

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50126528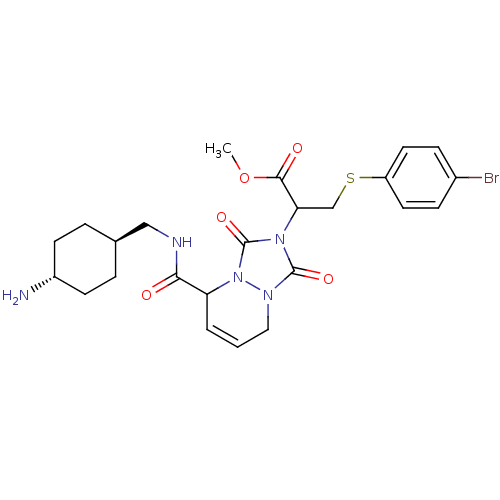 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071565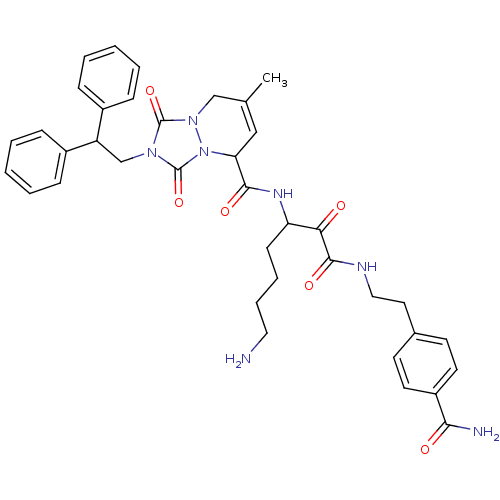 (2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126525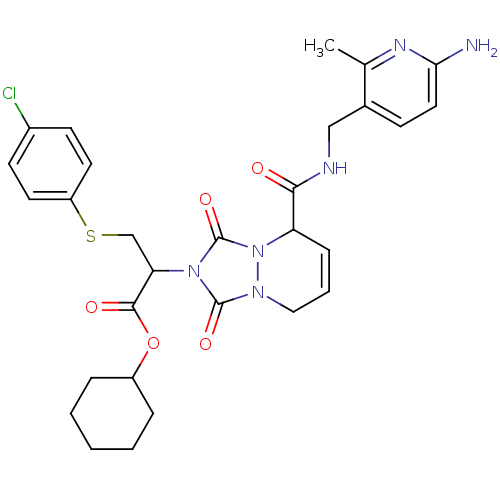 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126521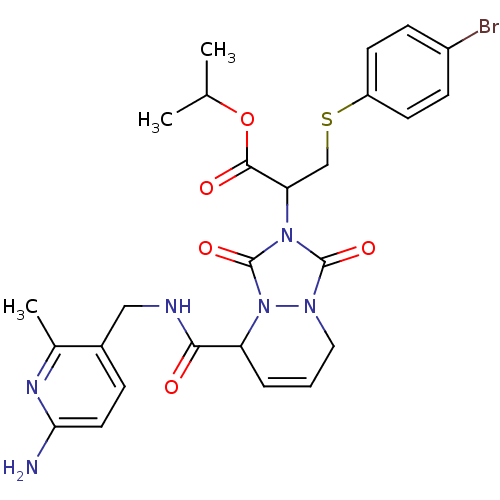 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071575 (2,2-Dibutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126508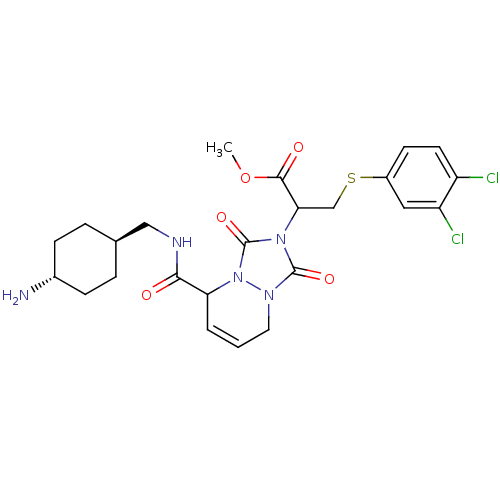 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126520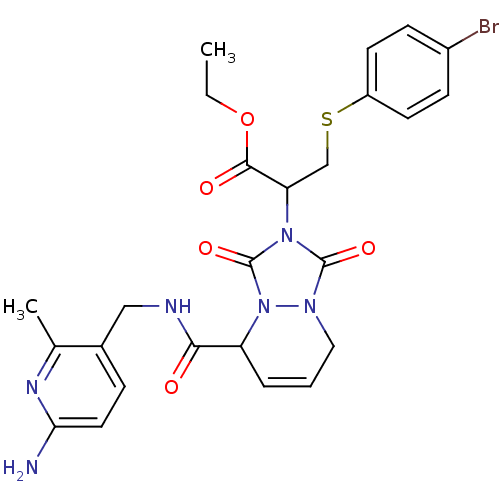 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071570 (8-Isobutyl-2-(4-methoxy-phenyl)-1,3-dioxo-2,3,5,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071573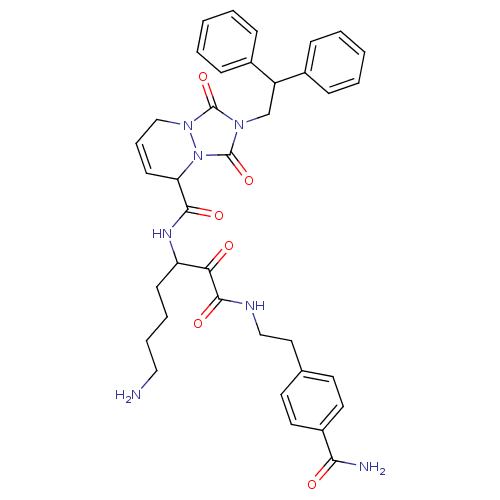 (2-(2,2-Diphenyl-ethyl)-1,3-dioxo-2,3,5,8-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126526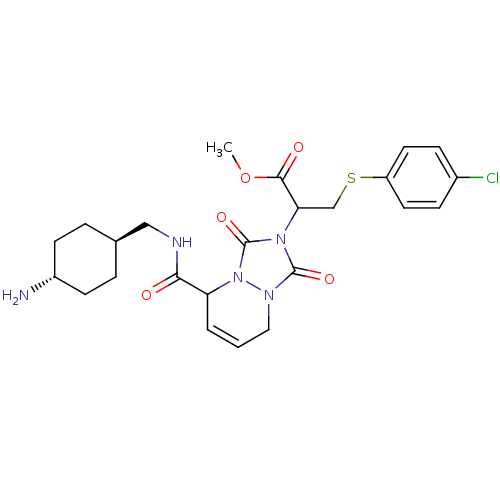 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071571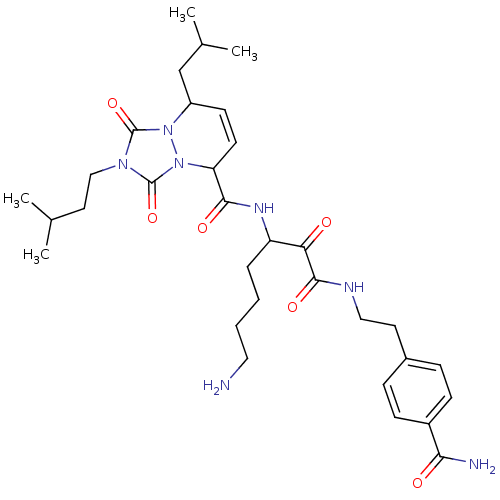 (8-Isobutyl-2-(3-methyl-butyl)-1,3-dioxo-2,3,5,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126500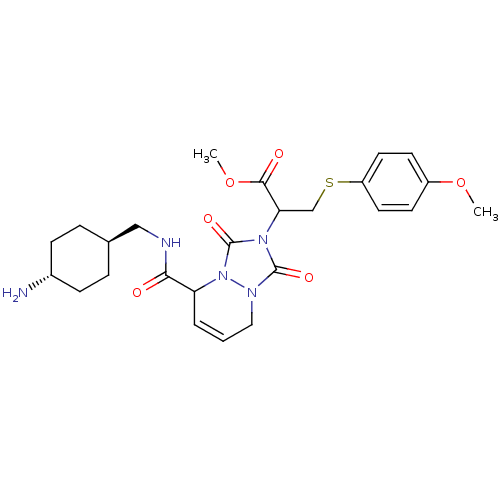 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126523 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126514 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071568 (2-Amino-2-benzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071567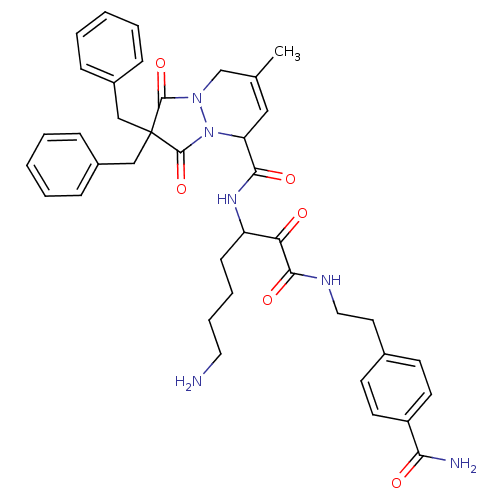 (2,2-Dibenzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126529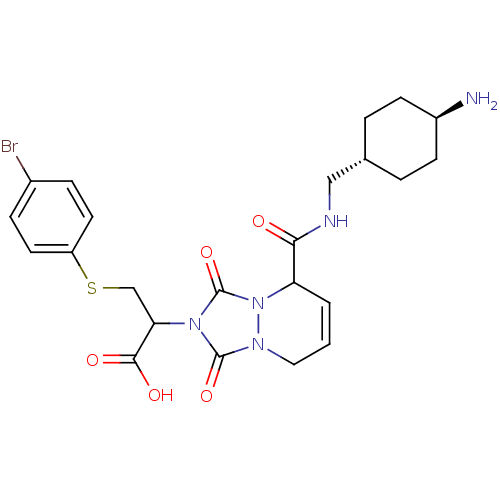 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126532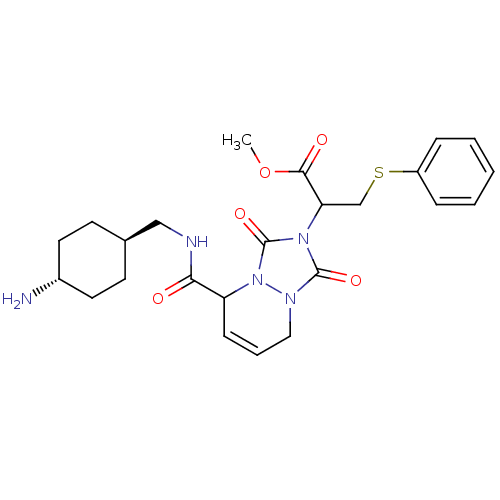 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071566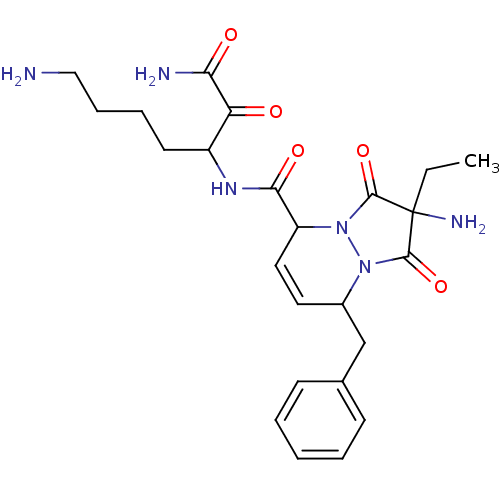 (2-Amino-8-benzyl-2-ethyl-1,3-dioxo-2,3,5,8-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126534 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071574 (2,2-Diisobutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126505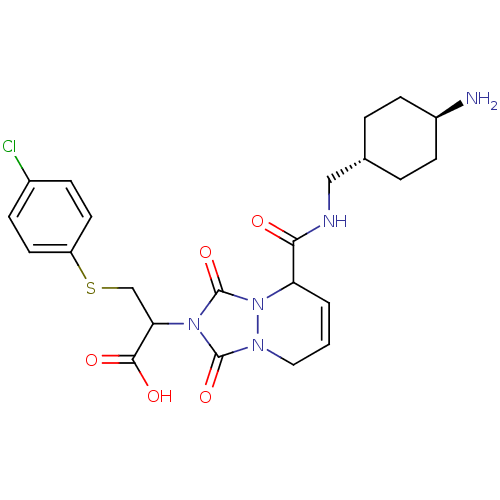 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50216213 (CHEMBL306744) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the tryptase | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126519 (2-[1-(4-Chloro-phenylsulfanylmethyl)-2-morpholin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126513 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126506 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126510 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126501 (2-[1-(3,4-Dichloro-phenylsulfanylmethyl)-2-oxo-2-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071564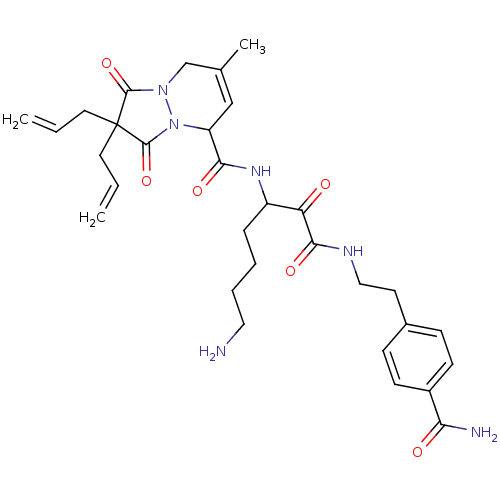 (2,2-Diallyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18428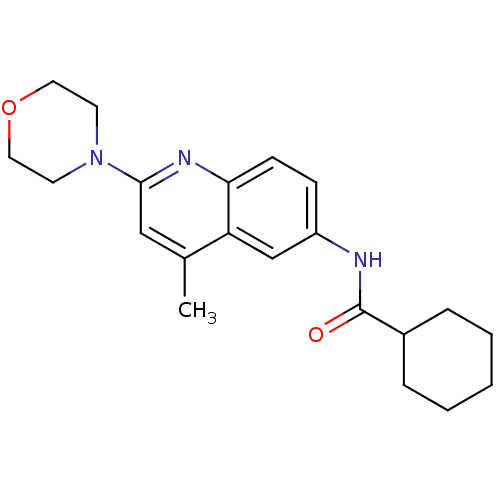 (Aminoquinoline compound, 1 | N-[4-methyl-2-(morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.2 | 31 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50071572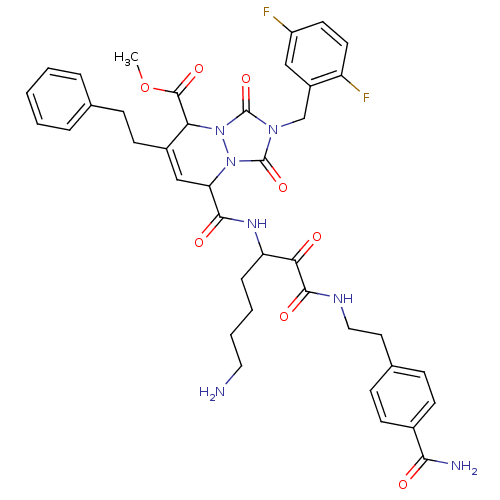 (8-{5-Amino-1-[2-(4-carbamoyl-phenyl)-ethylaminooxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Kallikrein | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126503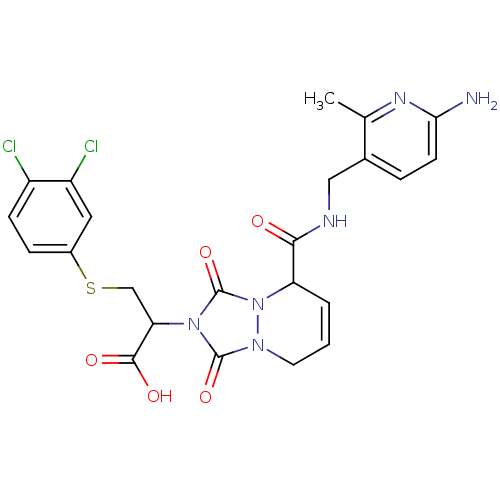 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126516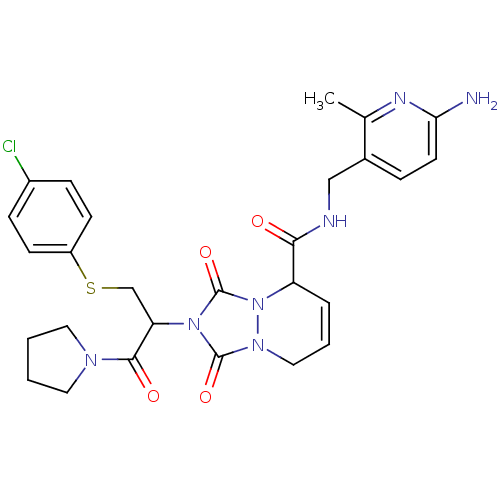 (2-[1-(4-Chloro-phenylsulfanylmethyl)-2-oxo-2-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126522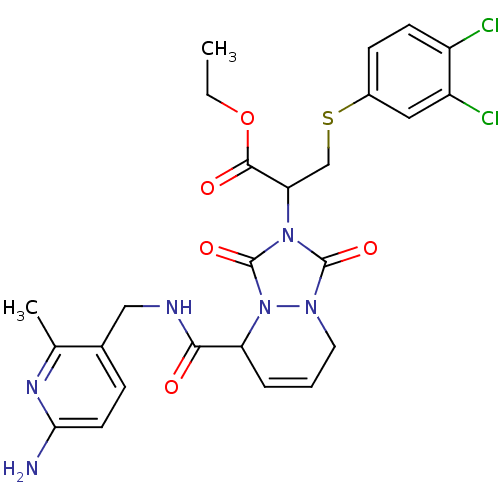 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126511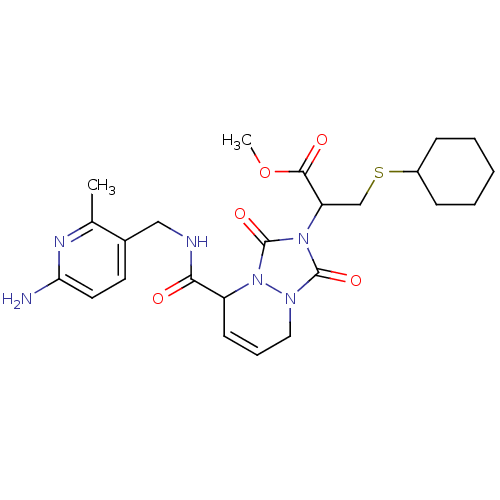 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126512 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126517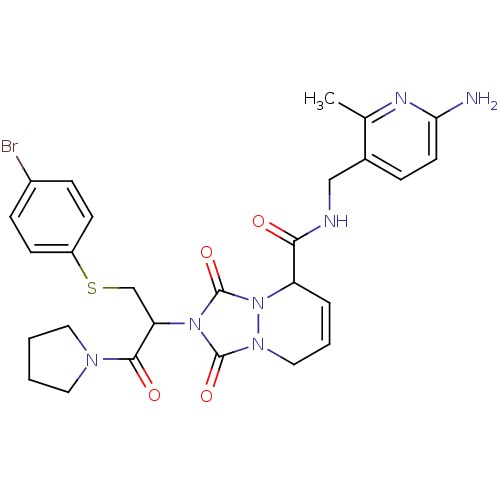 (2-[1-(4-Bromo-phenylsulfanylmethyl)-2-oxo-2-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50071563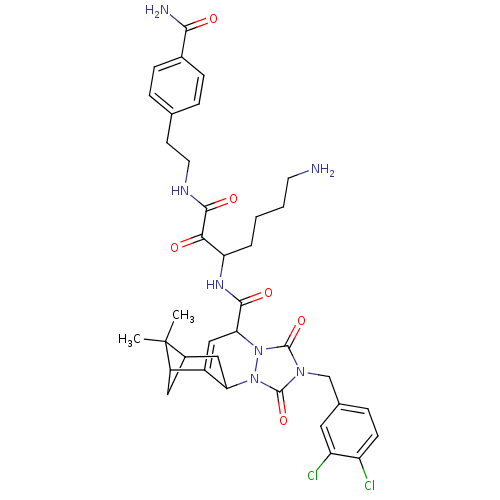 (4-(2-{6-amino-2-[7-(3,4-dichlorobenzyl)-13,13-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Kallikrein | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126531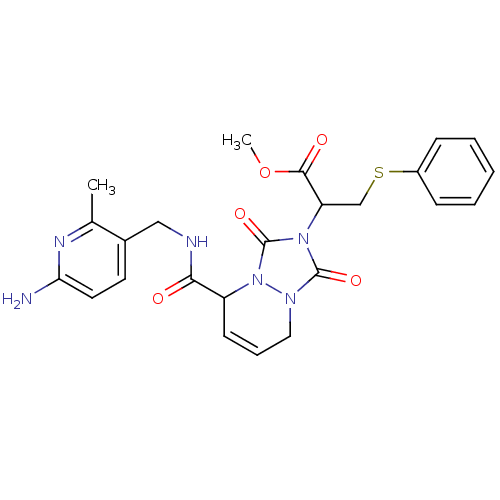 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126535 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126507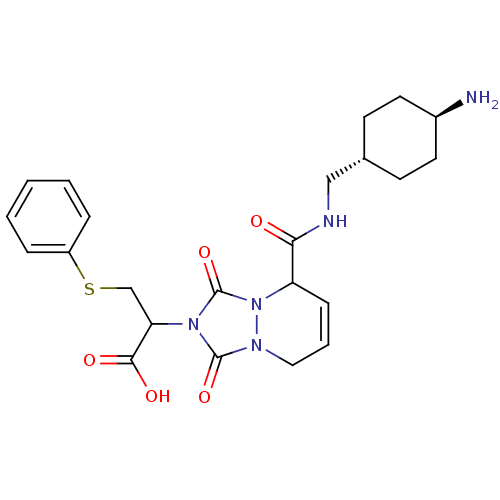 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126515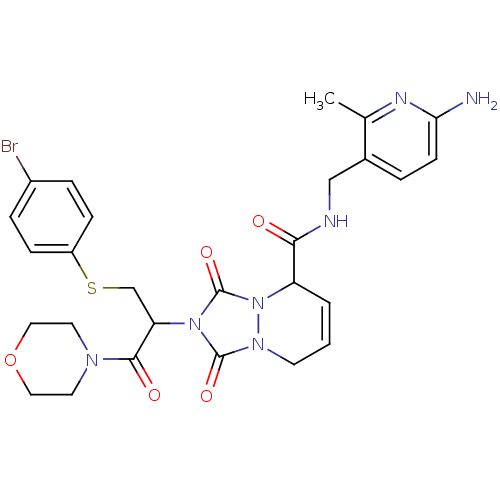 (2-[1-(4-Bromo-phenylsulfanylmethyl)-2-morpholin-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18438 (4-benzenesulfonamido-N-(5-ethyl-1,3,4-thiadiazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 52 | -41.0 | 103 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126533 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126518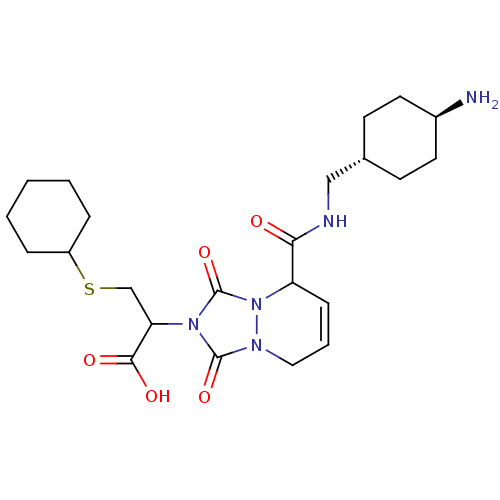 