

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arginase (Schistosoma mansoni (flatworms)) | BDBM130378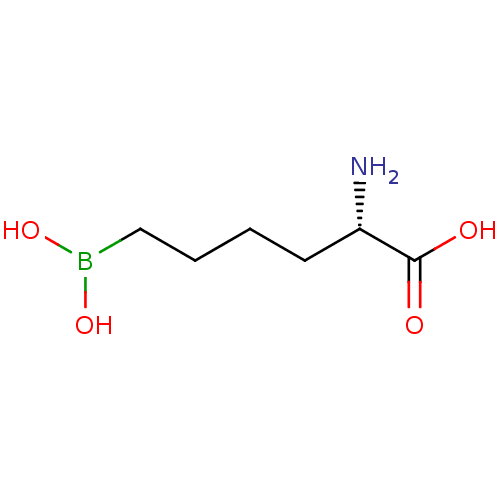 (2(S)-amino-6-boronohexanoic acid (ABH)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
University of Pennsylvania | Assay Description Briefly, 0.5-50 mM L-arginine (pH 8.5) was added to a solution of 50 mM 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) (pH 8.5) and 100 &... | Biochemistry 53: 4671-4684 (2014) Article DOI: 10.1021/bi5004519 BindingDB Entry DOI: 10.7270/Q2RR1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Schistosoma mansoni (flatworms)) | BDBM130379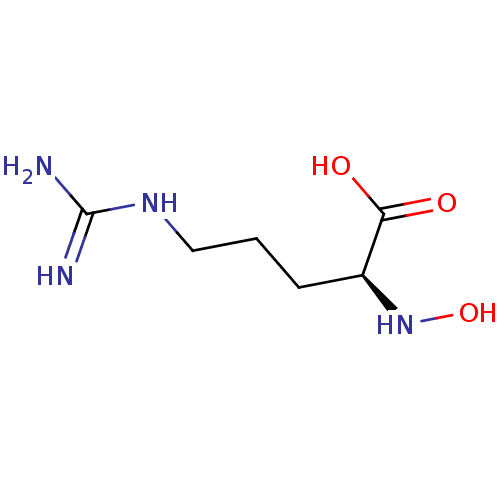 (N-hydroxy-L-arginine (NOHA)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
