 Found 2114 hits with Last Name = 'unwalla' and Initial = 'rj'
Found 2114 hits with Last Name = 'unwalla' and Initial = 'rj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50031895
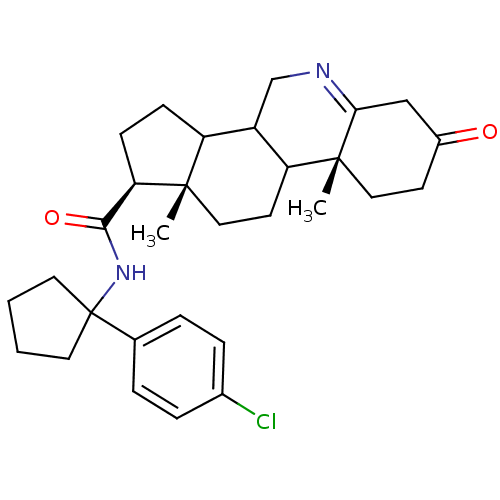
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC1(CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H39ClN2O2/c1-28-16-12-24-22(18-32-26-17-21(34)11-15-29(24,26)2)23(28)9-10-25(28)27(35)33-30(13-3-4-14-30)19-5-7-20(31)8-6-19/h5-8,22-25H,3-4,9-18H2,1-2H3,(H,33,35)/t22?,23?,24?,25-,28+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity (in vitro) |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50031877
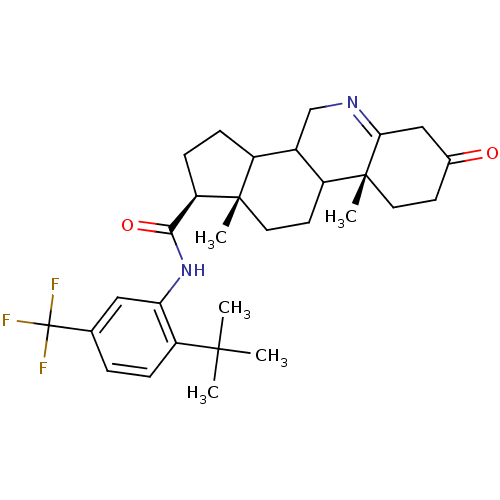
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C)C(F)(F)F |t:20| Show InChI InChI=1S/C30H39F3N2O2/c1-27(2,3)22-7-6-17(30(31,32)33)14-24(22)35-26(37)23-9-8-20-19-16-34-25-15-18(36)10-12-29(25,5)21(19)11-13-28(20,23)4/h6-7,14,19-21,23H,8-13,15-16H2,1-5H3,(H,35,37)/t19?,20?,21?,23-,28+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Rattus norvegicus) | BDBM50031895

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC1(CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H39ClN2O2/c1-28-16-12-24-22(18-32-26-17-21(34)11-15-29(24,26)2)23(28)9-10-25(28)27(35)33-30(13-3-4-14-30)19-5-7-20(31)8-6-19/h5-8,22-25H,3-4,9-18H2,1-2H3,(H,33,35)/t22?,23?,24?,25-,28+,29-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031874
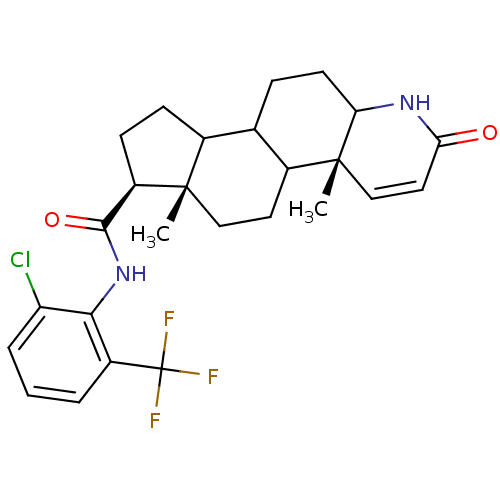
((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CC[C@@H]2C(=O)Nc1c(Cl)cccc1C(F)(F)F |c:12| Show InChI InChI=1S/C26H30ClF3N2O2/c1-24-12-10-16-14(6-9-20-25(16,2)13-11-21(33)31-20)15(24)7-8-18(24)23(34)32-22-17(26(28,29)30)4-3-5-19(22)27/h3-5,11,13-16,18,20H,6-10,12H2,1-2H3,(H,31,33)(H,32,34)/t14?,15?,16?,18-,20?,24+,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Steroid 5-alpha-reductase type I was evaluated |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Rattus norvegicus) | BDBM50031877

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C)C(F)(F)F |t:20| Show InChI InChI=1S/C30H39F3N2O2/c1-27(2,3)22-7-6-17(30(31,32)33)14-24(22)35-26(37)23-9-8-20-19-16-34-25-15-18(36)10-12-29(25,5)21(19)11-13-28(20,23)4/h6-7,14,19-21,23H,8-13,15-16H2,1-5H3,(H,35,37)/t19?,20?,21?,23-,28+,29-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031883
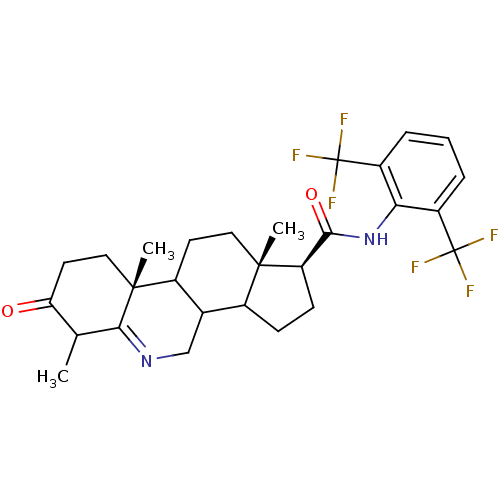
