 Found 116 hits with Last Name = 'vendome' and Initial = 'j'
Found 116 hits with Last Name = 'vendome' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241831

(CHEMBL4062273 | US10626113, Compound M | US1089975...)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H21ClN4O2/c1-14(29)28-8-7-21-18(13-28)23(17-9-15(11-25)3-5-20(17)27-21)26-12-16-4-6-22(30-2)19(24)10-16/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241840
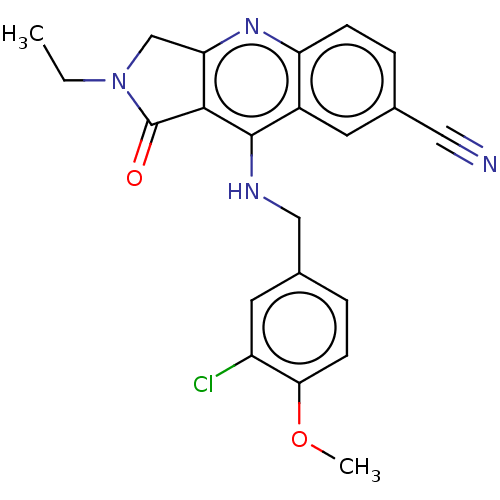
(CHEMBL4072903 | US10899756, Compound K)Show SMILES CCN1Cc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O)C#N Show InChI InChI=1S/C22H19ClN4O2/c1-3-27-12-18-20(22(27)28)21(15-8-13(10-24)4-6-17(15)26-18)25-11-14-5-7-19(29-2)16(23)9-14/h4-9H,3,11-12H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241832
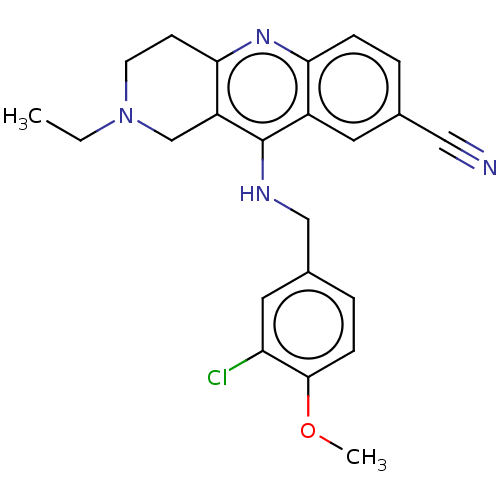
(CHEMBL4083986 | US10626113, Compound C | US1089975...)Show SMILES CCN1CCc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1)C#N Show InChI InChI=1S/C23H23ClN4O/c1-3-28-9-8-21-18(14-28)23(17-10-15(12-25)4-6-20(17)27-21)26-13-16-5-7-22(29-2)19(24)11-16/h4-7,10-11H,3,8-9,13-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241842
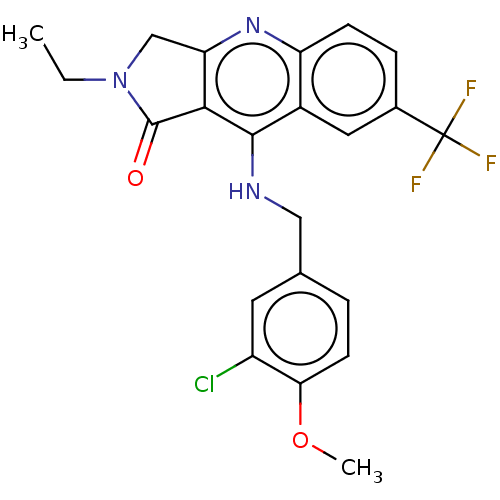
(CHEMBL4064315 | US10899756, Compound M)Show SMILES CCN1Cc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O)C(F)(F)F Show InChI InChI=1S/C22H19ClF3N3O2/c1-3-29-11-17-19(21(29)30)20(27-10-12-4-7-18(31-2)15(23)8-12)14-9-13(22(24,25)26)5-6-16(14)28-17/h4-9H,3,10-11H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of adenylate cyclase via Adenosine A1 receptor in rat fat cell membranes |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241835
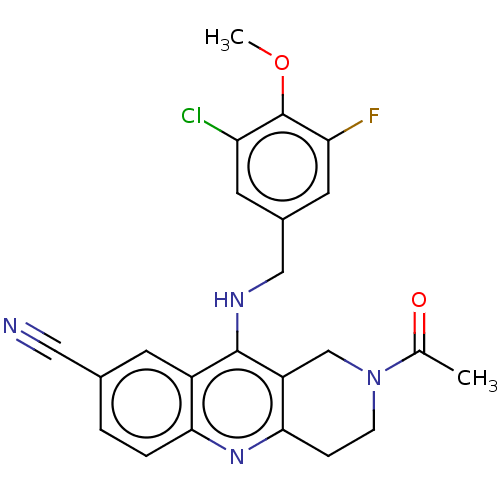
(CHEMBL4092717 | US10899756, Compound AC)Show SMILES COc1c(F)cc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H20ClFN4O2/c1-13(30)29-6-5-21-17(12-29)22(16-7-14(10-26)3-4-20(16)28-21)27-11-15-8-18(24)23(31-2)19(25)9-15/h3-4,7-9H,5-6,11-12H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50242157
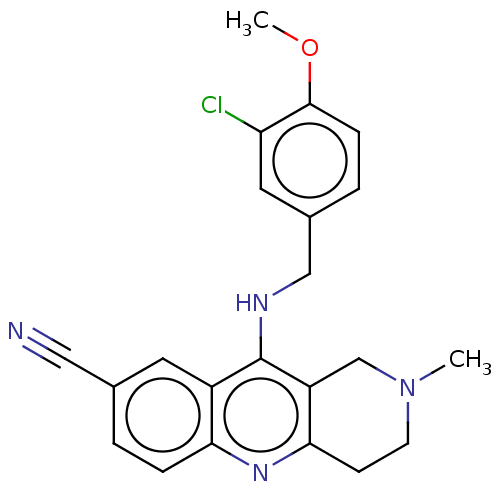
(CHEMBL4070894 | US10626113, Compound B | US1089975...)Show SMILES COc1ccc(CNc2c3CN(C)CCc3nc3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H21ClN4O/c1-27-8-7-20-17(13-27)22(16-9-14(11-24)3-5-19(16)26-20)25-12-15-4-6-21(28-2)18(23)10-15/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of K+ stimulated gastric ATPase |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241834
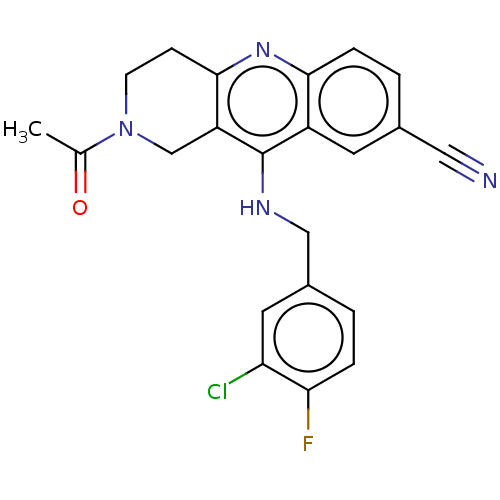
(CHEMBL4065036 | US10626113, Compound J | US1089975...)Show SMILES CC(=O)N1CCc2nc3ccc(cc3c(NCc3ccc(F)c(Cl)c3)c2C1)C#N Show InChI InChI=1S/C22H18ClFN4O/c1-13(29)28-7-6-21-17(12-28)22(16-8-14(10-25)3-5-20(16)27-21)26-11-15-2-4-19(24)18(23)9-15/h2-5,8-9H,6-7,11-12H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241837