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18431 (Aminoquinoline compound, 16 | N-[4-methyl-2-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 55 | -40.9 | 133 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18430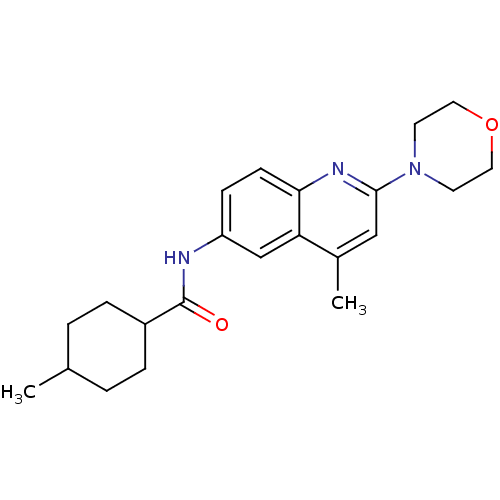 (4-methyl-N-[4-methyl-2-(morpholin-4-yl)quinolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | -40.8 | 63 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126527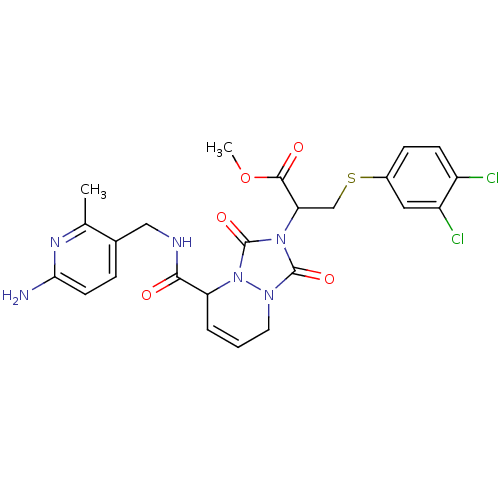 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126509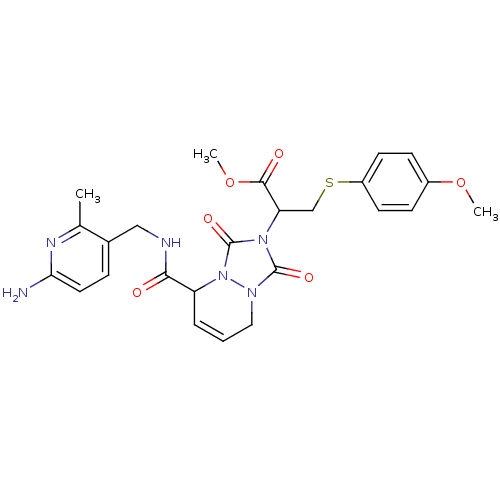 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126502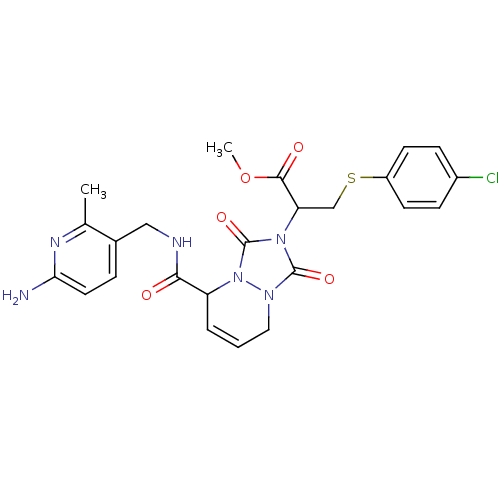 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2210 total ) | Next | Last >> |