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)Nc4c(cccc4C(F)(F)F)C(F)(F)F)C3CN=C12 |t:39| Show InChI InChI=1S/C28H32F6N2O2/c1-14-21(37)10-12-26(3)17-9-11-25(2)16(15(17)13-35-23(14)26)7-8-20(25)24(38)36-22-18(27(29,30)31)5-4-6-19(22)28(32,33)34/h4-6,14-17,20H,7-13H2,1-3H3,(H,36,38)/t14?,15?,16?,17?,20-,25+,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50407405
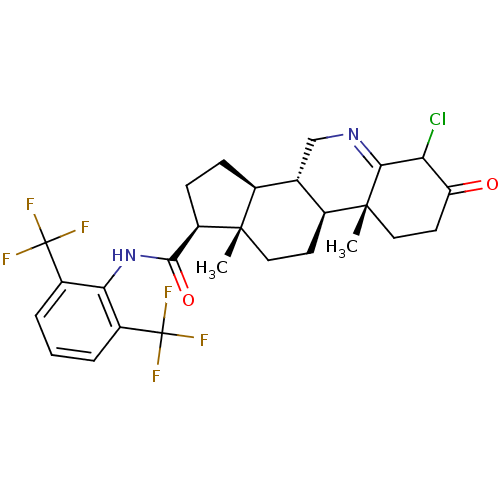
(CHEMBL2115222)Show SMILES C[C@]12CC[C@H]3[C@@H](CN=C4C(Cl)C(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)Nc1c(cccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H29ClF6N2O2/c1-24-10-8-15-13(12-35-22-20(28)19(37)9-11-25(15,22)2)14(24)6-7-18(24)23(38)36-21-16(26(29,30)31)4-3-5-17(21)27(32,33)34/h3-5,13-15,18,20H,6-12H2,1-2H3,(H,36,38)/t13-,14-,15-,18+,20?,24-,25+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50407405

(CHEMBL2115222)Show SMILES C[C@]12CC[C@H]3[C@@H](CN=C4C(Cl)C(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)Nc1c(cccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H29ClF6N2O2/c1-24-10-8-15-13(12-35-22-20(28)19(37)9-11-25(15,22)2)14(24)6-7-18(24)23(38)36-21-16(26(29,30)31)4-3-5-17(21)27(32,33)34/h3-5,13-15,18,20H,6-12H2,1-2H3,(H,36,38)/t13-,14-,15-,18+,20?,24-,25+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50031896
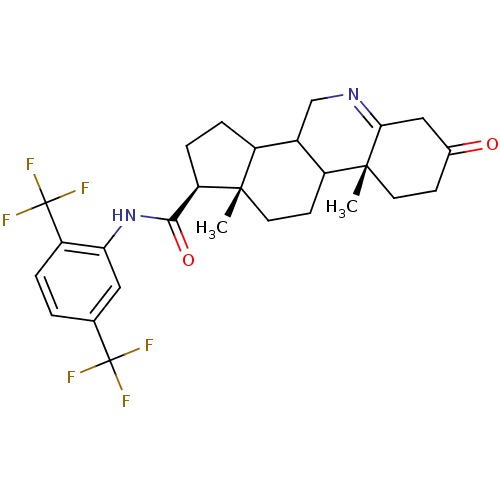
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)Nc1cc(ccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H30F6N2O2/c1-24-10-8-18-16(13-34-22-12-15(36)7-9-25(18,22)2)17(24)5-6-20(24)23(37)35-21-11-14(26(28,29)30)3-4-19(21)27(31,32)33/h3-4,11,16-18,20H,5-10,12-13H2,1-2H3,(H,35,37)/t16?,17?,18?,20-,24+,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031889
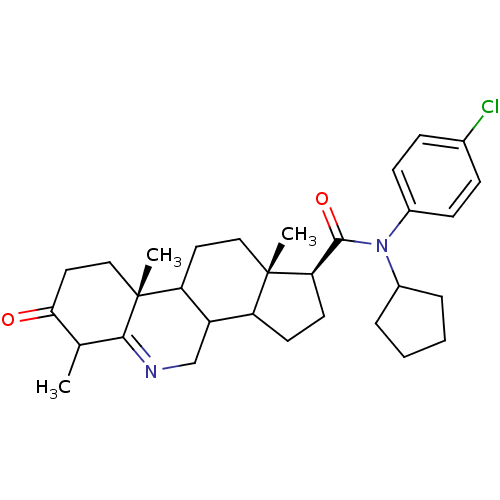
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)N(C4CCCC4)c4ccc(Cl)cc4)C3CN=C12 |t:38| Show InChI InChI=1S/C31H41ClN2O2/c1-19-27(35)15-17-31(3)25-14-16-30(2)24(23(25)18-33-28(19)31)12-13-26(30)29(36)34(21-6-4-5-7-21)22-10-8-20(32)9-11-22/h8-11,19,21,23-26H,4-7,12-18H2,1-3H3/t19?,23?,24?,25?,26-,30+,31-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031878
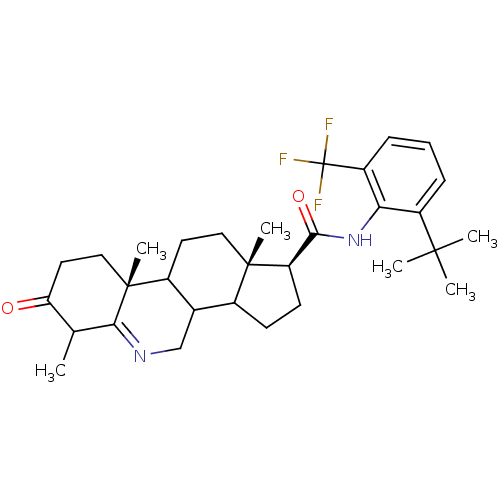
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)Nc4c(cccc4C(F)(F)F)C(C)(C)C)C3CN=C12 |t:39| Show InChI InChI=1S/C31H41F3N2O2/c1-17-24(37)13-15-30(6)20-12-14-29(5)19(18(20)16-35-26(17)30)10-11-23(29)27(38)36-25-21(28(2,3)4)8-7-9-22(25)31(32,33)34/h7-9,17-20,23H,10-16H2,1-6H3,(H,36,38)/t17?,18?,19?,20?,23-,29+,30-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031897
