

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM14073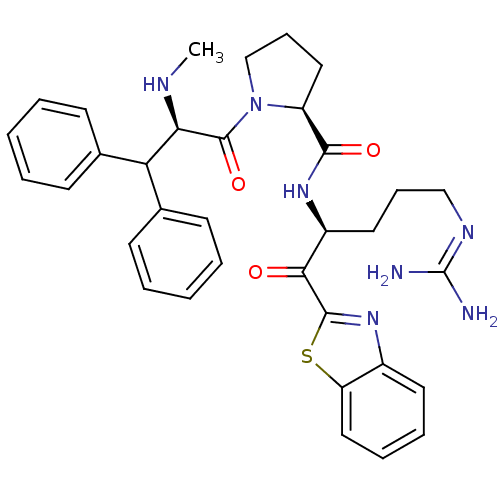 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | -72.4 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14065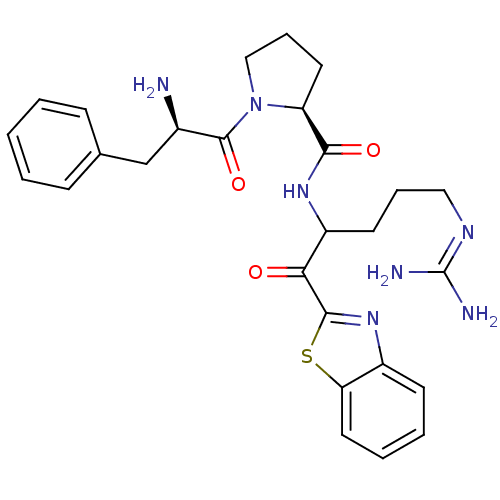 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00550 | -66.9 | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14127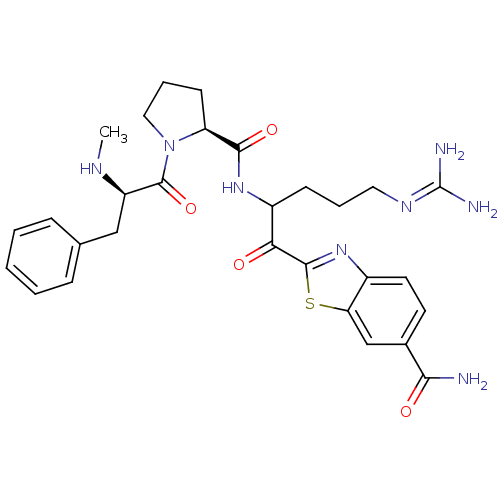 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14076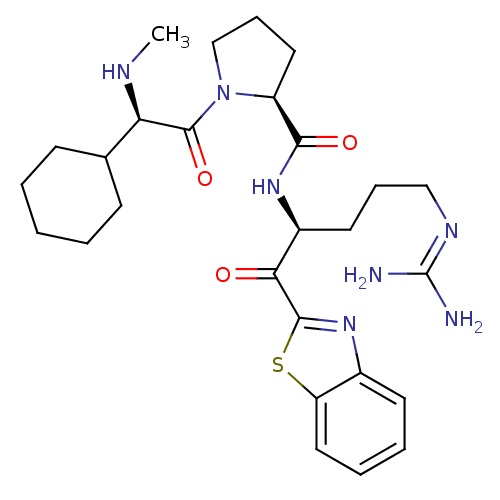 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -63.8 | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50173133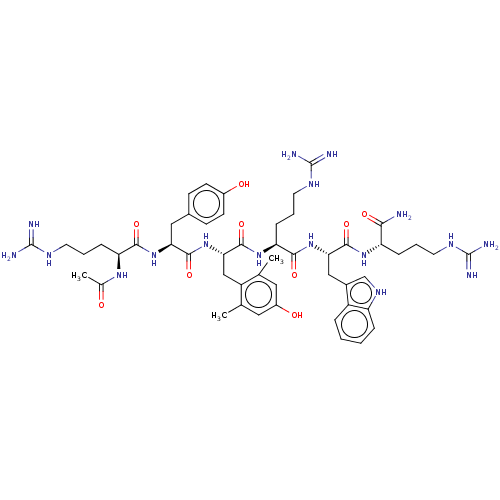 (CHEMBL3810319) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counti... | J Med Chem 59: 3777-92 (2016) Article DOI: 10.1021/acs.jmedchem.5b01976 BindingDB Entry DOI: 10.7270/Q2T72KC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50173132 (CHEMBL3808650) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counti... | J Med Chem 59: 3777-92 (2016) Article DOI: 10.1021/acs.jmedchem.5b01976 BindingDB Entry DOI: 10.7270/Q2T72KC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50070386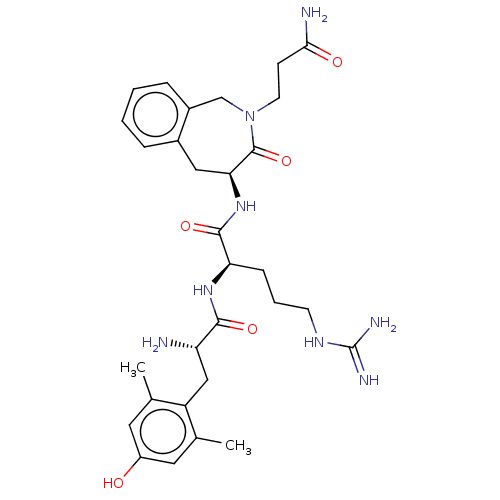 (CHEMBL3408737) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in HEK293 cell membrane incubated for 1 hr by TopCount scintillation counti... | J Med Chem 59: 3777-92 (2016) Article DOI: 10.1021/acs.jmedchem.5b01976 BindingDB Entry DOI: 10.7270/Q2T72KC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14066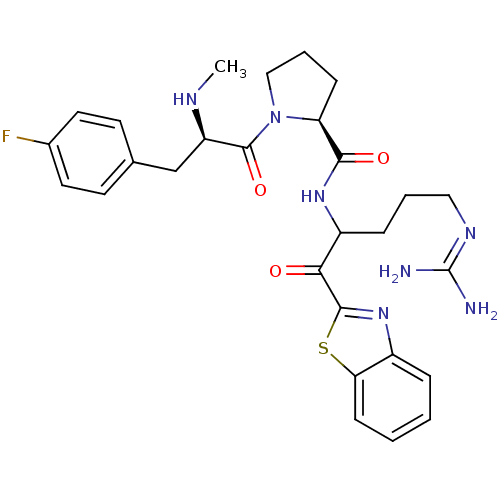 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -58.9 | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14123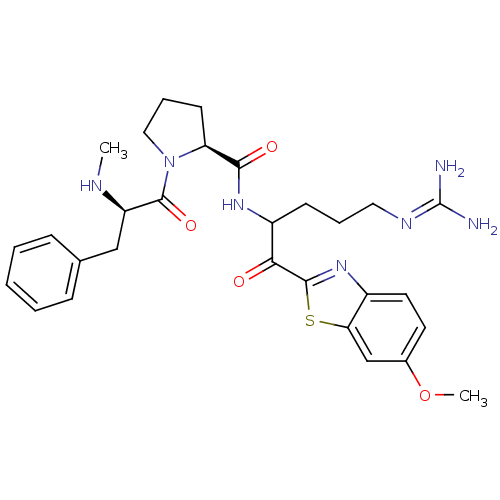 ((2S)-N-[5-carbamimidamido-1-(6-methoxy-1,3-benzoth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14125 ((2S)-N-[5-carbamimidamido-1-(6-fluoro-1,3-benzothi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14068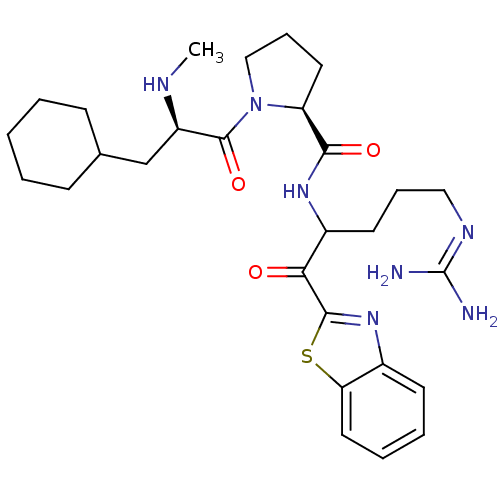 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | -57.9 | 48 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14071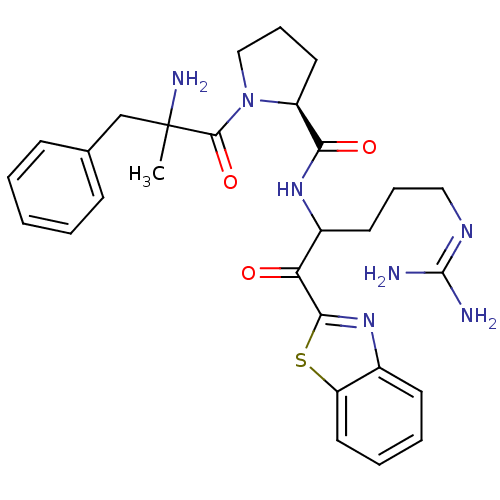 ((2S)-1-(2-amino-2-benzylpropanoyl)-N-[1-(1,3-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -57.6 | 3.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14063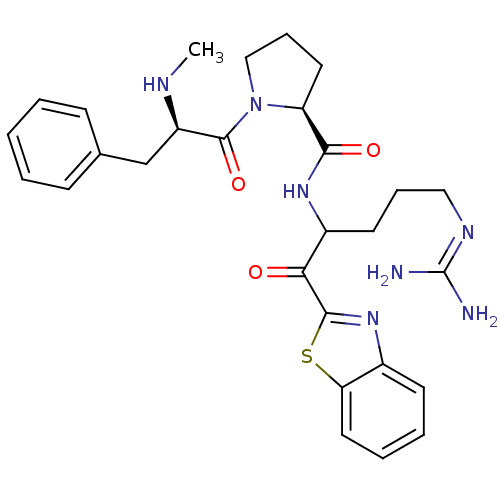 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -57.6 | 29 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14124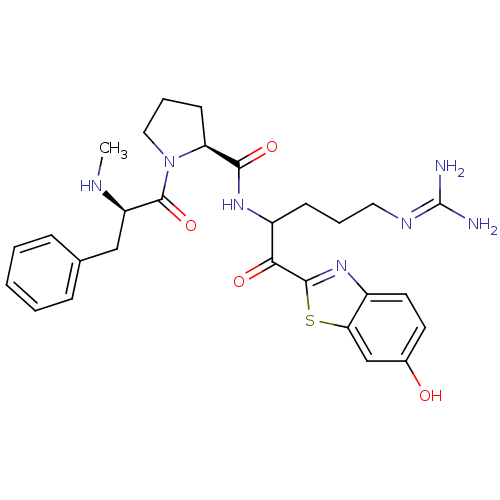 ((2S)-N-[5-carbamimidamido-1-(6-hydroxy-1,3-benzoth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500683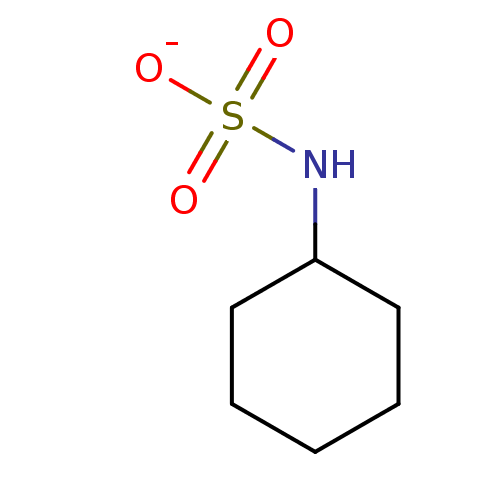 (CHEBI:82431 | E952 | Sodium