(CHEMBL4102913 | US10626113, Compound G | US1089975...)Show InChI InChI=1S/C21H19ClN4O/c1-27-20-5-3-14(9-17(20)22)11-25-21-15-8-13(10-23)2-4-18(15)26-19-6-7-24-12-16(19)21/h2-5,8-9,24H,6-7,11-12H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241843
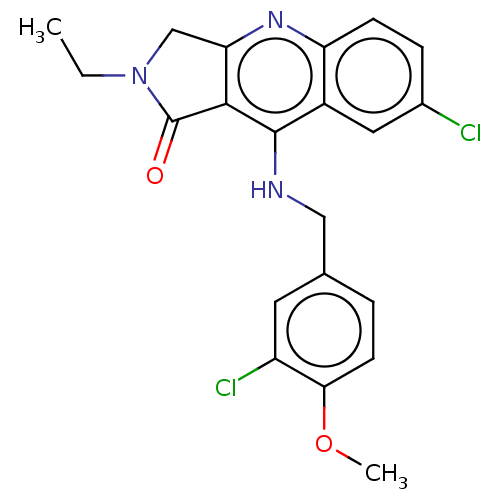
(CHEMBL4102250 | US10899756, Compound N)Show SMILES CCN1Cc2nc3ccc(Cl)cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O Show InChI InChI=1S/C21H19Cl2N3O2/c1-3-26-11-17-19(21(26)27)20(14-9-13(22)5-6-16(14)25-17)24-10-12-4-7-18(28-2)15(23)8-12/h4-9H,3,10-11H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241839
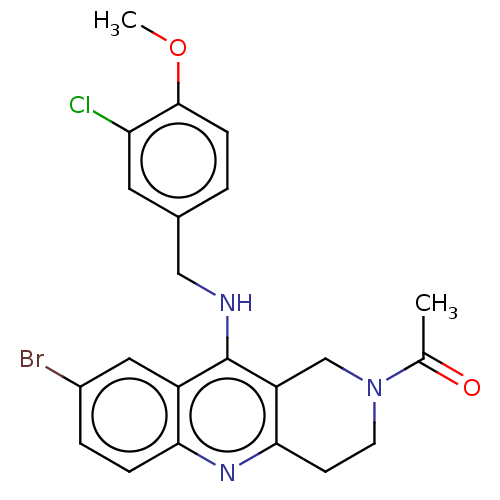
(CHEMBL4083346)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(Br)cc23)C(C)=O)cc1Cl Show InChI InChI=1S/C22H21BrClN3O2/c1-13(28)27-8-7-20-17(12-27)22(16-10-15(23)4-5-19(16)26-20)25-11-14-3-6-21(29-2)18(24)9-14/h3-6,9-10H,7-8,11-12H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241841