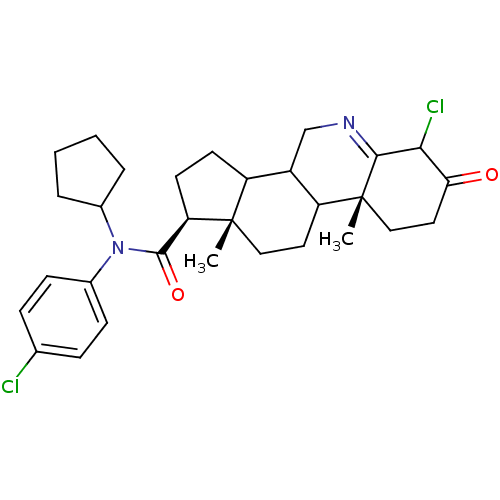
((1S,9aR,11aS)-6-Chloro-9a,11a-dimethyl-7-oxo-2,3,3...)Show SMILES C[C@]12CCC3C(CN=C4C(Cl)C(=O)CC[C@]34C)C1CC[C@@H]2C(=O)N(C1CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H38Cl2N2O2/c1-29-15-13-23-21(17-33-27-26(32)25(35)14-16-30(23,27)2)22(29)11-12-24(29)28(36)34(19-5-3-4-6-19)20-9-7-18(31)8-10-20/h7-10,19,21-24,26H,3-6,11-17H2,1-2H3/t21?,22?,23?,24-,26?,29+,30-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Rattus norvegicus) | BDBM50031896

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)Nc1cc(ccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H30F6N2O2/c1-24-10-8-18-16(13-34-22-12-15(36)7-9-25(18,22)2)17(24)5-6-20(24)23(37)35-21-11-14(26(28,29)30)3-4-19(21)27(31,32)33/h3-4,11,16-18,20H,5-10,12-13H2,1-2H3,(H,35,37)/t16?,17?,18?,20-,24+,25-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031903
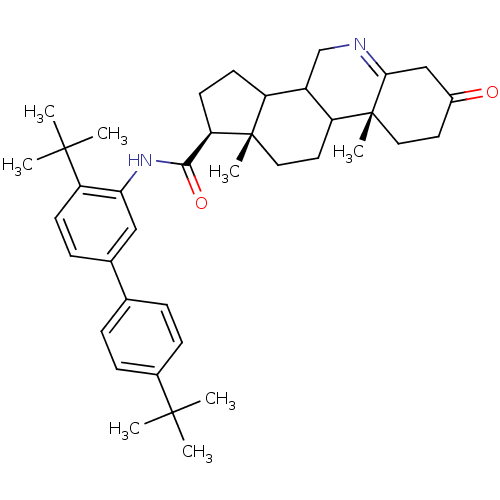
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1)-c1ccc(c(NC(=O)[C@H]2CCC3C4CN=C5CC(=O)CC[C@]5(C)C4CC[C@]23C)c1)C(C)(C)C |t:25| Show InChI InChI=1S/C39H52N2O2/c1-36(2,3)26-12-9-24(10-13-26)25-11-14-31(37(4,5)6)33(21-25)41-35(43)32-16-15-29-28-23-40-34-22-27(42)17-19-39(34,8)30(28)18-20-38(29,32)7/h9-14,21,28-30,32H,15-20,22-23H2,1-8H3,(H,41,43)/t28?,29?,30?,32-,38+,39-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031879
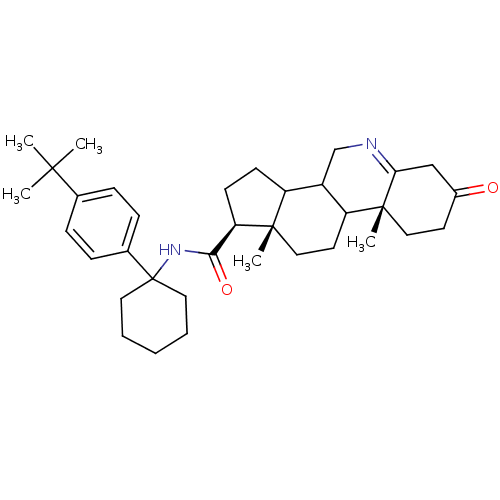
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1)C1(CCCCC1)NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:27| Show InChI InChI=1S/C35H50N2O2/c1-32(2,3)23-9-11-24(12-10-23)35(17-7-6-8-18-35)37-31(39)29-14-13-27-26-22-36-30-21-25(38)15-19-34(30,5)28(26)16-20-33(27,29)4/h9-12,26-29H,6-8,13-22H2,1-5H3,(H,37,39)/t26?,27?,28?,29-,33+,34-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031875
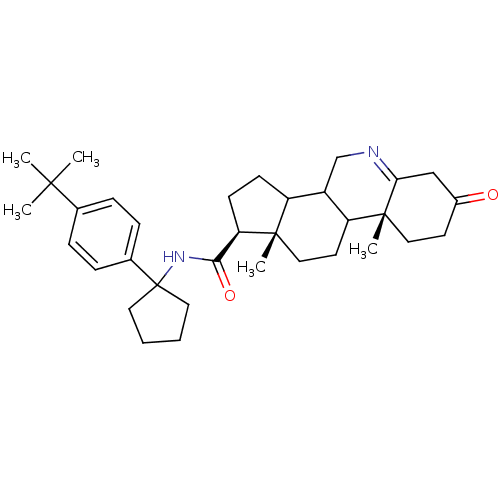
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1)C1(CCCC1)NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:26| Show InChI InChI=1S/C34H48N2O2/c1-31(2,3)22-8-10-23(11-9-22)34(16-6-7-17-34)36-30(38)28-13-12-26-25-21-35-29-20-24(37)14-18-33(29,5)27(25)15-19-32(26,28)4/h8-11,25-28H,6-7,12-21H2,1-5H3,(H,36,38)/t25?,26?,27?,28-,32+,33-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031893
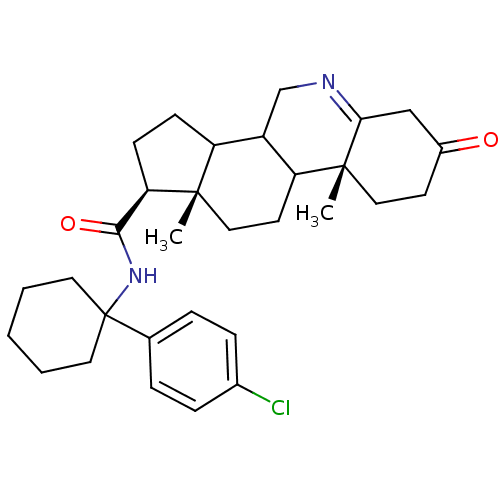
