

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346448 (2-(2-(((2S,6S,13S,E)-13-Amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331042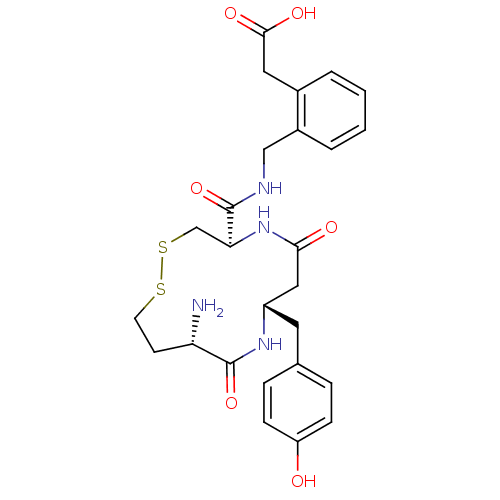 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346455 (2-(2-(((2S,5S,13S,E)-13-Amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331046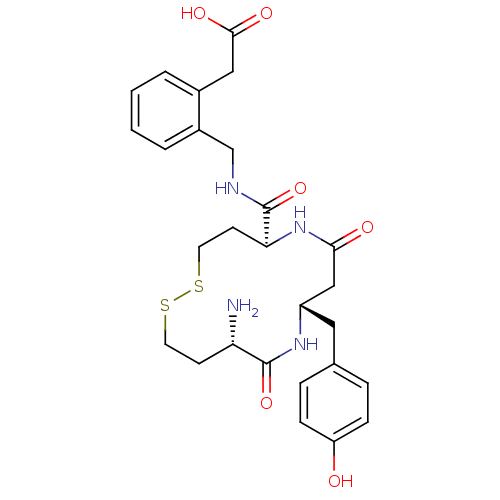 (2-(2-(((5S,9S,12S)-12-amino-9-(4-hydroxybenzyl)-7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]Angiotensin 4 from human recombinant IRAP expressed in CHOK1 cells | Bioorg Med Chem 16: 6924-35 (2008) Article DOI: 10.1016/j.bmc.2008.05.046 BindingDB Entry DOI: 10.7270/Q2B857XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415629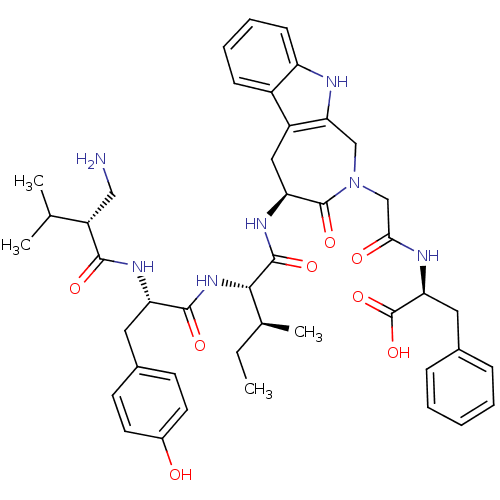 (CHEMBL1077583) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331035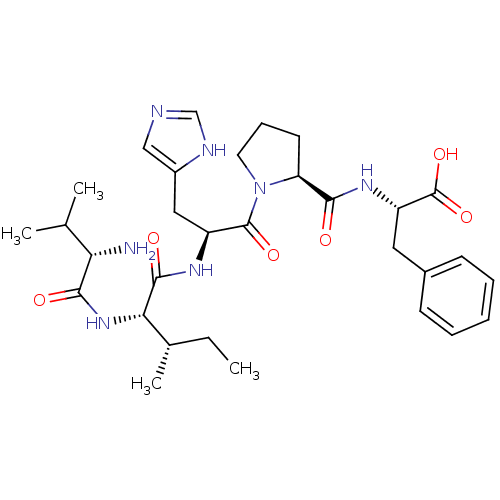 ((S)-2-((S)-1-((S)-2-((2S,3S)-2-((S)-2-amino-3-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331046 (2-(2-(((5S,9S,12S)-12-amino-9-(4-hydroxybenzyl)-7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415628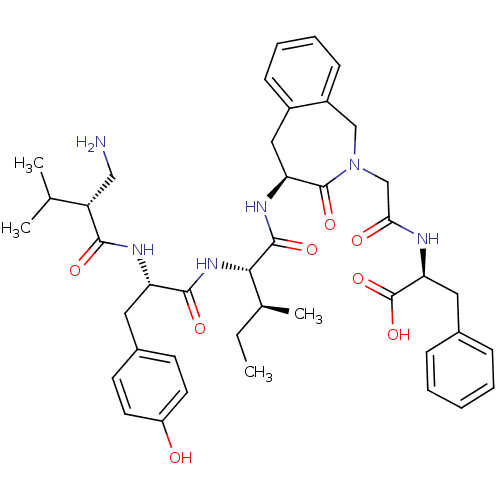 (CHEMBL1077582) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331044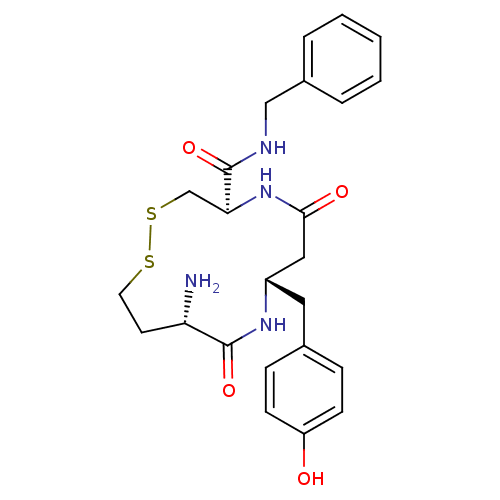 ((4R,8S,11S)-11-amino-N-benzyl-8-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331048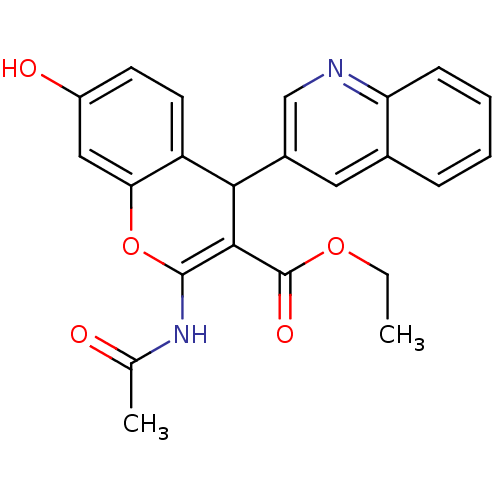 (CHEMBL1277437 | ethyl 2-acetamido-7-hydroxy-4-(qui...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50478359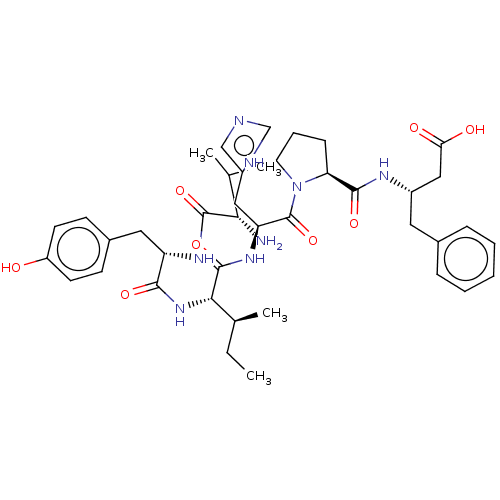 (CHEMBL408871) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331048 (CHEMBL1277437 | ethyl 2-acetamido-7-hydroxy-4-(qui...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331038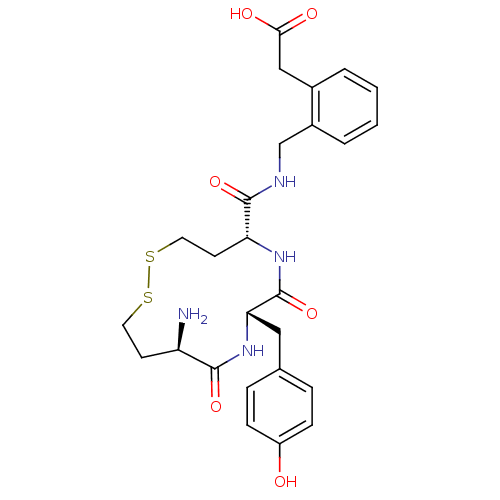 (2-(2-(((5R,8S,11R)-11-amino-8-(4-hydroxybenzyl)-7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331044 ((4R,8S,11S)-11-amino-N-benzyl-8-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346461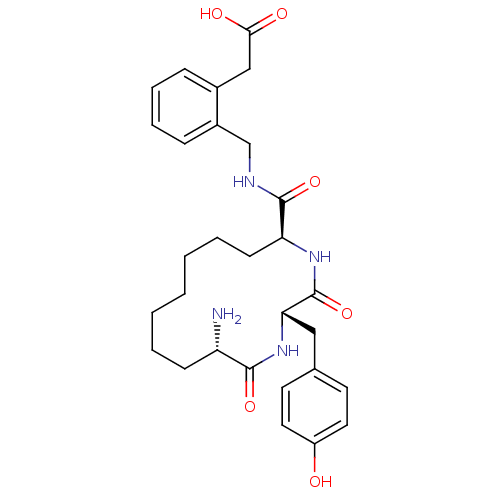 (2-(2-(((2S,5S,13S)-13-amino-2-(4-hydroxybenzyl)-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331038 (2-(2-(((5R,8S,11R)-11-amino-8-(4-hydroxybenzyl)-7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415633 (CHEMBL259019) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415633 (CHEMBL259019) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346452 (2-(2-(((2S,6S,13S,Z)-13-amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331047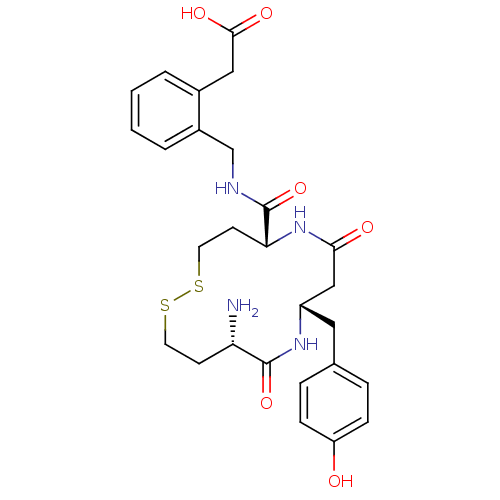 (2-(2-(((5R,9S,12S)-12-amino-9-(4-hydroxybenzyl)-7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331042 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346456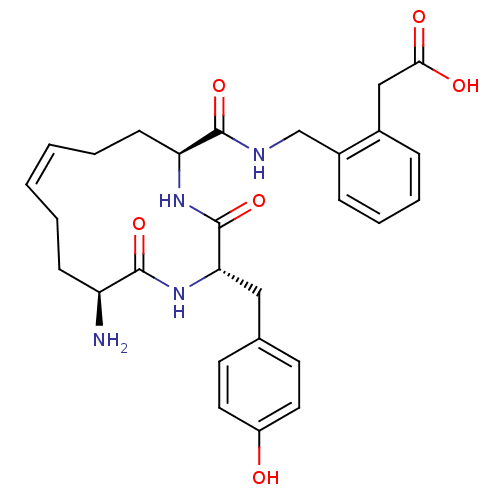 (2-(2-(((2S,5S,12S,Z)-12-amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346451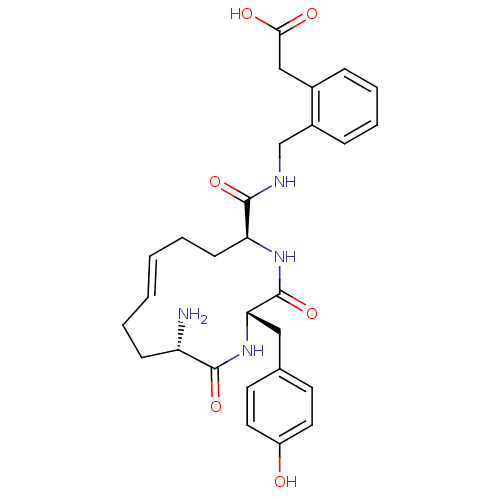 (2-(2-(((2S,5S,12S,E)-12-amino-2-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415627 (CHEMBL1077592) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415632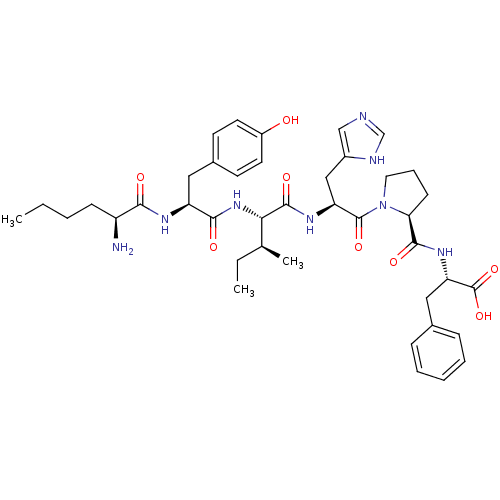 (CHEMBL1077586) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415631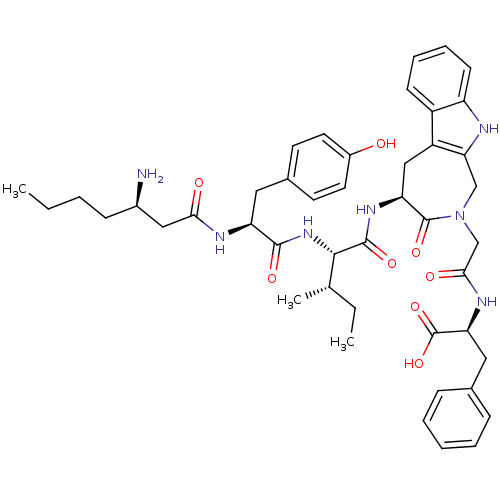 (CHEMBL1077585) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331040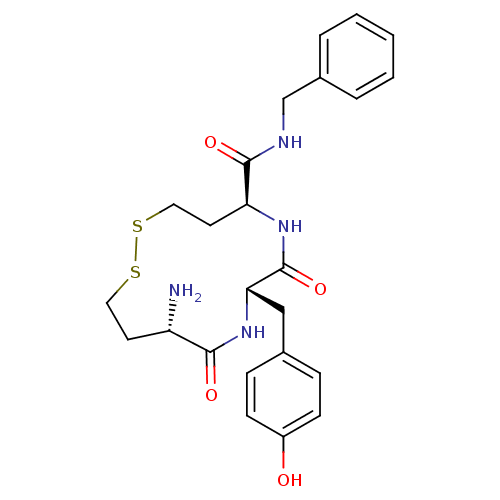 ((5S,8S,11S)-11-amino-N-benzyl-8-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | Bioorg Med Chem 16: 6924-35 (2008) Article DOI: 10.1016/j.bmc.2008.05.046 BindingDB Entry DOI: 10.7270/Q2B857XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331035 ((S)-2-((S)-1-((S)-2-((2S,3S)-2-((S)-2-amino-3-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50346453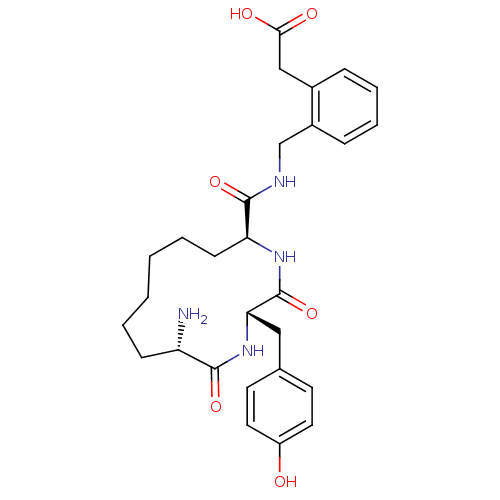 (2-(2-(((2S,5S,12S)-12-amino-2-(4-hydroxybenzyl)-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... | J Med Chem 54: 3779-92 (2011) Article DOI: 10.1021/jm200036n BindingDB Entry DOI: 10.7270/Q2348KPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50478364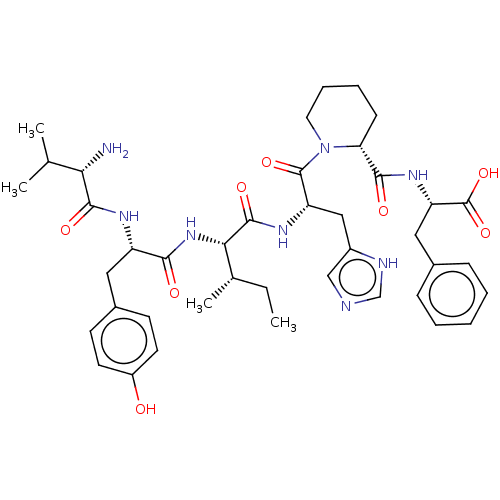 (CHEMBL248592) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331045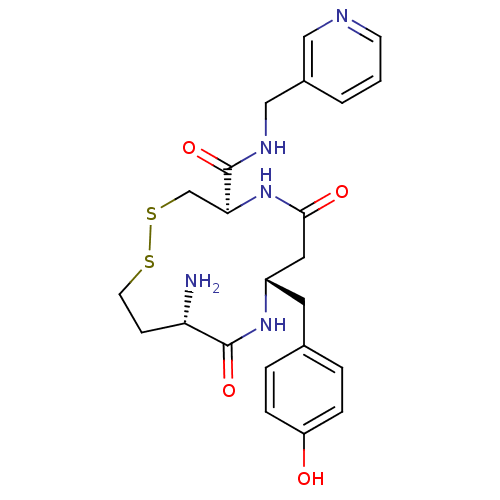 ((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,10-diox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331040 ((5S,8S,11S)-11-amino-N-benzyl-8-(4-hydroxybenzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415634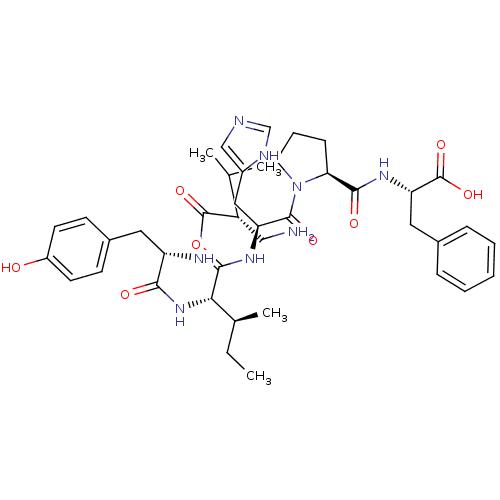 (CHEMBL260622) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415634 (CHEMBL260622) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50478356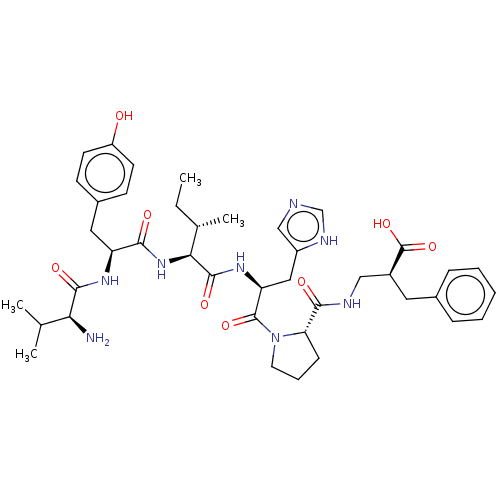 (CHEMBL408132) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415630 (CHEMBL1077584) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50478366 (CHEMBL258480) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant APN expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50415628 (CHEMBL1077582) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]valsartan from human recombinant AT1 receptor expressed in CHO cells after 40 mins by liquid scintillation counting | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50478362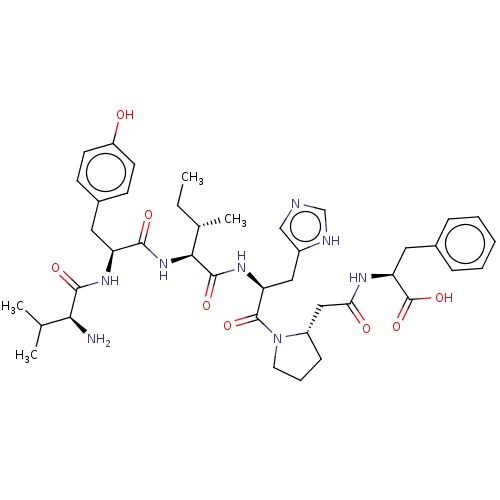 (CHEMBL261417) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50478360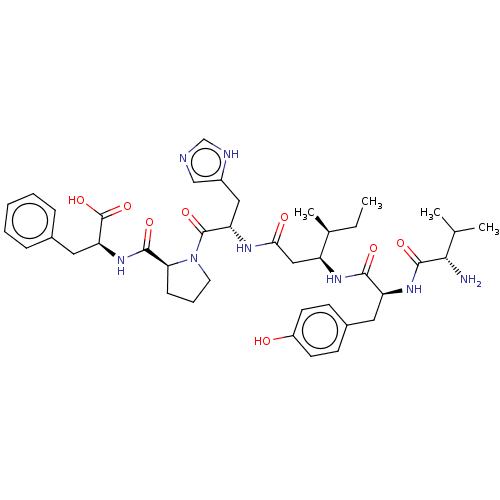 (CHEMBL261731) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50478354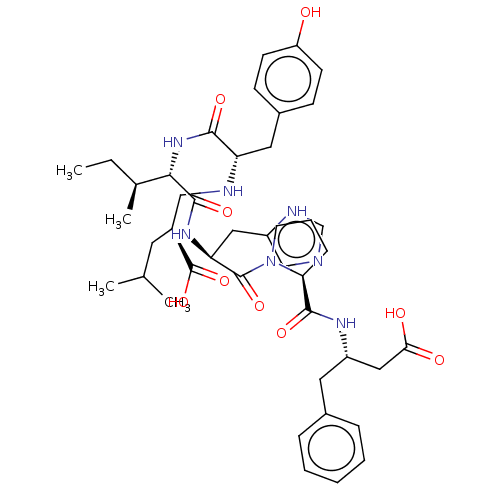 (CHEMBL261422) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50478367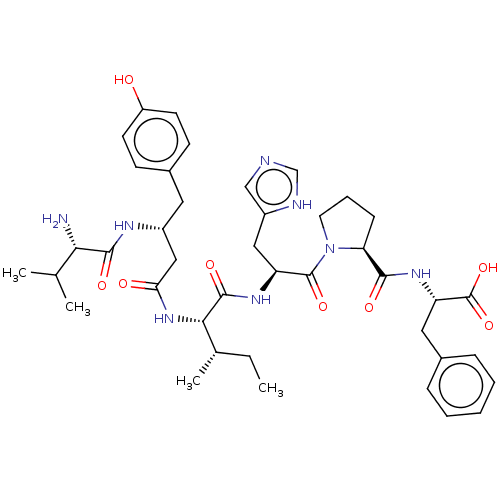 (CHEMBL259382) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50478357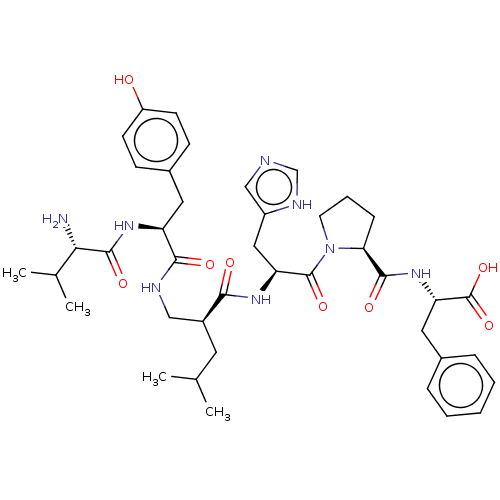 (CHEMBL428457) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50478357 (CHEMBL428457) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of human recombinant IRAP expressed in HEK293 cells | J Med Chem 51: 2291-6 (2008) Article DOI: 10.1021/jm701490g BindingDB Entry DOI: 10.7270/Q25M68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415626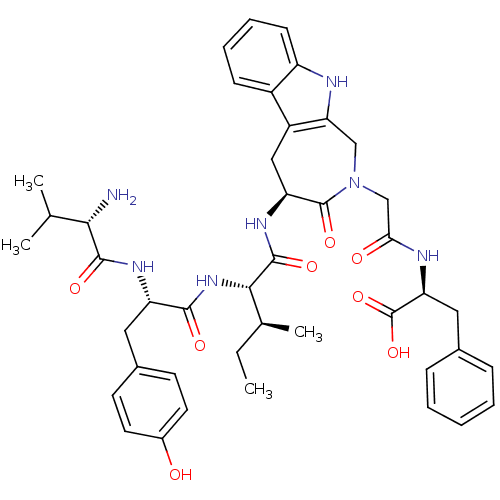 (CHEMBL1077591) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 195 total ) | Next | Last >> |