Cyclamate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500684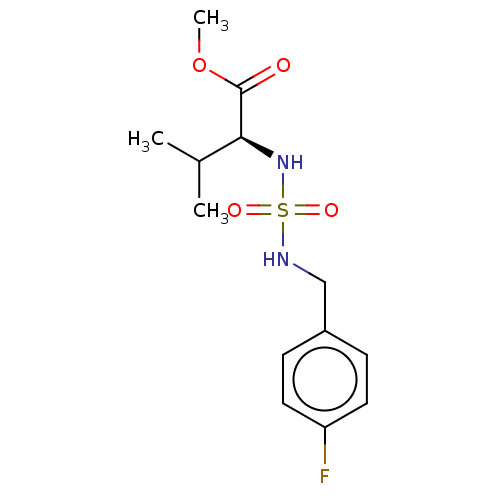 (CHEMBL3752046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500679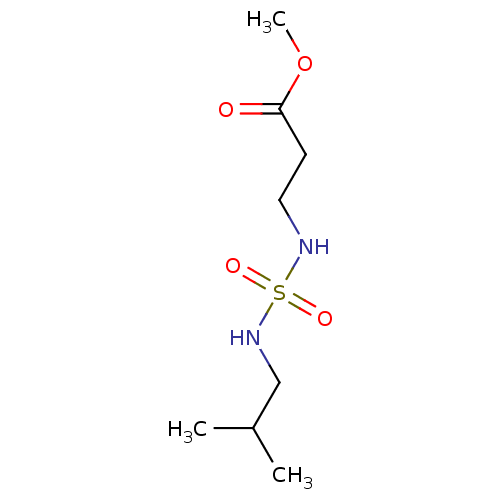 (CHEMBL3752852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500678 (CHEMBL3752328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14065 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14127 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500682 (CHEMBL3754185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500677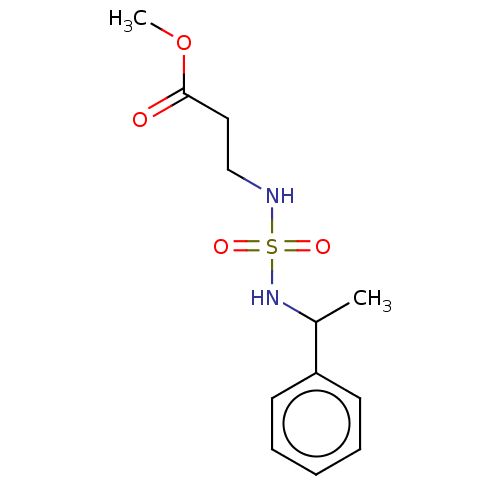 (CHEMBL3754060) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14086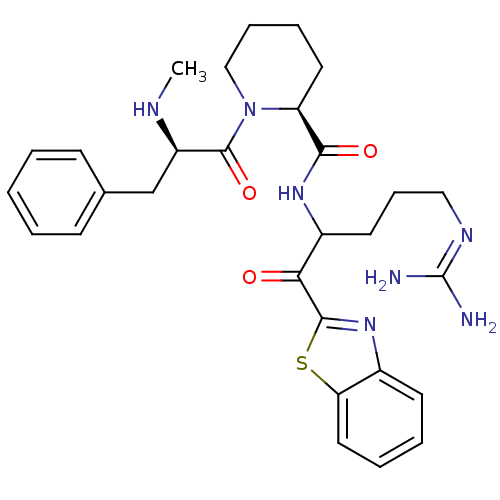 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14072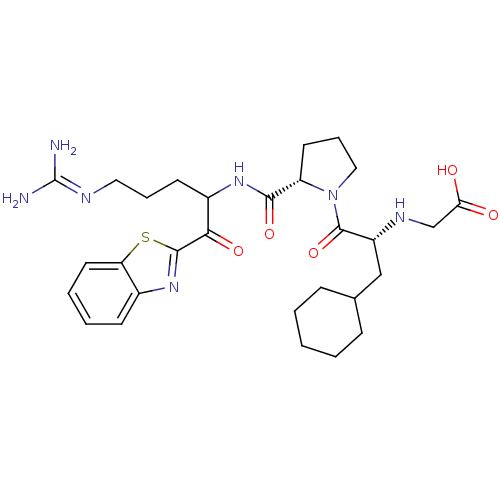 (2-ketobenzothiazole 12 | 2-{[(2R)-1-[(2S)-2-{[1-(1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.360 | -56.1 | 29 