(CHEMBL4092040 | US10899756, Compound L)Show SMILES CCN1Cc2nc3ccc(OC)cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O Show InChI InChI=1S/C22H22ClN3O3/c1-4-26-12-18-20(22(26)27)21(15-10-14(28-2)6-7-17(15)25-18)24-11-13-5-8-19(29-3)16(23)9-13/h5-10H,4,11-12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
Cone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha'
(Homo sapiens (Human)) | BDBM50241840

(CHEMBL4072903 | US10899756, Compound K)Show SMILES CCN1Cc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O)C#N Show InChI InChI=1S/C22H19ClN4O2/c1-3-27-12-18-20(22(27)28)21(15-8-13(10-24)4-6-17(15)26-18)25-11-14-5-7-19(29-2)16(23)9-14/h4-9H,3,11-12H2,1-2H3,(H,25,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of K+ stimulated gastric ATPase |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50242154

(CHEMBL4099788 | US10626113, Compound A | US1089975...)Show SMILES COc1ccc(CNc2c3CN(C)CCc3nc3ccc(Br)cc23)cc1Cl Show InChI InChI=1S/C21H21BrClN3O/c1-26-8-7-19-16(12-26)21(15-10-14(22)4-5-18(15)25-19)24-11-13-3-6-20(27-2)17(23)9-13/h3-6,9-10H,7-8,11-12H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of K+ stimulated gastric ATPase |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
Cone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha'
(Homo sapiens (Human)) | BDBM50241831

(CHEMBL4062273 | US10626113, Compound M | US1089975...)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H21ClN4O2/c1-14(29)28-8-7-21-18(13-28)23(17-9-15(11-25)3-5-20(17)27-21)26-12-16-4-6-22(30-2)19(24)10-16/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,26,27) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE6C using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241838
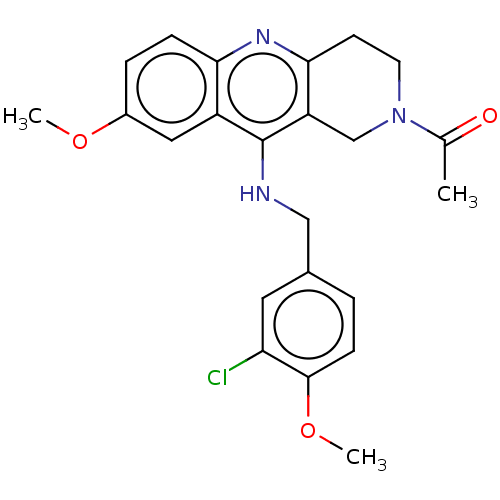
(CHEMBL4061516)Show SMILES COc1ccc2nc3CCN(Cc3c(NCc3ccc(OC)c(Cl)c3)c2c1)C(C)=O Show InChI InChI=1S/C23H24ClN3O3/c1-14(28)27-9-8-21-18(13-27)23(17-11-16(29-2)5-6-20(17)26-21)25-12-15-4-7-22(30-3)19(24)10-15/h4-7,10-11H,8-9,12-13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50242155

(CHEMBL4100445 | US10899756, Compound AA)Show SMILES CC(=O)N1CCc2nc3ccc(cc3c(NCc3ccnc(Cl)c3)c2C1)C#N Show InChI InChI=1S/C21H18ClN5O/c1-13(28)27-7-5-19-17(12-27)21(25-11-15-4-6-24-20(22)9-15)16-8-14(10-23)2-3-18(16)26-19/h2-4,6,8-9H,5,7,11-12H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of K+ stimulated gastric ATPase |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50242158