University of Pennsylvania | Assay Description Briefly, 0.5-50 mM L-arginine (pH 8.5) was added to a solution of 50 mM 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) (pH 8.5) and 100 &... | Biochemistry 53: 4671-4684 (2014) Article DOI: 10.1021/bi5004519 BindingDB Entry DOI: 10.7270/Q2RR1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509011 (CHEMBL4538713) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509016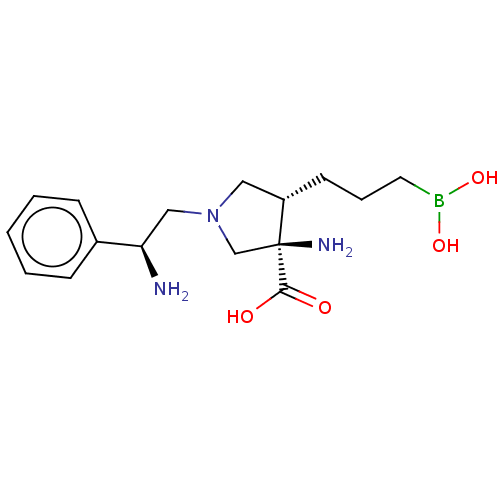 (CHEMBL4450972) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509014 (CHEMBL4557975) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16469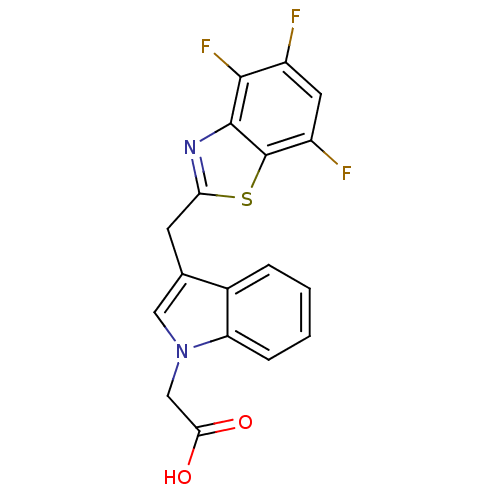 (2-{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16485 (2-{6-methoxy-3-[(4,5,7-trifluoro-1,3-benzothiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16491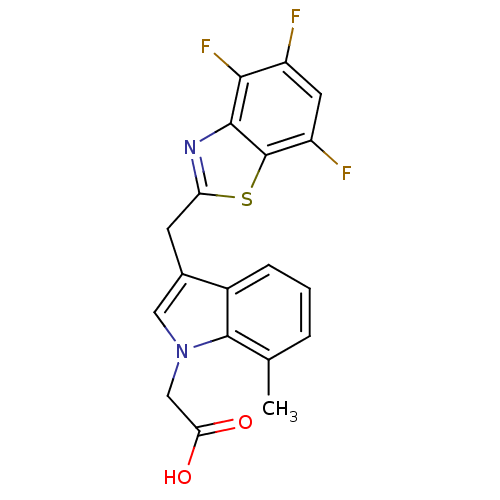 (2-{7-methyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16482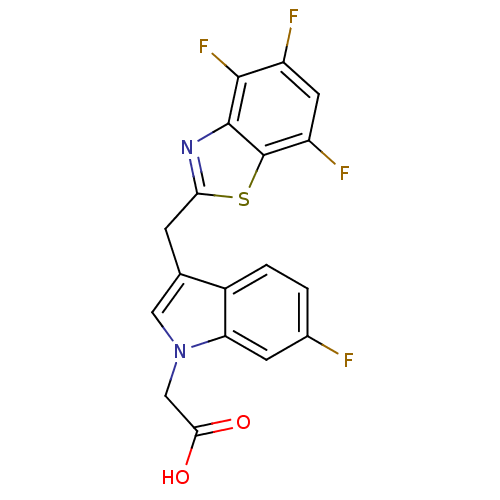 (2-{6-fluoro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16488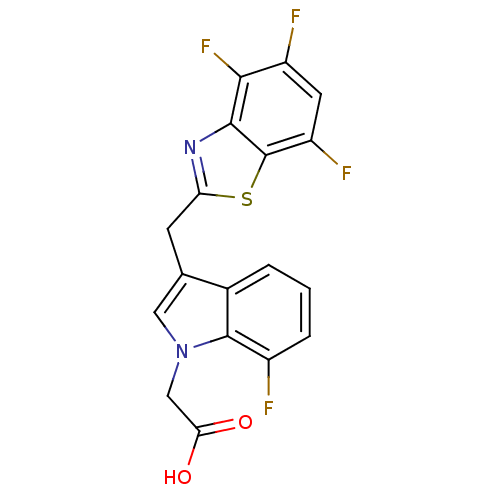 (2-{7-fluoro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509010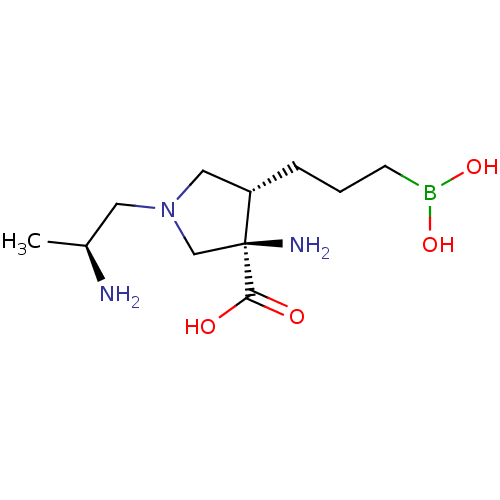 (CHEMBL4518246) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16496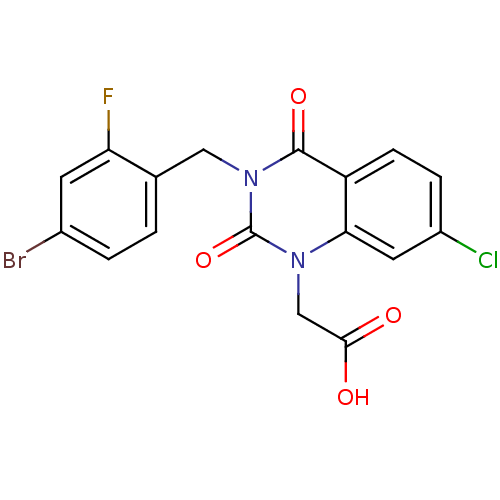 (2-{3-[(4-bromo-2-fluorophenyl)methyl]-7-chloro-2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16470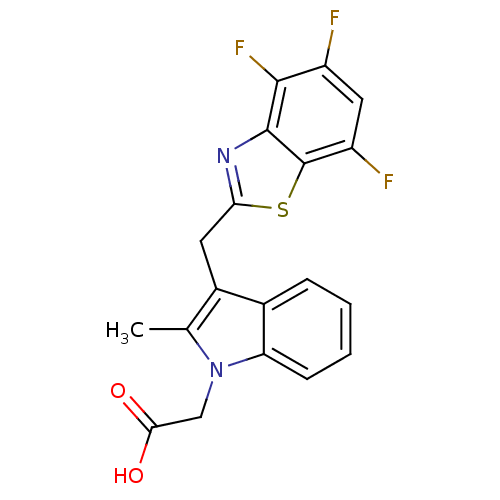 (2-{2-methyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16476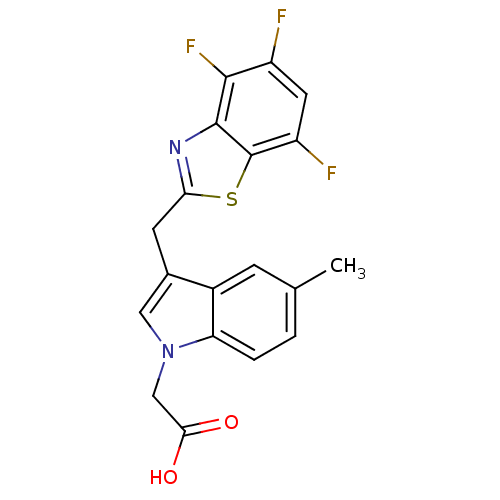 (2-{5-methyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16477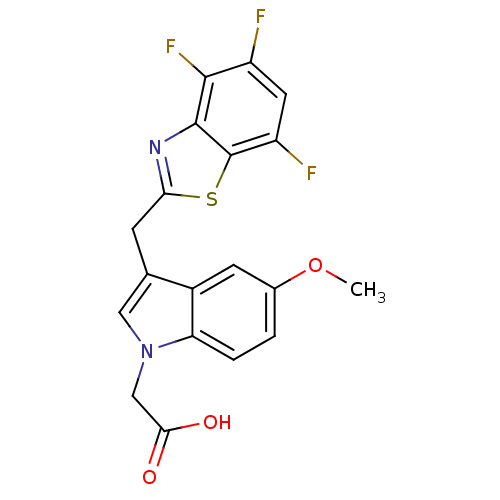 (2-{5-methoxy-3-[(4,5,7-trifluoro-1,3-benzothiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16481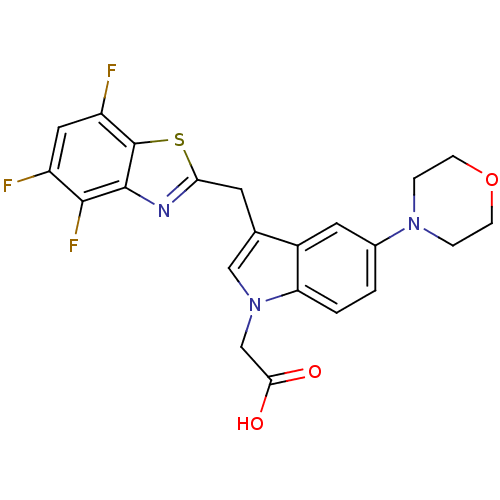 (2-[5-(morpholin-4-yl)-3-[(4,5,7-trifluoro-1,3-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16483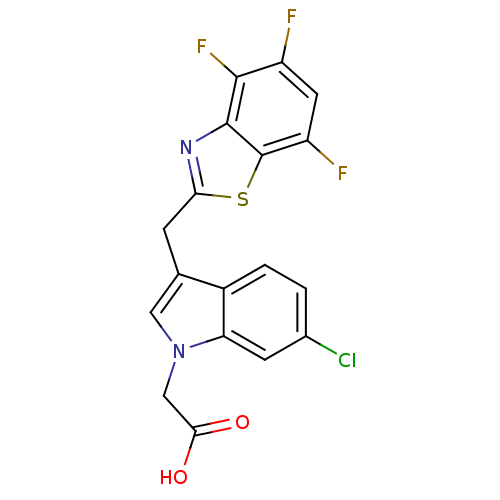 (2-{6-chloro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16493 (2-{3-[2-(4,5,7-trifluoro-1,3-benzothiazol-2-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452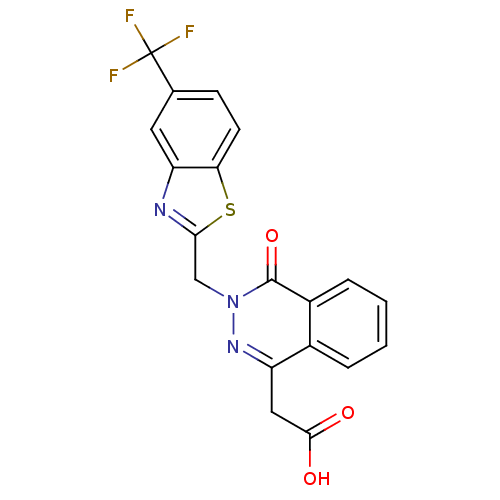 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16489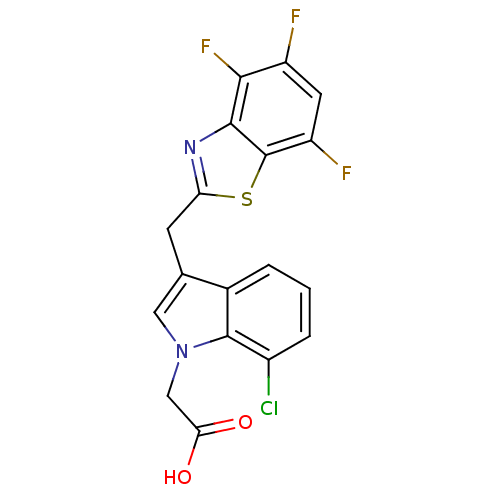 (2-{7-chloro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16464 (2-{3-[(5-fluoro-1,3-benzothiazol-2-yl)methyl]-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509018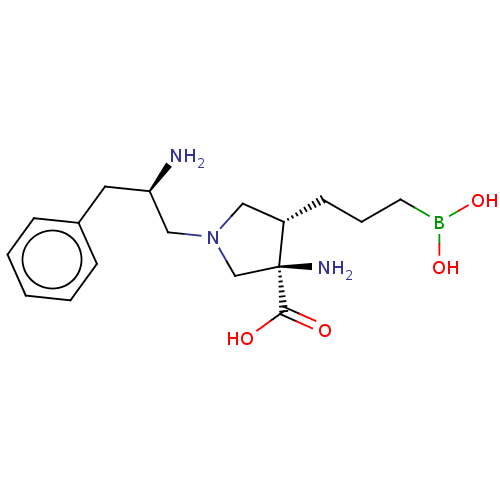 (CHEMBL4461464) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16473 (2-{5-chloro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509012 (CHEMBL4572850) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509015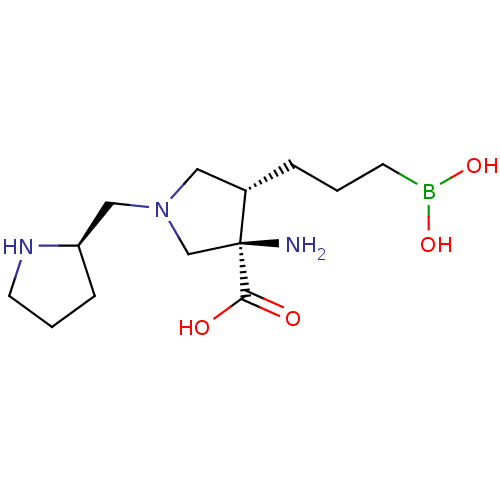 (CHEMBL4594157) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16484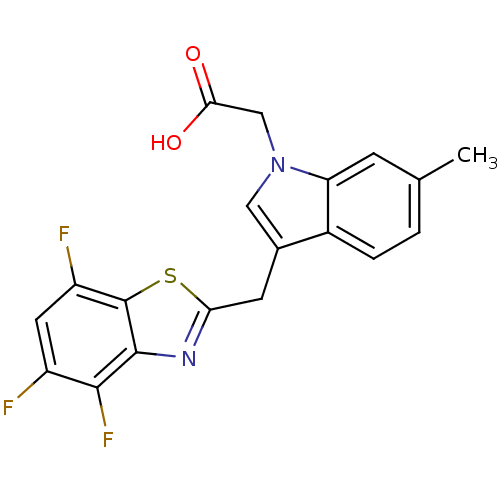 (2-{6-methyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16472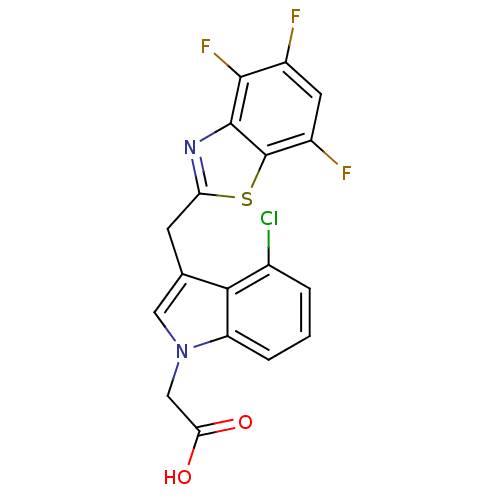 (2-{4-chloro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509017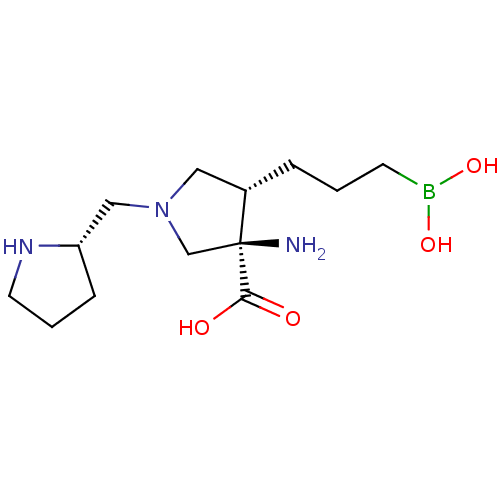 (CHEMBL4578964) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16474 (2-{5-fluoro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16478 (2-[5-(benzyloxy)-3-[(4,5,7-trifluoro-1,3-benzothia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16314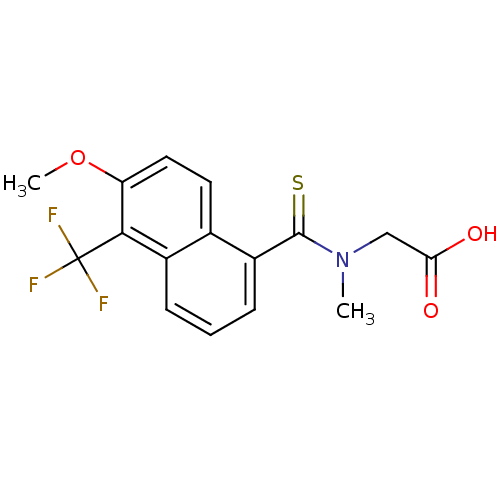 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16475 (2-{5-bromo-3-[(4,5,7-trifluoro-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16490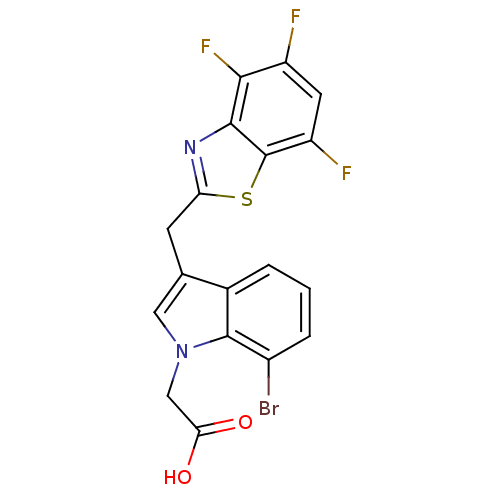 (2-{7-bromo-3-[(4,5,7-trifluoro-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16487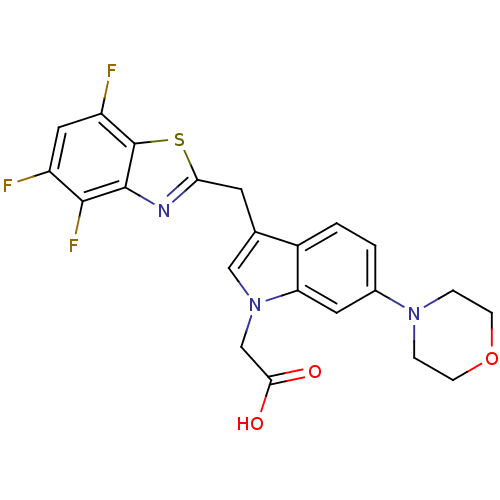 (2-[6-(morpholin-4-yl)-3-[(4,5,7-trifluoro-1,3-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509009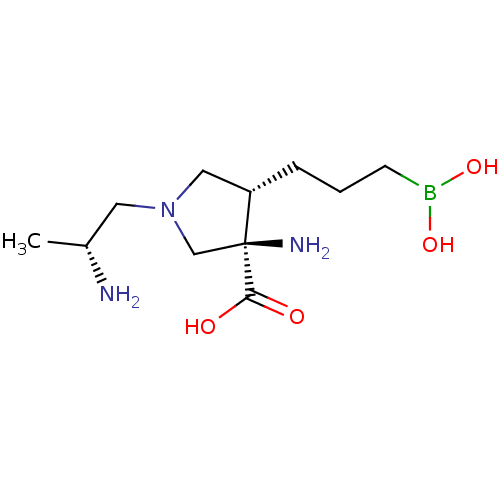 (CHEMBL4530103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16494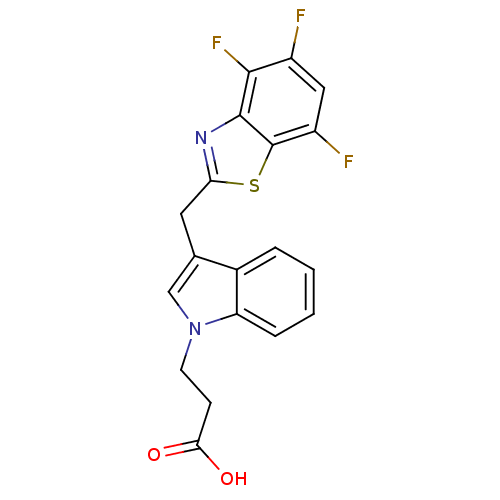 (3-{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439246 (CHEMBL2418830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509019 (CHEMBL4459462) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509003 (CHEMBL4556601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439247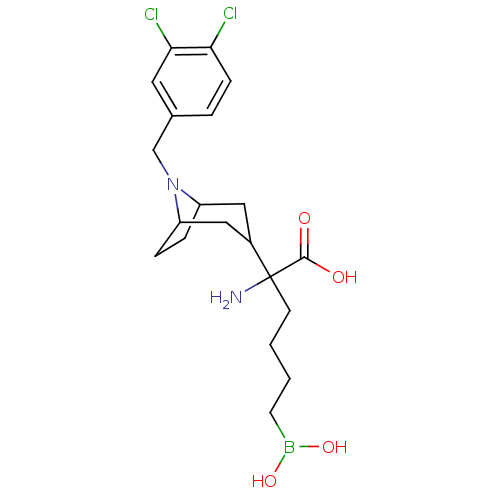 (CHEMBL2418831) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439245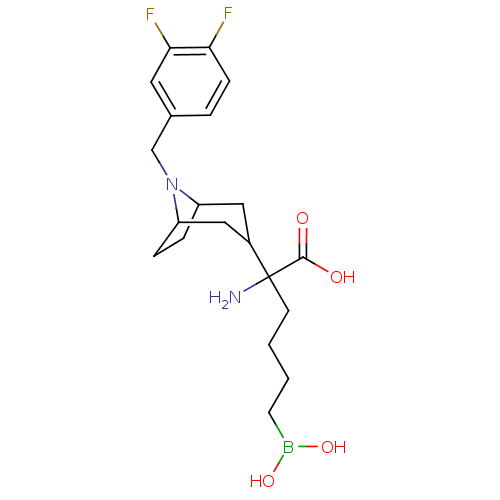 (CHEMBL2418991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439247 (CHEMBL2418831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439244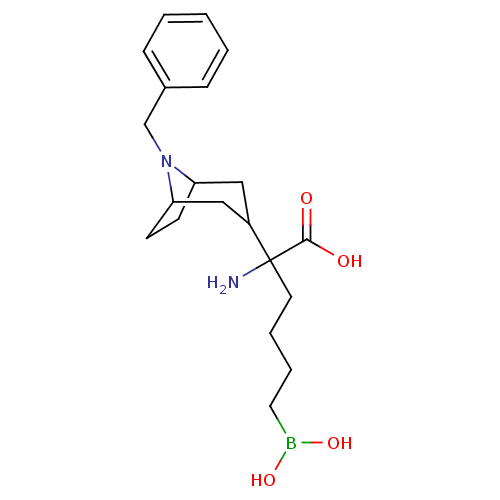 (CHEMBL2418829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16486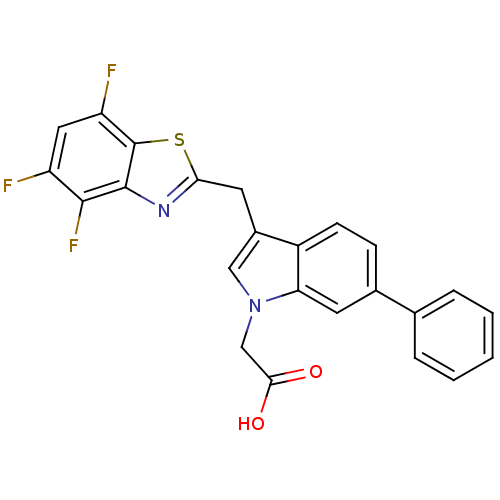 (2-{6-phenyl-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16479 (2-{5-phenoxy-3-[(4,5,7-trifluoro-1,3-benzothiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439246 (CHEMBL2418830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50509013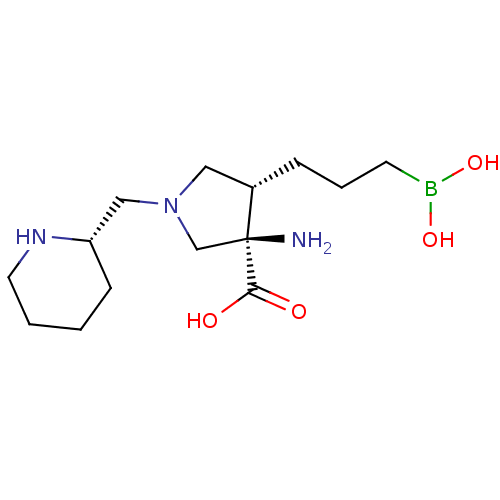 (CHEMBL4434802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 BindingDB Entry DOI: 10.7270/Q2X92FM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439245 (CHEMBL2418991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16463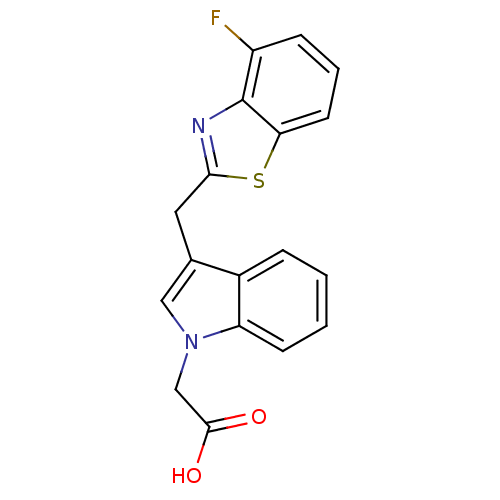 (2-{3-[(4-fluoro-1,3-benzothiazol-2-yl)methyl]-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439244 (CHEMBL2418829) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 617 total ) | Next | Last >> |