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14126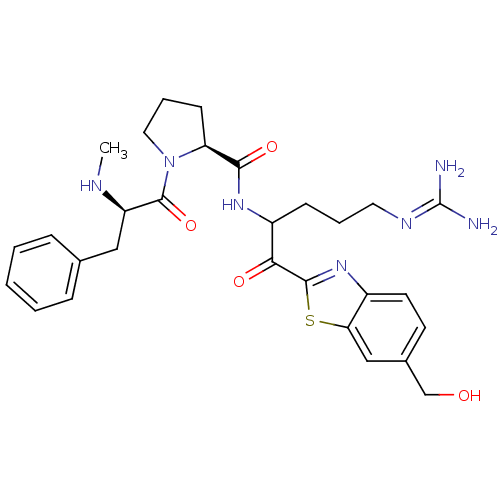 ((2S)-N-{5-carbamimidamido-1-[6-(hydroxymethyl)-1,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500675 (CHEMBL3752037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500676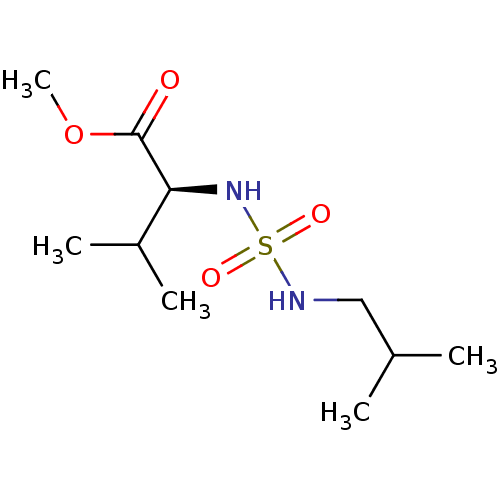 (CHEMBL3754636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500681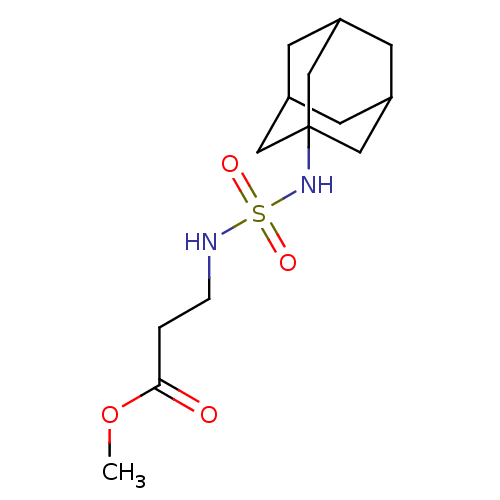 (CHEMBL3754723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50500674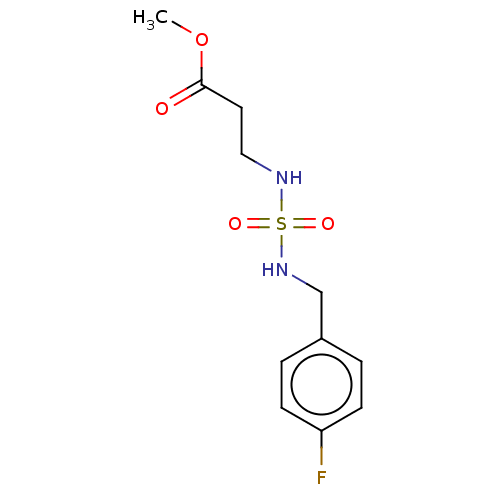 (CHEMBL3752196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14075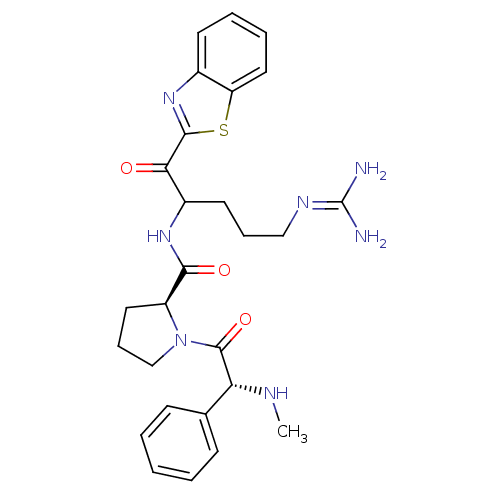 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.460 | -55.4 | 34 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50173126 (CHEMBL3809510) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in HEK293 cell membrane incubated for 1 hr by TopCount scintillation