(CHEMBL4084285 | US10899756, Compound 0)Show SMILES CCN1Cc2nc3ccccc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O Show InChI InChI=1S/C21H20ClN3O2/c1-3-25-12-17-19(21(25)26)20(14-6-4-5-7-16(14)24-17)23-11-13-8-9-18(27-2)15(22)10-13/h4-10H,3,11-12H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of K+ stimulated gastric ATPase |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50587109
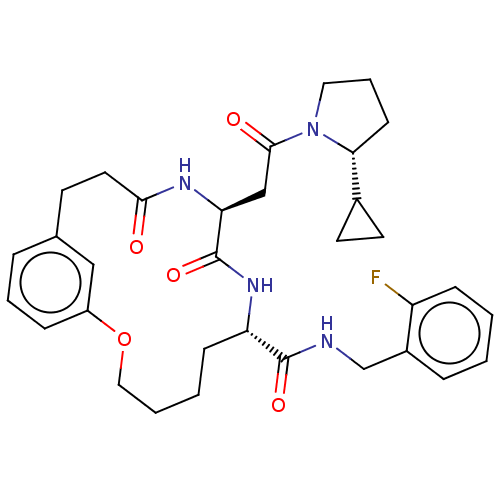
(CHEMBL5079787)Show SMILES Fc1ccccc1CNC(=O)[C@@H]1CCCCOc2cccc(CCC(=O)N[C@@H](CC(=O)N3CCC[C@@H]3C3CC3)C(=O)N1)c2 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human 20S immunoproteasome beta-5i subunit using Suc-LLVY-AMC as substrate measured over 1.5 to 2 hrs by plate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00296
BindingDB Entry DOI: 10.7270/Q2MG7TFF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50587109

(CHEMBL5079787)Show SMILES Fc1ccccc1CNC(=O)[C@@H]1CCCCOc2cccc(CCC(=O)N[C@@H](CC(=O)N3CCC[C@@H]3C3CC3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human 20S constitutive proteasome beta-5c subunit using Suc-LLVY-AMC as substrate measured over 1.5 to 2 hrs by plate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00296
BindingDB Entry DOI: 10.7270/Q2MG7TFF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50242159
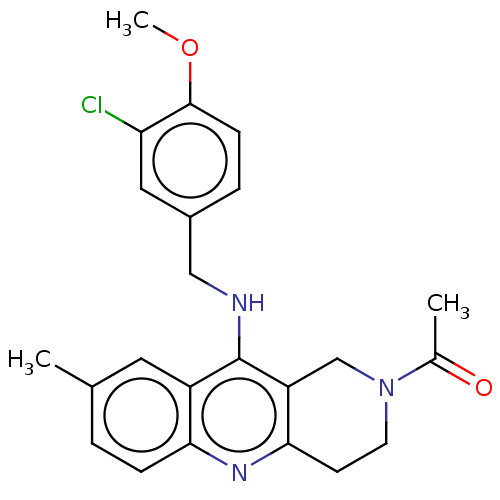
(CHEMBL4084966)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(C)cc23)C(C)=O)cc1Cl Show InChI InChI=1S/C23H24ClN3O2/c1-14-4-6-20-17(10-14)23(18-13-27(15(2)28)9-8-21(18)26-20)25-12-16-5-7-22(29-3)19(24)11-16/h4-7,10-11H,8-9,12-13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of gastric H+/K+ ATPase |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50242156

(CHEMBL4096417 | US10899756, Compound P)Show SMILES COc1ccc(CNc2c3C(=O)N(Cc3nc3ccccc23)C(C)(C)C)cc1Cl Show InChI InChI=1S/C23H24ClN3O2/c1-23(2,3)27-13-18-20(22(27)28)21(15-7-5-6-8-17(15)26-18)25-12-14-9-10-19(29-4)16(24)11-14/h5-11H,12-13H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 333 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of K+ stimulated gastric ATPase |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241833