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC1(CCCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C31H41ClN2O2/c1-29-17-13-25-23(19-33-27-18-22(35)12-16-30(25,27)2)24(29)10-11-26(29)28(36)34-31(14-4-3-5-15-31)20-6-8-21(32)9-7-20/h6-9,23-26H,3-5,10-19H2,1-2H3,(H,34,36)/t23?,24?,25?,26-,29+,30-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031887
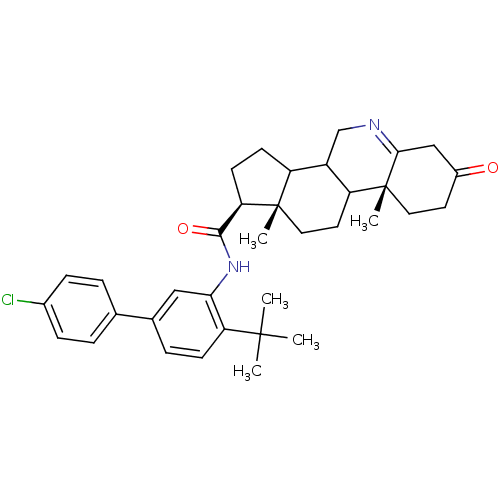
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C)-c1ccc(Cl)cc1 |t:20| Show InChI InChI=1S/C35H43ClN2O2/c1-33(2,3)28-11-8-22(21-6-9-23(36)10-7-21)18-30(28)38-32(40)29-13-12-26-25-20-37-31-19-24(39)14-16-35(31,5)27(25)15-17-34(26,29)4/h6-11,18,25-27,29H,12-17,19-20H2,1-5H3,(H,38,40)/t25?,26?,27?,29-,34+,35-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031898
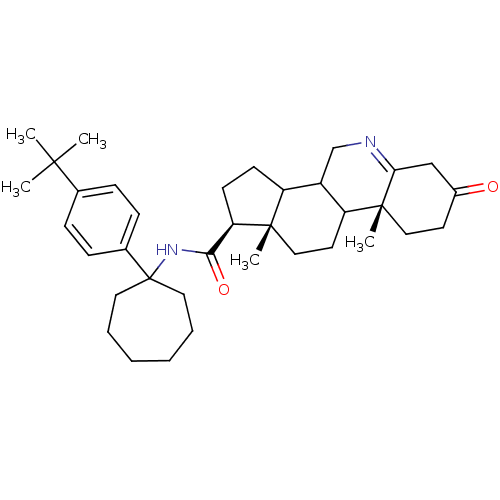
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1)C1(CCCCCC1)NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:28| Show InChI InChI=1S/C36H52N2O2/c1-33(2,3)24-10-12-25(13-11-24)36(18-8-6-7-9-19-36)38-32(40)30-15-14-28-27-23-37-31-22-26(39)16-20-35(31,5)29(27)17-21-34(28,30)4/h10-13,27-30H,6-9,14-23H2,1-5H3,(H,38,40)/t27?,28?,29?,30-,34+,35-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031896

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)Nc1cc(ccc1C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H30F6N2O2/c1-24-10-8-18-16(13-34-22-12-15(36)7-9-25(18,22)2)17(24)5-6-20(24)23(37)35-21-11-14(26(28,29)30)3-4-19(21)27(31,32)33/h3-4,11,16-18,20H,5-10,12-13H2,1-2H3,(H,35,37)/t16?,17?,18?,20-,24+,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031885
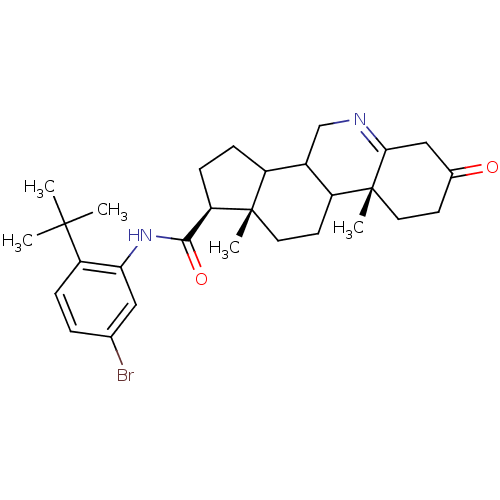
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(Br)cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:21| Show InChI InChI=1S/C29H39BrN2O2/c1-27(2,3)22-7-6-17(30)14-24(22)32-26(34)23-9-8-20-19-16-31-25-15-18(33)10-12-29(25,5)21(19)11-13-28(20,23)4/h6-7,14,19-21,23H,8-13,15-16H2,1-5H3,(H,32,34)/t19?,20?,21?,23-,28+,29-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031886
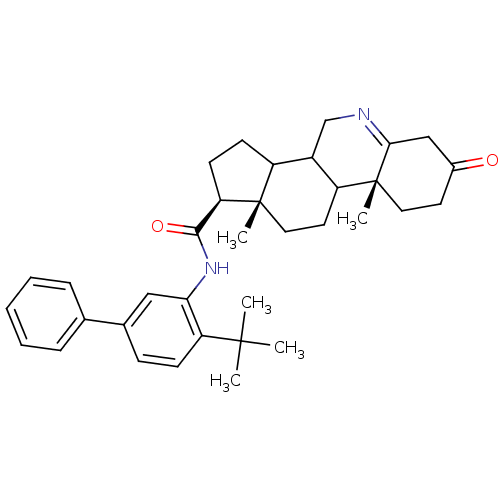
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C)-c1ccccc1 |t:20| Show InChI InChI=1S/C35H44N2O2/c1-33(2,3)28-12-11-23(22-9-7-6-8-10-22)19-30(28)37-32(39)29-14-13-26-25-21-36-31-20-24(38)15-17-35(31,5)27(25)16-18-34(26,29)4/h6-12,19,25-27,29H,13-18,20-21H2,1-5H3,(H,37,39)/t25?,26?,27?,29-,34+,35-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031899
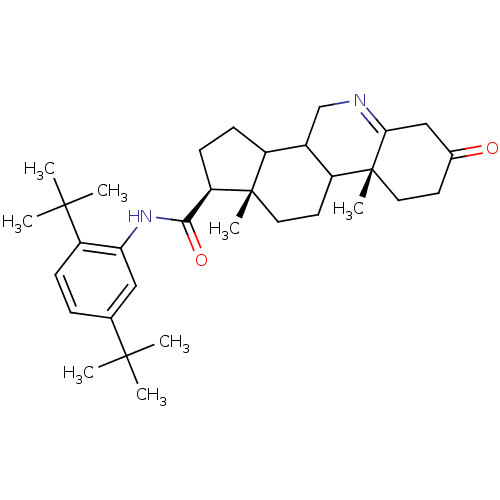
