((S)-5-butyl-9-(1-(4,6-dimethyl-2-(trifluoromethyl)...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)nc(nc1C)C(F)(F)F |r| Show InChI InChI=1S/C32H48F3N5O4/c1-5-6-7-25-21-39(20-24-8-18-43-19-9-24)29(42)44-31(25)12-16-40(17-13-31)30(4)10-14-38(15-11-30)27(41)26-22(2)36-28(32(33,34)35)37-23(26)3/h24-25H,5-21H2,1-4H3/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310738
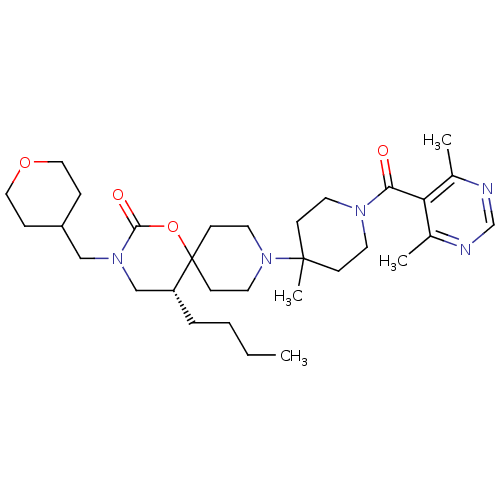
((R)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310725
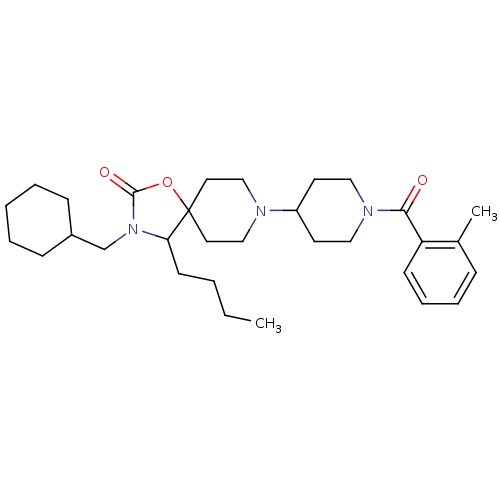
(4-butyl-3-(cyclohexylmethyl)-8-(1-(2-methylbenzoyl...)Show SMILES CCCCC1N(CC2CCCCC2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1ccccc1C Show InChI InChI=1S/C31H47N3O3/c1-3-4-14-28-31(37-30(36)34(28)23-25-11-6-5-7-12-25)17-21-32(22-18-31)26-15-19-33(20-16-26)29(35)27-13-9-8-10-24(27)2/h8-10,13,25-26,28H,3-7,11-12,14-23H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310728

(3-benzyl-4-butyl-8-(1-(2,6-dimethylbenzoyl)piperid...)Show SMILES CCCCC1N(Cc2ccccc2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C32H43N3O3/c1-4-5-14-28-32(38-31(37)35(28)23-26-12-7-6-8-13-26)17-21-33(22-18-32)27-15-19-34(20-16-27)30(36)29-24(2)10-9-11-25(29)3/h6-13,27-28H,4-5,14-23H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310729

(3-(cyclohexylmethyl)-8-(1-(2,6-dimethylbenzoyl)pip...)Show SMILES CCCC1N(CC2CCCCC2)C(=O)OC11CCN(CC1)C1CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C31H47N3O3/c1-4-9-27-31(37-30(36)34(27)22-25-12-6-5-7-13-25)16-20-32(21-17-31)26-14-18-33(19-15-26)29(35)28-23(2)10-8-11-24(28)3/h8,10-11,25-27H,4-7,9,12-22H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50440020
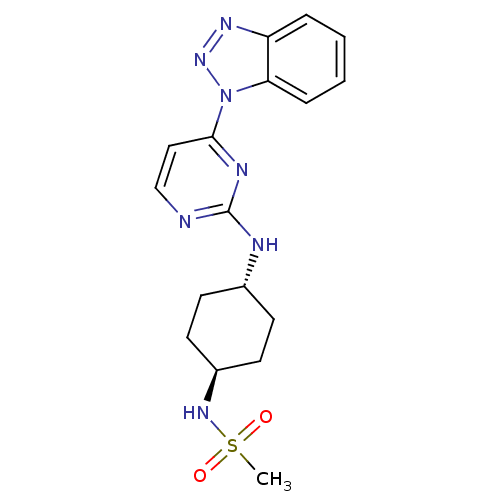
(CHEMBL2425655)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1nnc2ccccc12 |r,wU:8.11,wD:5.4,(4.67,-9.66,;3.88,-8.32,;3.09,-6.98,;2.54,-9.1,;5.22,-7.53,;6.57,-8.3,;6.58,-9.85,;7.92,-10.61,;9.25,-9.83,;9.24,-8.29,;7.92,-7.53,;10.6,-10.6,;11.94,-9.82,;11.94,-8.28,;13.27,-7.51,;14.6,-8.27,;14.6,-9.82,;13.28,-10.59,;15.94,-10.58,;17.36,-10.03,;18.33,-11.22,;17.5,-12.52,;17.91,-14,;16.83,-15.08,;15.33,-14.67,;14.94,-13.22,;16.02,-12.12,)| Show InChI InChI=1S/C17H21N7O2S/c1-27(25,26)22-13-8-6-12(7-9-13)19-17-18-11-10-16(20-17)24-15-5-3-2-4-14(15)21-23-24/h2-5,10-13,22H,6-9H2,1H3,(H,18,19,20)/t12-,13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant human CDK1/GST-tagged cyclin B expressed in baculovirus infected insect cells assessed as inhibition of 6XHis-tagged ... |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50310739