(CHEMBL4073617 | US10626113, Compound I | US1089975...)Show SMILES CC(=O)N1CCc2nc3ccc(cc3c(NCc3ccc(OC(F)(F)F)c(Cl)c3)c2C1)C#N Show InChI InChI=1S/C23H18ClF3N4O2/c1-13(32)31-7-6-20-17(12-31)22(16-8-14(10-28)2-4-19(16)30-20)29-11-15-3-5-21(18(24)9-15)33-23(25,26)27/h2-5,8-9H,6-7,11-12H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 337 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50587110
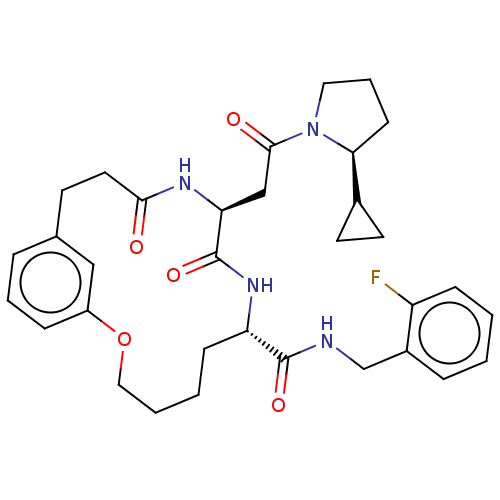
(CHEMBL5079934)Show SMILES Fc1ccccc1CNC(=O)[C@@H]1CCCCOc2cccc(CCC(=O)N[C@@H](CC(=O)N3CCC[C@H]3C3CC3)C(=O)N1)c2 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human 20S immunoproteasome beta-5i subunit using Suc-LLVY-AMC as substrate measured over 1.5 to 2 hrs by plate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00296
BindingDB Entry DOI: 10.7270/Q2MG7TFF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50587110

(CHEMBL5079934)Show SMILES Fc1ccccc1CNC(=O)[C@@H]1CCCCOc2cccc(CCC(=O)N[C@@H](CC(=O)N3CCC[C@H]3C3CC3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human 20S constitutive proteasome beta-5c subunit using Suc-LLVY-AMC as substrate measured over 1.5 to 2 hrs by plate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00296
BindingDB Entry DOI: 10.7270/Q2MG7TFF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600764
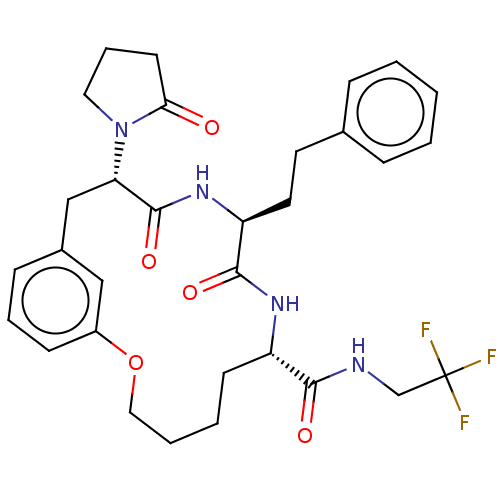
(CHEMBL5182017)Show SMILES FC(F)(F)CNC(=O)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600773
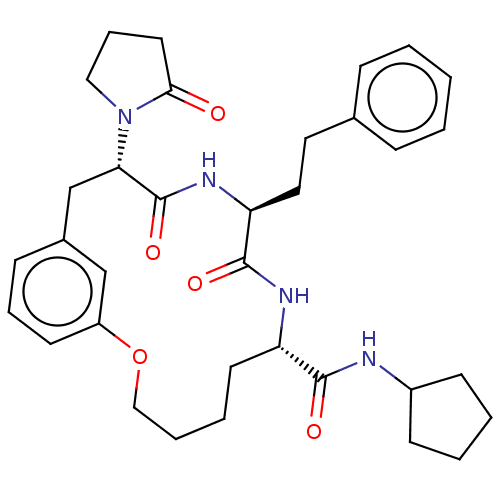
(CHEMBL5174759 | US20240043470, Compound 3-08)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600775
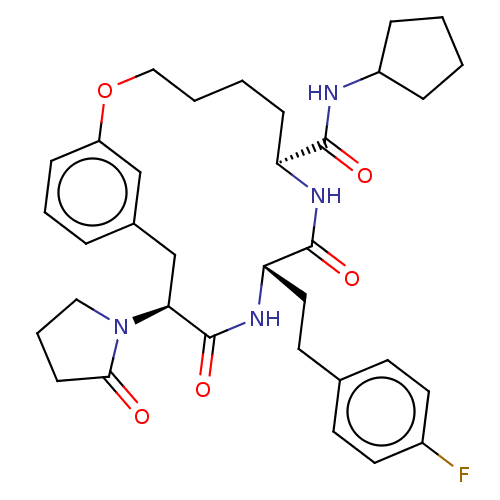
(CHEMBL5207942 | US20240043470, Compound 3-28)Show SMILES Fc1ccc(CC[C@@H]2NC(=O)[C@H](Cc3cccc(OCCCC[C@H](NC2=O)C(=O)NC2CCCC2)c3)N2CCCC2=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600767
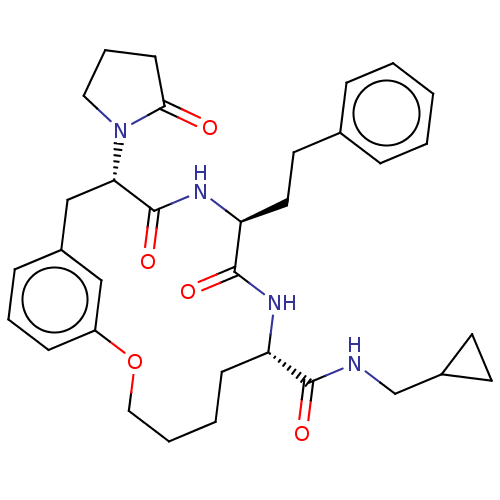
(CHEMBL5191353 | US20240043470, Compound 3-07)Show SMILES O=C(NCC1CC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600776