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(c(NC(=O)[C@H]2CCC3C4CN=C5CC(=O)CC[C@]5(C)C4CC[C@]23C)c1)C(C)(C)C |t:18| Show InChI InChI=1S/C33H48N2O2/c1-30(2,3)20-9-10-25(31(4,5)6)27(17-20)35-29(37)26-12-11-23-22-19-34-28-18-21(36)13-15-33(28,8)24(22)14-16-32(23,26)7/h9-10,17,22-24,26H,11-16,18-19H2,1-8H3,(H,35,37)/t22?,23?,24?,26-,32+,33-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031888
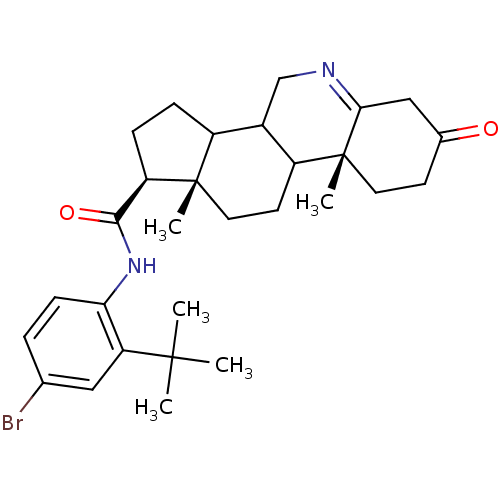
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1cc(Br)ccc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:21| Show InChI InChI=1S/C29H39BrN2O2/c1-27(2,3)23-14-17(30)6-9-24(23)32-26(34)22-8-7-20-19-16-31-25-15-18(33)10-12-29(25,5)21(19)11-13-28(20,22)4/h6,9,14,19-22H,7-8,10-13,15-16H2,1-5H3,(H,32,34)/t19?,20?,21?,22-,28+,29-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031884
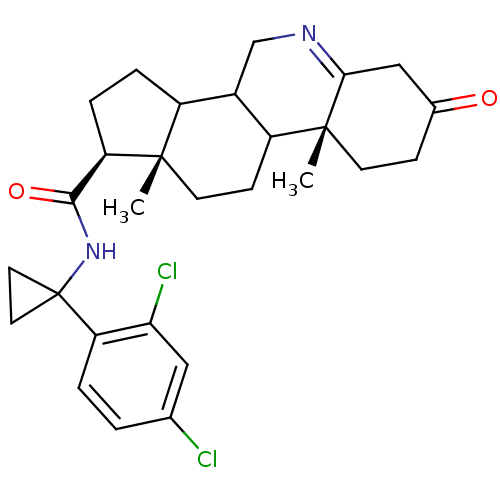
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC1(CC1)c1ccc(Cl)cc1Cl |t:7| Show InChI InChI=1S/C28H34Cl2N2O2/c1-26-10-8-20-18(15-31-24-14-17(33)7-9-27(20,24)2)19(26)5-6-22(26)25(34)32-28(11-12-28)21-4-3-16(29)13-23(21)30/h3-4,13,18-20,22H,5-12,14-15H2,1-2H3,(H,32,34)/t18?,19?,20?,22-,26+,27-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031895

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC1(CCCC1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C30H39ClN2O2/c1-28-16-12-24-22(18-32-26-17-21(34)11-15-29(24,26)2)23(28)9-10-25(28)27(35)33-30(13-3-4-14-30)19-5-7-20(31)8-6-19/h5-8,22-25H,3-4,9-18H2,1-2H3,(H,33,35)/t22?,23?,24?,25-,28+,29-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity (in vitro) |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031902
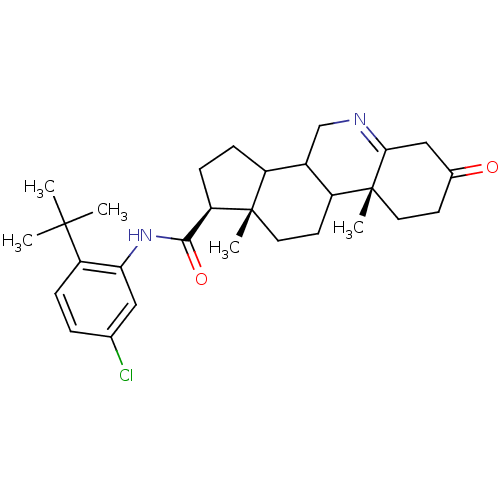
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(Cl)cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:21| Show InChI InChI=1S/C29H39ClN2O2/c1-27(2,3)22-7-6-17(30)14-24(22)32-26(34)23-9-8-20-19-16-31-25-15-18(33)10-12-29(25,5)21(19)11-13-28(20,23)4/h6-7,14,19-21,23H,8-13,15-16H2,1-5H3,(H,32,34)/t19?,20?,21?,23-,28+,29-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031880
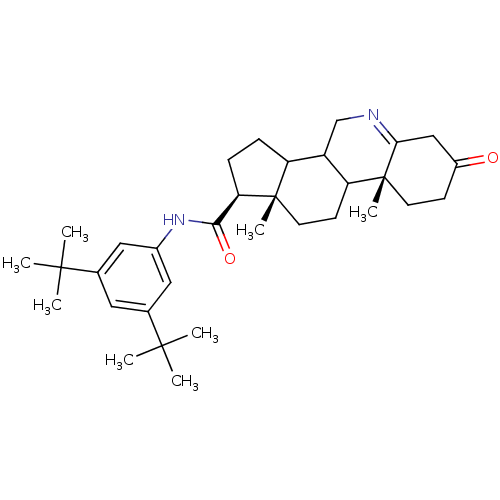
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1cc(NC(=O)[C@H]2CCC3C4CN=C5CC(=O)CC[C@]5(C)C4CC[C@]23C)cc(c1)C(C)(C)C |t:16| Show InChI InChI=1S/C33H48N2O2/c1-30(2,3)20-15-21(31(4,5)6)17-22(16-20)35-29(37)27-10-9-25-24-19-34-28-18-23(36)11-13-33(28,8)26(24)12-14-32(25,27)7/h15-17,24-27H,9-14,18-19H2,1-8H3,(H,35,37)/t24?,25?,26?,27-,32+,33-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85153
