 Found 24 hits with Last Name = 'vig' and Initial = 'r'
Found 24 hits with Last Name = 'vig' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM1
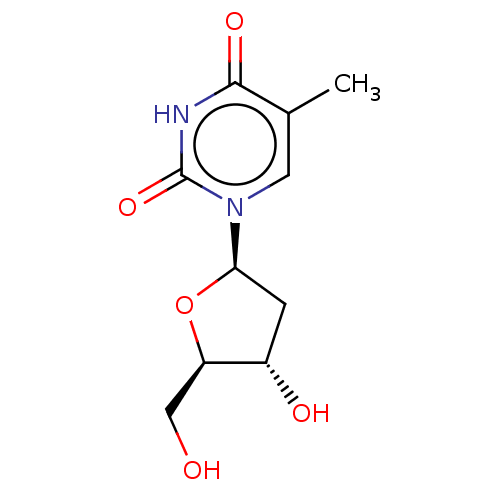
(dT | thymidine)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3,11H,1-2H3,(H2,7,13)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Genetica Biochimica ed Evoluzionistica
Curated by ChEMBL
| Assay Description
Inhibition of HSV-1 thymidine kinase |
J Med Chem 35: 4214-20 (1992)
BindingDB Entry DOI: 10.7270/Q2J67HJP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 354 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 927 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC9 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC7 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50070471
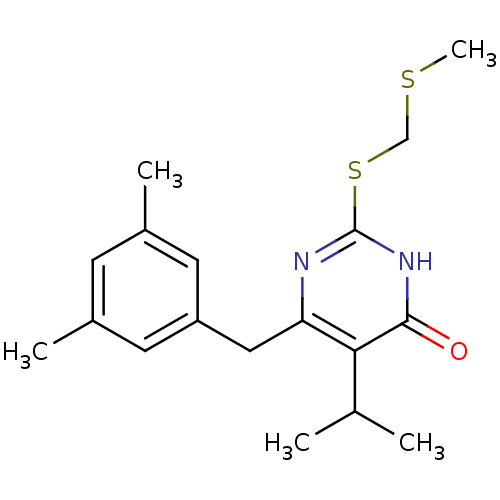
(6-(3,5-Dimethyl-benzyl)-5-isopropyl-2-methylsulfan...)Show SMILES CSCSc1nc(Cc2cc(C)cc(C)c2)c(C(C)C)c(=O)[nH]1 Show InChI InChI=1S/C18H24N2OS2/c1-11(2)16-15(9-14-7-12(3)6-13(4)8-14)19-18(20-17(16)21)23-10-22-5/h6-8,11H,9-10H2,1-5H3,(H,19,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for enzymatic inhibitory activity against recombinant HIV reverse transcriptase |
Bioorg Med Chem Lett 8: 1461-6 (1999)
BindingDB Entry DOI: 10.7270/Q24X56XN |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50070469

(6-Benzyl-5-isopropyl-2-methylsulfanylmethylsulfany...)Show InChI InChI=1S/C16H20N2OS2/c1-11(2)14-13(9-12-7-5-4-6-8-12)17-16(18-15(14)19)21-10-20-3/h4-8,11H,9-10H2,1-3H3,(H,17,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for enzymatic inhibitory activity against recombinant HIV reverse transcriptase |
Bioorg Med Chem Lett 8: 1461-6 (1999)
BindingDB Entry DOI: 10.7270/Q24X56XN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50070468

(6-Benzyl-5-ethyl-2-methylsulfanylmethylsulfanyl-3H...)Show InChI InChI=1S/C15H18N2OS2/c1-3-12-13(9-11-7-5-4-6-8-11)16-15(17-14(12)18)20-10-19-2/h4-8H,3,9-10H2,1-2H3,(H,16,17,18) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for enzymatic inhibitory activity against recombinant HIV reverse transcriptase |
Bioorg Med Chem Lett 8: 1461-6 (1999)
BindingDB Entry DOI: 10.7270/Q24X56XN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50070470

(6-Benzyl-5-methyl-2-methylsulfanylmethylsulfanyl-3...)Show InChI InChI=1S/C14H16N2OS2/c1-10-12(8-11-6-4-3-5-7-11)15-14(16-13(10)17)19-9-18-2/h3-7H,8-9H2,1-2H3,(H,15,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for enzymatic inhibitory activity against recombinant HIV reverse transcriptase |
Bioorg Med Chem Lett 8: 1461-6 (1999)
BindingDB Entry DOI: 10.7270/Q24X56XN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for enzymatic inhibitory activity against recombinant HIV reverse transcriptase |
Bioorg Med Chem Lett 8: 1461-6 (1999)
BindingDB Entry DOI: 10.7270/Q24X56XN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data