(CHEMBL5198676 | US20240043470, Compound 3-42)Show SMILES FC1(F)CCN(CC[C@@H]2NC(=O)[C@H](Cc3cccc(OCCCC[C@H](NC2=O)C(=O)NC2CCCC2)c3)N2CCCC2=O)CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50583310
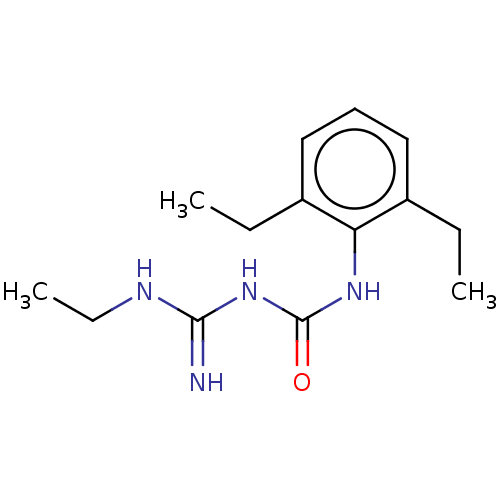
(CHEMBL5082711) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Nav1.5 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600762

(CHEMBL5180427 | US20240043470, Compound 1-53)Show SMILES CC(=O)N[C@H]1Cc2cccc(Oc3cccc(C[C@H](NC(=O)[C@H](CCc4ccccc4)NC1=O)C(=O)NCC(F)(F)F)c3)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600765
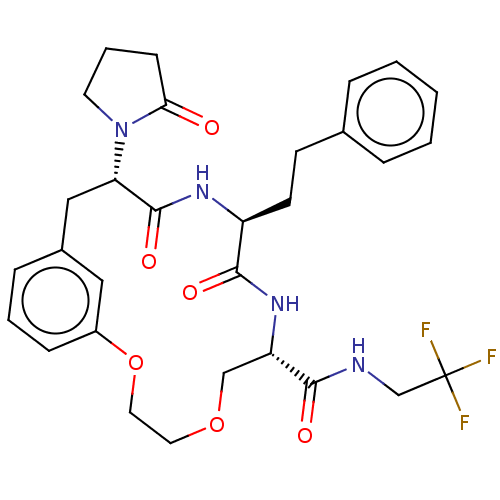
(CHEMBL5175549 | US20240043470, Compound 3-09)Show SMILES FC(F)(F)CNC(=O)[C@@H]1COCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50583310

(CHEMBL5082711) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Cav1.2 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600766

(CHEMBL5193963 | US20240043470, Compound 3-10)Show SMILES FC(F)CNC(=O)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](CCc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600768
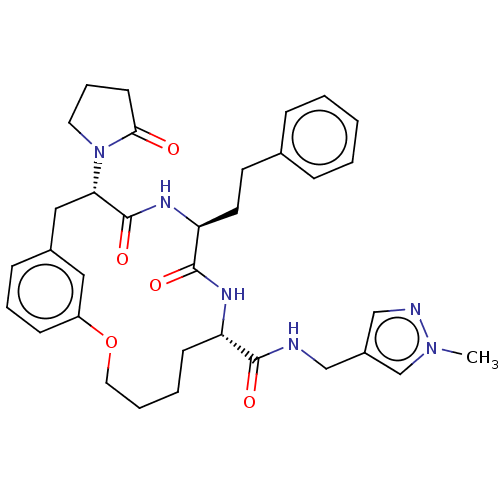
(CHEMBL5195365 | US20240043470, Compound 3-11)Show SMILES Cn1cc(CNC(=O)[C@@H]2CCCCOc3cccc(C[C@H](N4CCCC4=O)C(=O)N[C@@H](CCc4ccccc4)C(=O)N2)c3)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600774