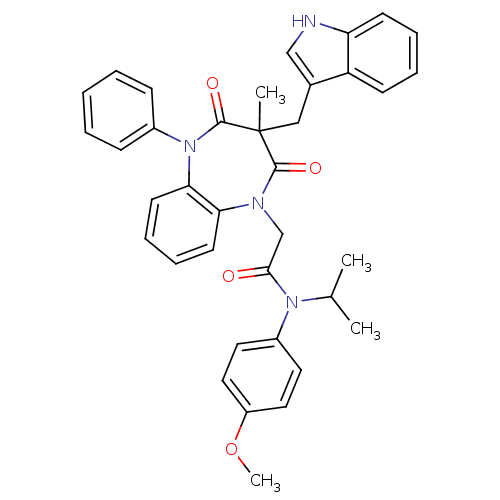
(CCK-A Agonist 20)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C37H36N4O4/c1-25(2)40(28-18-20-29(45-4)21-19-28)34(42)24-39-32-16-10-11-17-33(32)41(27-12-6-5-7-13-27)36(44)37(3,35(39)43)22-26-23-38-31-15-9-8-14-30(26)31/h5-21,23,25,38H,22,24H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031877

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccc(cc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C)C(F)(F)F |t:20| Show InChI InChI=1S/C30H39F3N2O2/c1-27(2,3)22-7-6-17(30(31,32)33)14-24(22)35-26(37)23-9-8-20-19-16-34-25-15-18(36)10-12-29(25,5)21(19)11-13-28(20,23)4/h6-7,14,19-21,23H,8-13,15-16H2,1-5H3,(H,35,37)/t19?,20?,21?,23-,28+,29-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85145
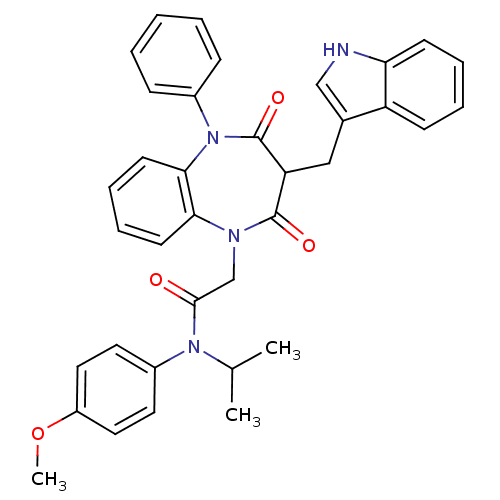
(CCK-A Agonist 15)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C36H34N4O4/c1-24(2)39(27-17-19-28(44-3)20-18-27)34(41)23-38-32-15-9-10-16-33(32)40(26-11-5-4-6-12-26)36(43)30(35(38)42)21-25-22-37-31-14-8-7-13-29(25)31/h4-20,22,24,30,37H,21,23H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85152
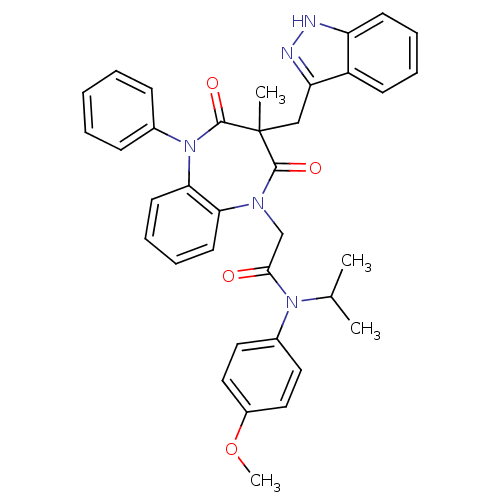
(CCK-A Agonist 23)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2n[nH]c3ccccc23)C1=O Show InChI InChI=1S/C36H35N5O4/c1-24(2)40(26-18-20-27(45-4)21-19-26)33(42)23-39-31-16-10-11-17-32(31)41(25-12-6-5-7-13-25)35(44)36(3,34(39)43)22-30-28-14-8-9-15-29(28)37-38-30/h5-21,24H,22-23H2,1-4H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85148

(CCK-A Agonist 21)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2c[nH]c3ccccc23)(OC)C1=O Show InChI InChI=1S/C37H36N4O5/c1-25(2)40(28-18-20-29(45-3)21-19-28)34(42)24-39-32-16-10-11-17-33(32)41(27-12-6-5-7-13-27)36(44)37(46-4,35(39)43)22-26-23-38-31-15-9-8-14-30(26)31/h5-21,23,25,38H,22,24H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85141
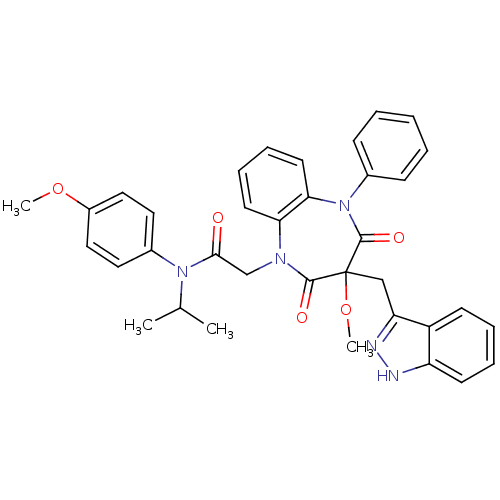
(CCK-A Agonist 24)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)(OC)C1=O Show InChI InChI=1S/C36H35N5O5/c1-24(2)40(26-18-20-27(45-3)21-19-26)33(42)23-39-31-16-10-11-17-32(31)41(25-12-6-5-7-13-25)35(44)36(46-4,34(39)43)22-30-28-14-8-9-15-29(28)37-38-30/h5-21,24H,22-23H2,1-4H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85161
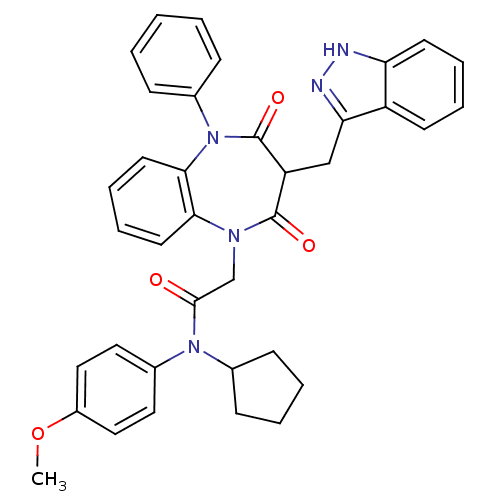