((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50310739

((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR4 |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50310739

((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR4 receptor |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50310739

((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 receptor |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50310739

((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR2b receptor |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50310739

((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 receptor |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 6
(Homo sapiens (Human)) | BDBM50310739

((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR6 receptor |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50440018
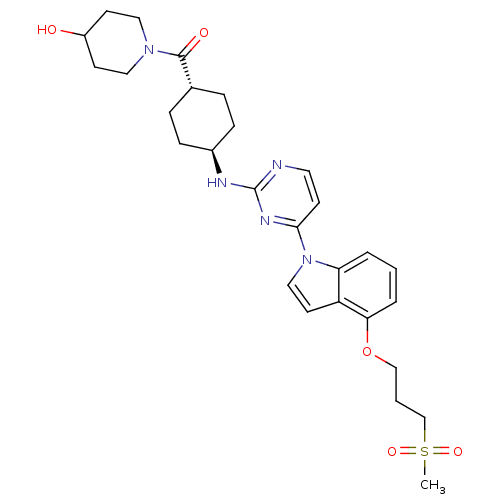
(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to TTK (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 16
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to STK16 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
NUAK family SNF1-like kinase 2
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to SNARK (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase family member 4
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to SgK085 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to RPS6KA1 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Interferon-induced, double-stranded RNA-activated protein kinase
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to PRKR (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to PDGFRB (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek7
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to NEK7 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek5
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to NEK5 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek1
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to NEK1 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to MKNK1 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 6
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to MEK6 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 4
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to MEK4 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to LIMK2 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to LIMK1 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 15
(Homo sapiens (Human)) | BDBM50440018

(CHEMBL2425628)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCC(O)CC2)n1 |r,wU:23.24,wD:26.31,(28.71,-17.43,;28.01,-18.78,;27.59,-20.26,;29.41,-19.4,;26.53,-18.36,;26.13,-16.88,;24.65,-16.47,;24.27,-14.99,;22.78,-14.58,;21.71,-15.67,;20.2,-15.25,;19.81,-13.8,;20.9,-12.71,;20.81,-11.17,;22.23,-10.62,;23.2,-11.81,;22.38,-13.1,;19.48,-10.41,;19.47,-8.86,;18.15,-8.1,;16.81,-8.87,;16.81,-10.41,;15.47,-11.19,;14.13,-10.42,;12.8,-11.2,;11.46,-10.44,;11.44,-8.89,;12.79,-8.11,;14.12,-8.88,;10.09,-8.13,;10.08,-6.58,;8.76,-8.89,;7.43,-8.12,;6.1,-8.9,;6.1,-10.44,;4.77,-11.21,;7.43,-11.21,;8.76,-10.44,;18.15,-11.18,)| Show InChI InChI=1S/C28H37N5O5S/c1-39(36,37)19-3-18-38-25-5-2-4-24-23(25)13-17-33(24)26-10-14-29-28(31-26)30-21-8-6-20(7-9-21)27(35)32-15-11-22(34)12-16-32/h2,4-5,10,13-14,17,20-22,34H,3,6-9,11-12,15-16,18-19H2,1H3,(H,29,30,31)/t20-,21- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to ERK8 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data