(CHEMBL5200145 | US20240043470, Compound 3-35)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCCC3=O)C(=O)N[C@@H](COc3ccccc3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600782
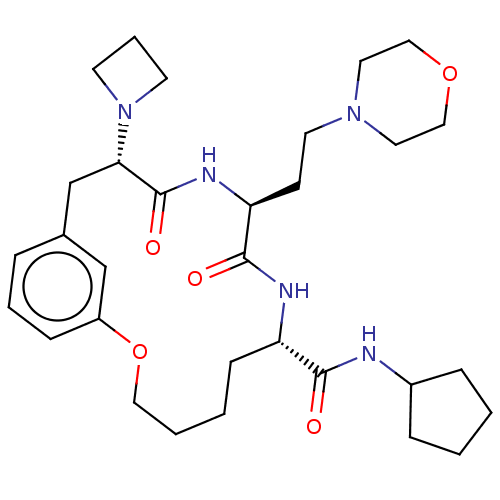
(CHEMBL5198080)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCC3)C(=O)N[C@@H](CCN3CCOCC3)C(=O)N1)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50600782

(CHEMBL5198080)Show SMILES O=C(NC1CCCC1)[C@@H]1CCCCOc2cccc(C[C@H](N3CCC3)C(=O)N[C@@H](CCN3CCOCC3)C(=O)N1)c2 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50587103
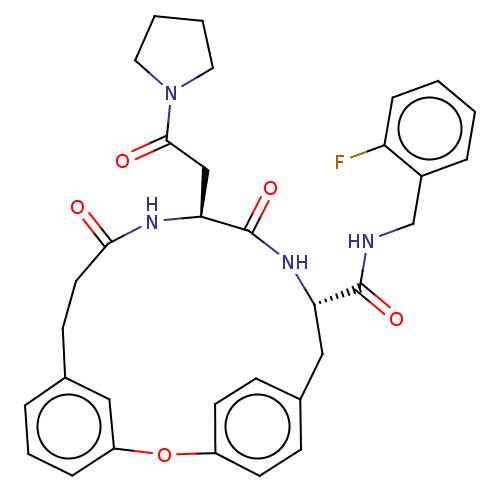
(CHEMBL5086113 | US20240043470, Compound 2-01-B)Show SMILES Fc1ccccc1CNC(=O)[C@@H]1Cc2ccc(Oc3cccc(CCC(=O)N[C@@H](CC(=O)N4CCCC4)C(=O)N1)c3)cc2 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human 20S immunoproteasome beta-5i subunit using Suc-LLVY-AMC as substrate measured over 1.5 to 2 hrs by plate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00296
BindingDB Entry DOI: 10.7270/Q2MG7TFF |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50583311
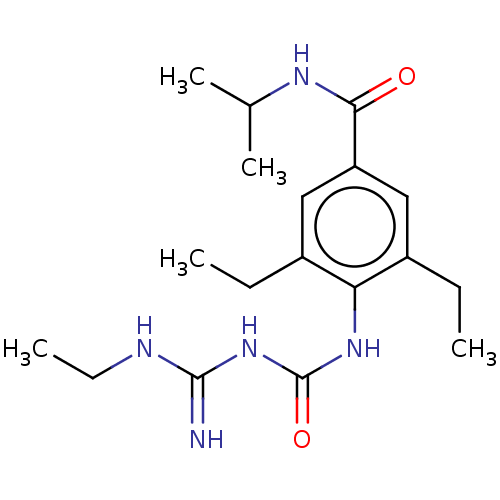
(CHEMBL5084930) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Nav1.5 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50583312
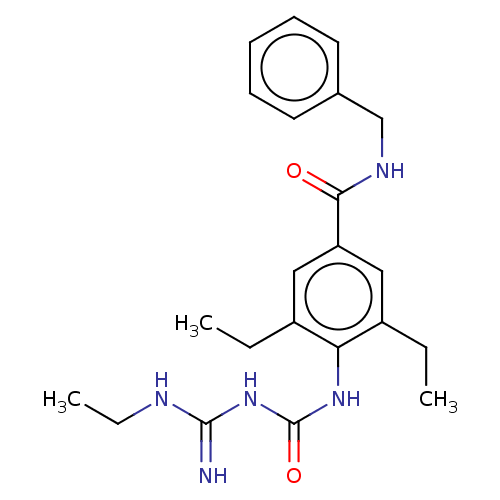
(CHEMBL5076143)Show SMILES CCNC(=N)NC(=O)Nc1c(CC)cc(cc1CC)C(=O)NCc1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Cav1.2 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50583312