(CCK-A Agonist 43)Show SMILES COc1ccc(cc1)N(C1CCCC1)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)C1=O Show InChI InChI=1S/C37H35N5O4/c1-46-28-21-19-27(20-22-28)41(25-13-5-6-14-25)35(43)24-40-33-17-9-10-18-34(33)42(26-11-3-2-4-12-26)37(45)30(36(40)44)23-32-29-15-7-8-16-31(29)38-39-32/h2-4,7-12,15-22,25,30H,5-6,13-14,23-24H2,1H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85155
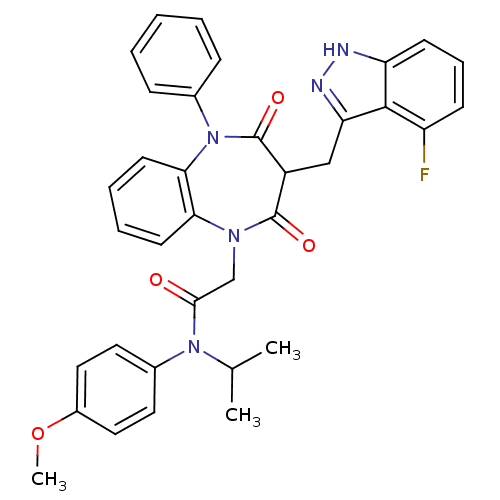
(CCK-A Agonist 31)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3cccc(F)c23)C1=O Show InChI InChI=1S/C35H32FN5O4/c1-22(2)40(24-16-18-25(45-3)19-17-24)32(42)21-39-30-14-7-8-15-31(30)41(23-10-5-4-6-11-23)35(44)26(34(39)43)20-29-33-27(36)12-9-13-28(33)37-38-29/h4-19,22,26H,20-21H2,1-3H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85147
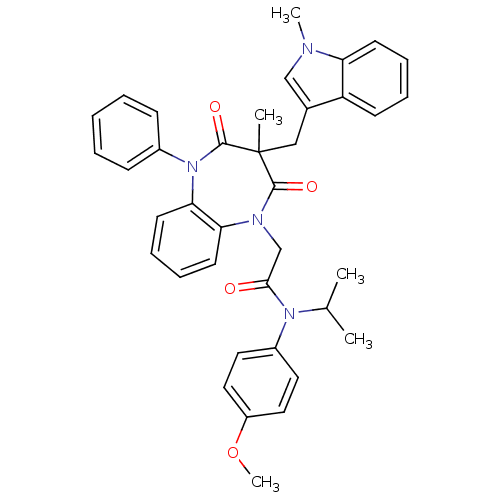
(CCK-A Agonist 22)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2cn(C)c3ccccc23)C1=O Show InChI InChI=1S/C38H38N4O4/c1-26(2)41(29-19-21-30(46-5)22-20-29)35(43)25-40-33-17-11-12-18-34(33)42(28-13-7-6-8-14-28)37(45)38(3,36(40)44)23-27-24-39(4)32-16-10-9-15-31(27)32/h6-22,24,26H,23,25H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85143
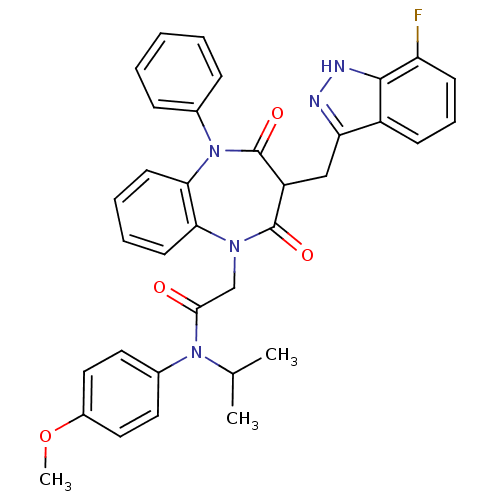
(CCK-A Agonist 34)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3c(F)cccc23)C1=O Show InChI InChI=1S/C35H32FN5O4/c1-22(2)40(24-16-18-25(45-3)19-17-24)32(42)21-39-30-14-7-8-15-31(30)41(23-10-5-4-6-11-23)35(44)27(34(39)43)20-29-26-12-9-13-28(36)33(26)38-37-29/h4-19,22,27H,20-21H2,1-3H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85162
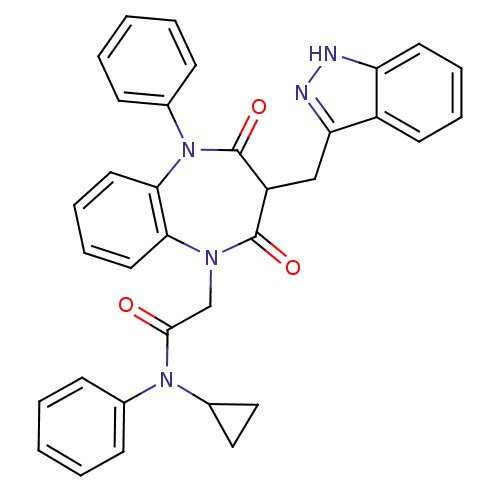
(CCK-A Agonist 44)Show SMILES O=C(CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)C1=O)N(C1CC1)c1ccccc1 Show InChI InChI=1S/C34H29N5O3/c40-32(38(25-19-20-25)23-11-3-1-4-12-23)22-37-30-17-9-10-18-31(30)39(24-13-5-2-6-14-24)34(42)27(33(37)41)21-29-26-15-7-8-16-28(26)35-36-29/h1-18,25,27H,19-22H2,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031901

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H30F6N2O2/c1-24-8-6-20-18(13-34-22-12-17(36)5-7-25(20,22)2)19(24)3-4-21(24)23(37)35-16-10-14(26(28,29)30)9-15(11-16)27(31,32)33/h9-11,18-21H,3-8,12-13H2,1-2H3,(H,35,37)/t18?,19?,20?,21-,24+,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50031891
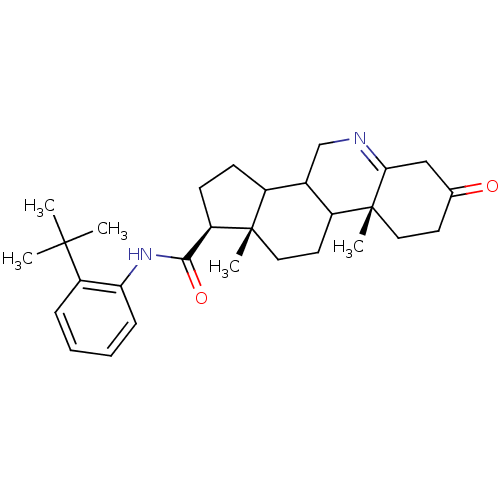
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccccc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:20| Show InChI InChI=1S/C29H40N2O2/c1-27(2,3)22-8-6-7-9-24(22)31-26(33)23-11-10-20-19-17-30-25-16-18(32)12-14-29(25,5)21(19)13-15-28(20,23)4/h6-9,19-21,23H,10-17H2,1-5H3,(H,31,33)/t19?,20?,21?,23-,28+,29-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant steroid 5-alpha-reductase type I |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85149