(CHEMBL5076143)Show SMILES CCNC(=N)NC(=O)Nc1c(CC)cc(cc1CC)C(=O)NCc1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Nav1.5 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50600763
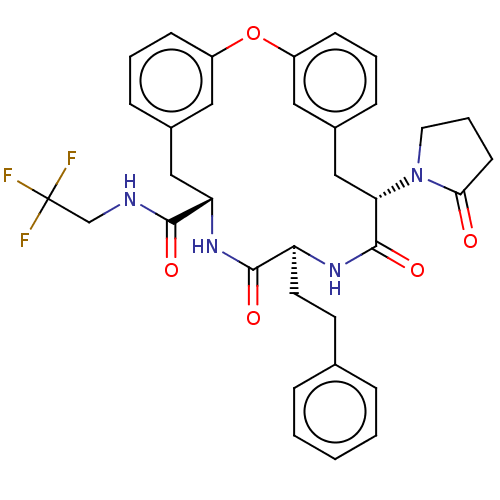
(CHEMBL5194112 | US20240043470, Compound 1-56)Show SMILES FC(F)(F)CNC(=O)[C@@H]1Cc2cccc(Oc3cccc(C[C@H](N4CCCC4=O)C(=O)N[C@@H](CCc4ccccc4)C(=O)N1)c3)c2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00611
BindingDB Entry DOI: 10.7270/Q20869BV |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50239382
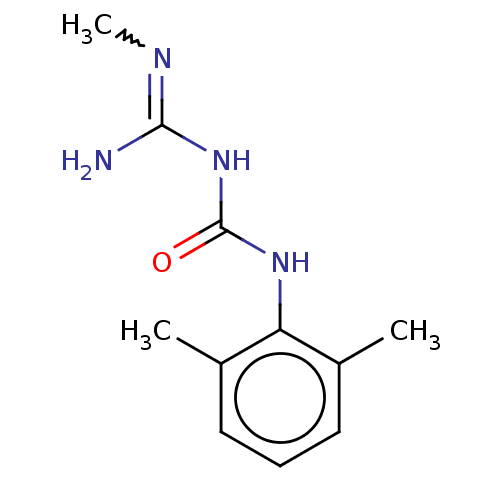
(Lidamidine)Show InChI InChI=1S/C11H16N4O/c1-7-5-4-6-8(2)9(7)14-11(16)15-10(12)13-3/h4-6H,1-3H3,(H4,12,13,14,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Cav1.2 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50583311

(CHEMBL5084930) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Cav1.2 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50583313
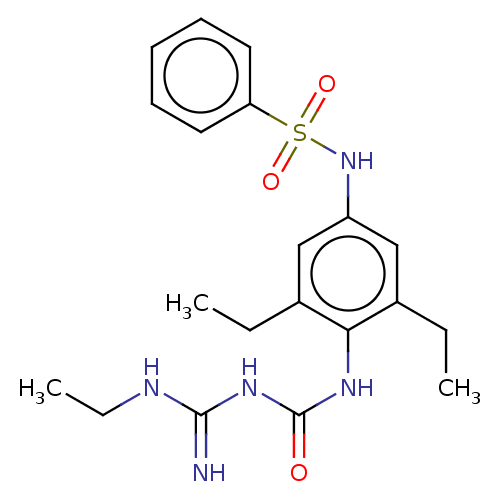
(CHEMBL5084225)Show SMILES CCNC(=N)NC(=O)Nc1c(CC)cc(NS(=O)(=O)c2ccccc2)cc1CC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Cav1.2 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50583314
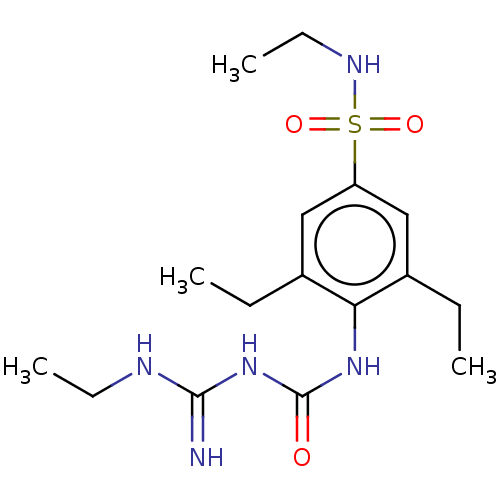
(CHEMBL5075910)Show SMILES CCNC(=N)NC(=O)Nc1c(CC)cc(cc1CC)S(=O)(=O)NCC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Cav1.2 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50239382

(Lidamidine)Show InChI InChI=1S/C11H16N4O/c1-7-5-4-6-8(2)9(7)14-11(16)15-10(12)13-3/h4-6H,1-3H3,(H4,12,13,14,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Nav1.5 expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50583314

(CHEMBL5075910)Show SMILES CCNC(=N)NC(=O)Nc1c(CC)cc(cc1CC)S(=O)(=O)NCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50583313

(CHEMBL5084225)Show SMILES CCNC(=N)NC(=O)Nc1c(CC)cc(NS(=O)(=O)c2ccccc2)cc1CC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG expressed in HEK cells by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01565
BindingDB Entry DOI: 10.7270/Q2MG7TD0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data