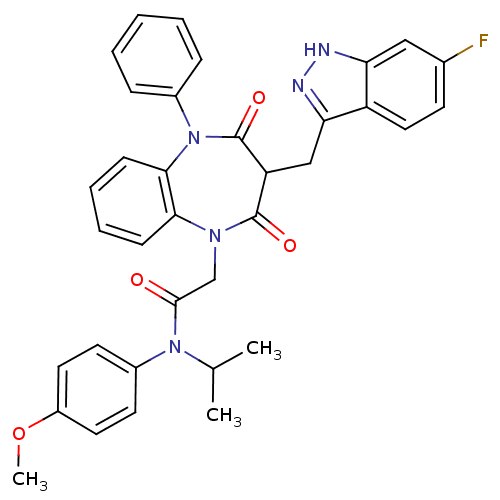
(CCK-A Agonist 33)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3cc(F)ccc23)C1=O Show InChI InChI=1S/C35H32FN5O4/c1-22(2)40(25-14-16-26(45-3)17-15-25)33(42)21-39-31-11-7-8-12-32(31)41(24-9-5-4-6-10-24)35(44)28(34(39)43)20-30-27-18-13-23(36)19-29(27)37-38-30/h4-19,22,28H,20-21H2,1-3H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50048248
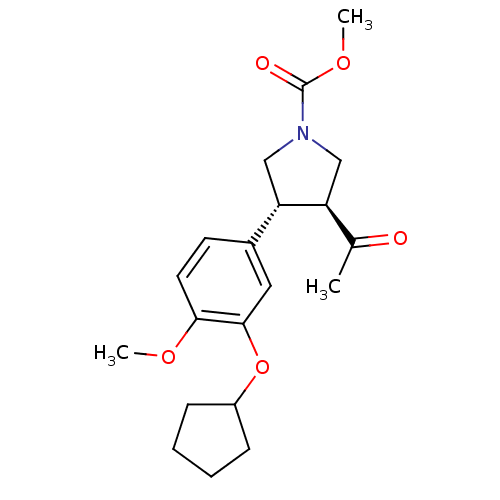
((3S,4R)-3-Acetyl-4-(3-cyclopentyloxy-4-methoxy-phe...)Show SMILES COC(=O)N1C[C@H]([C@@H](C1)c1ccc(OC)c(OC2CCCC2)c1)C(C)=O Show InChI InChI=1S/C20H27NO5/c1-13(22)16-11-21(20(23)25-3)12-17(16)14-8-9-18(24-2)19(10-14)26-15-6-4-5-7-15/h8-10,15-17H,4-7,11-12H2,1-3H3/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285059
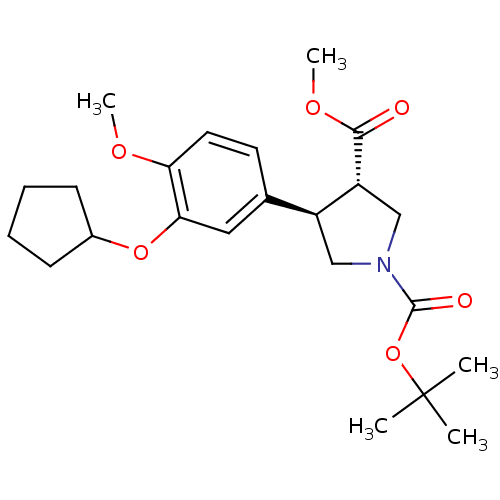
(1-(tert-butyl) 3-methyl 4-(3-cyclopentyloxy-4-meth...)Show SMILES COC(=O)[C@@H]1CN(C[C@H]1c1ccc(OC)c(OC2CCCC2)c1)C(=O)OC(C)(C)C Show InChI InChI=1S/C23H33NO6/c1-23(2,3)30-22(26)24-13-17(18(14-24)21(25)28-5)15-10-11-19(27-4)20(12-15)29-16-8-6-7-9-16/h10-12,16-18H,6-9,13-14H2,1-5H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85160
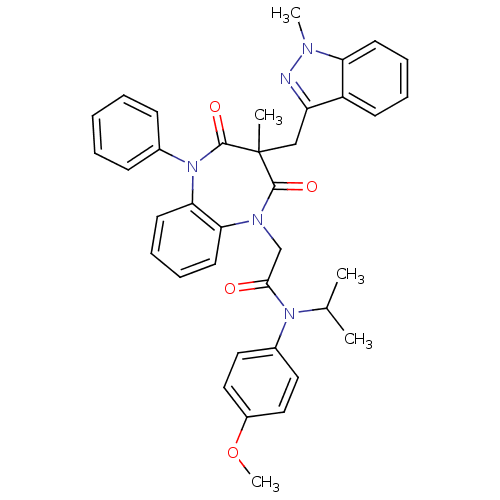
(CCK-A Agonist 27)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2nn(C)c3ccccc23)C1=O Show InChI InChI=1S/C37H37N5O4/c1-25(2)41(27-19-21-28(46-5)22-20-27)34(43)24-40-32-17-11-12-18-33(32)42(26-13-7-6-8-14-26)36(45)37(3,35(40)44)23-30-29-15-9-10-16-31(29)39(4)38-30/h6-22,25H,23-24H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285045
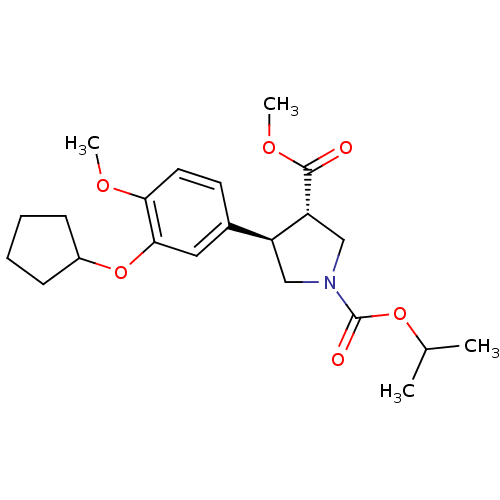
(1-isopropyl 3-methyl 4-(3-cyclopentyloxy-4-methoxy...)Show SMILES COC(=O)[C@@H]1CN(C[C@H]1c1ccc(OC)c(OC2CCCC2)c1)C(=O)OC(C)C Show InChI InChI=1S/C22H31NO6/c1-14(2)28-22(25)23-12-17(18(13-23)21(24)27-4)15-9-10-19(26-3)20(11-15)29-16-7-5-6-8-16/h9-11,14,16-18H,5-8,12-13H2,1-4H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85140
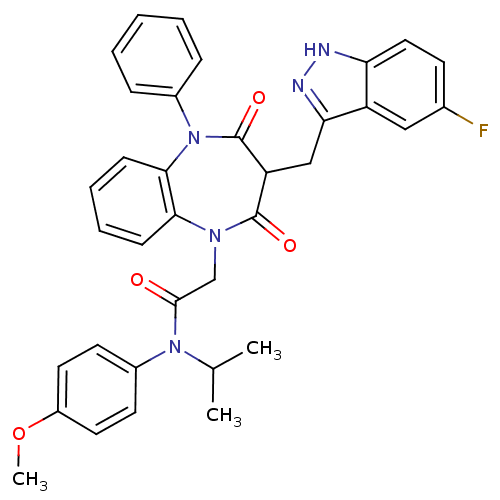
(CCK-A Agonist 32)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccc(F)cc23)C1=O Show InChI InChI=1S/C35H32FN5O4/c1-22(2)40(25-14-16-26(45-3)17-15-25)33(42)21-39-31-11-7-8-12-32(31)41(24-9-5-4-6-10-24)35(44)28(34(39)43)20-30-27-19-23(36)13-18-29(27)37-38-30/h4-19,22,28H,20-21H2,1-3H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data