coun... | J Med Chem 59: 3777-92 (2016) Article DOI: 10.1021/acs.jmedchem.5b01976 BindingDB Entry DOI: 10.7270/Q2T72KC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14122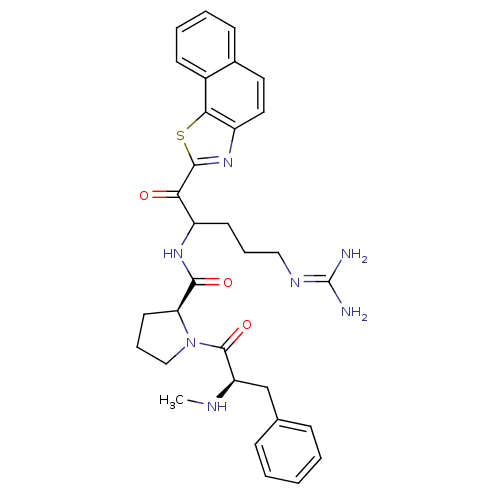 ((2S)-N-(5-carbamimidamido-1-oxo-1-{3-thia-5-azatri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50500678 (CHEMBL3752328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 12 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50500684 (CHEMBL3752046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of La Plata Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 12 preincubated for 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem 24: 894-901 (2016) Article DOI: 10.1016/j.bmc.2016.01.012 BindingDB Entry DOI: 10.7270/Q27H1NKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14139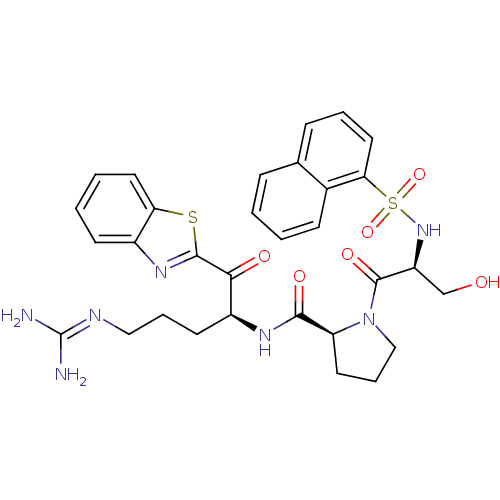 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.780 | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14092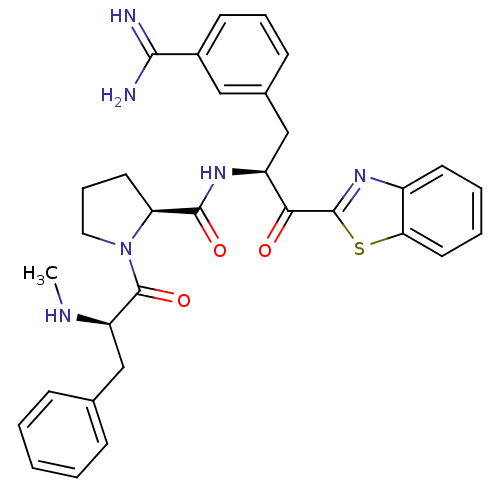 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-3-(3-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14074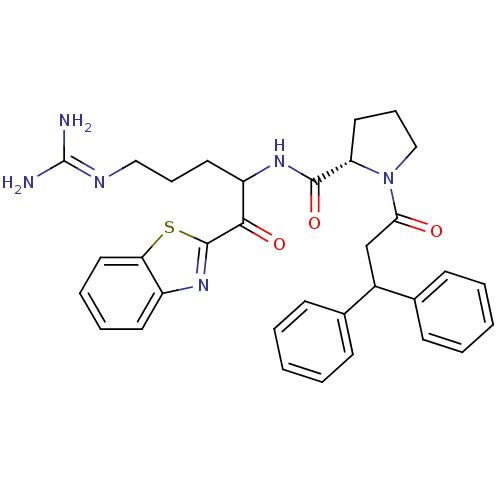 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | -53.2 | 11 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14076 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14117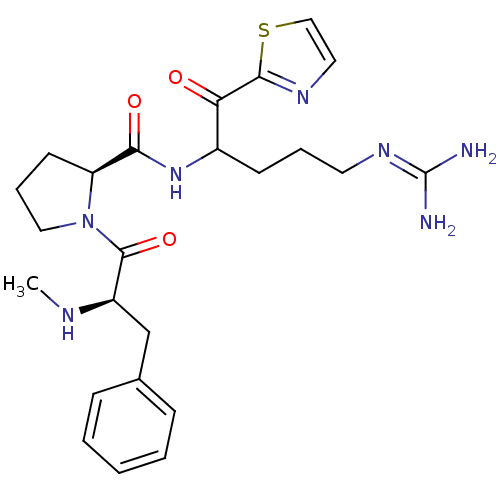 ((2S)-N-[5-carbamimidamido-1-oxo-1-(1,3-thiazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14128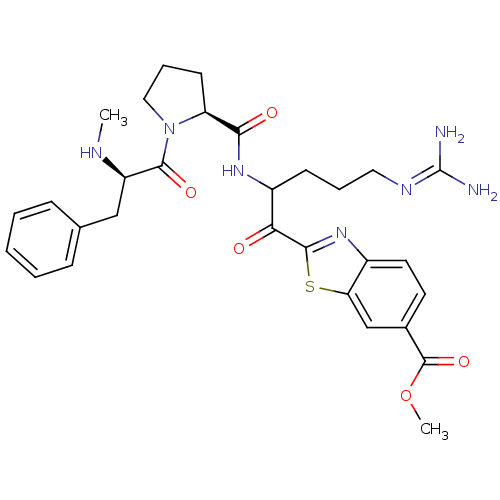 (2-ketobenzothiazole 68 | methyl 2-(5-carbamimidami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14137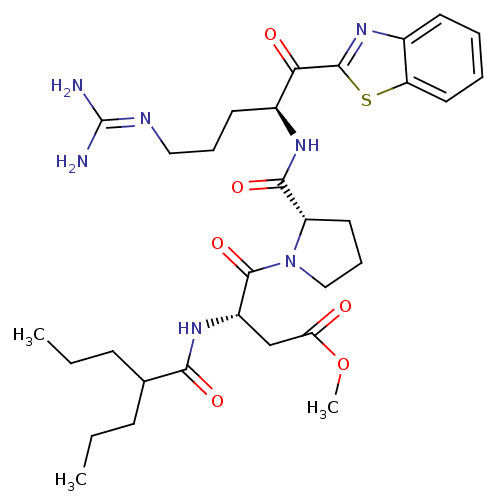 (2-ketobenzothiazole 75 | methyl (3S)-4-[(2S)-2-{[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146293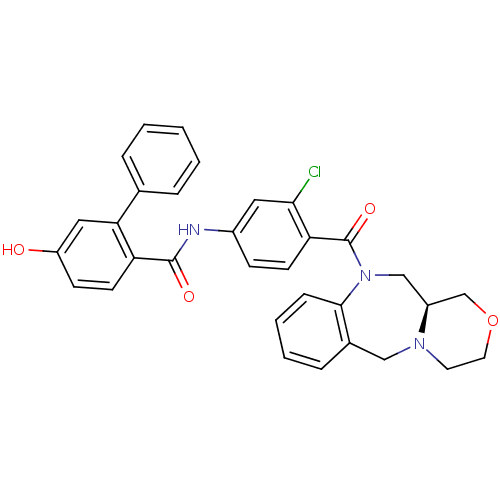 (5-Hydroxy-biphenyl-2-carboxylic acid [3-chloro-4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14138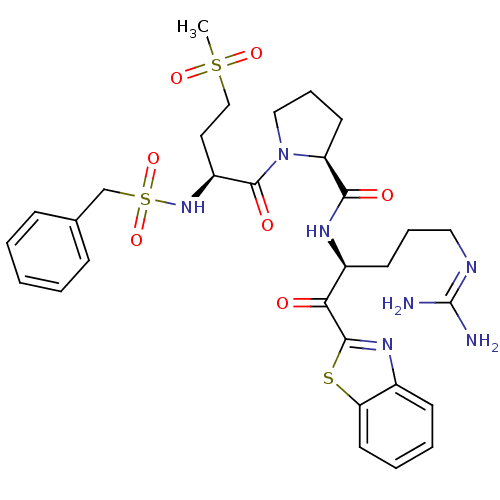 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14085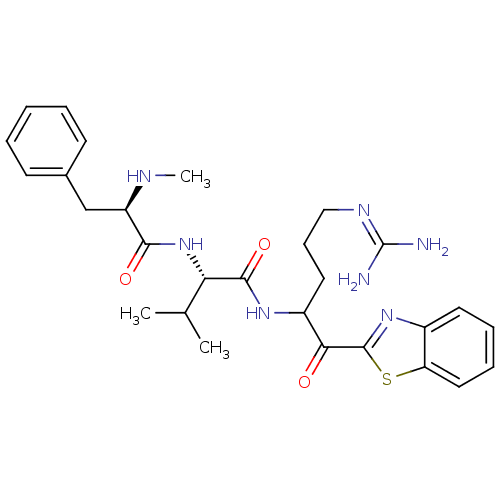 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14125 ((2S)-N-[5-carbamimidamido-1-(6-fluoro-1,3-benzothi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14094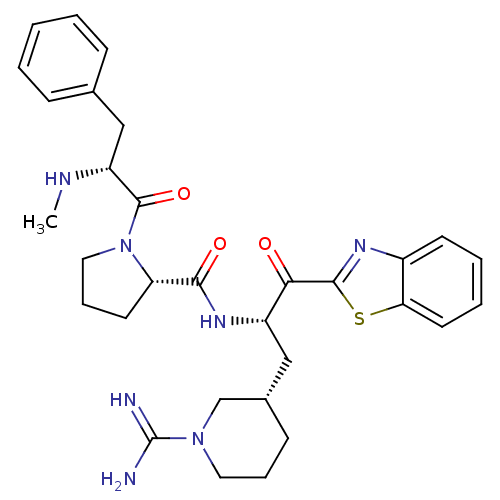 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-3-[(3S)-1-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14078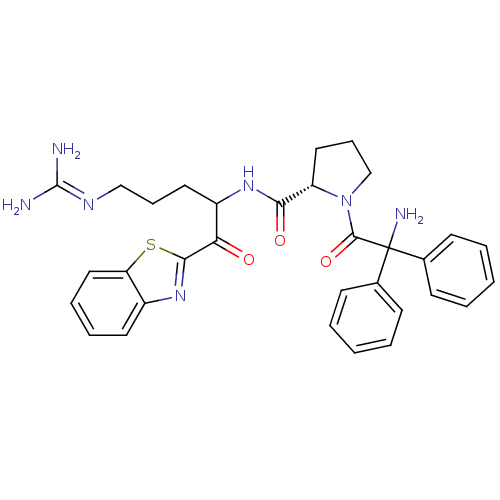 ((2S)-1-(2-amino-2,2-diphenylacetyl)-N-[1-(1,3-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14086 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14092 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-3-(3-carbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146309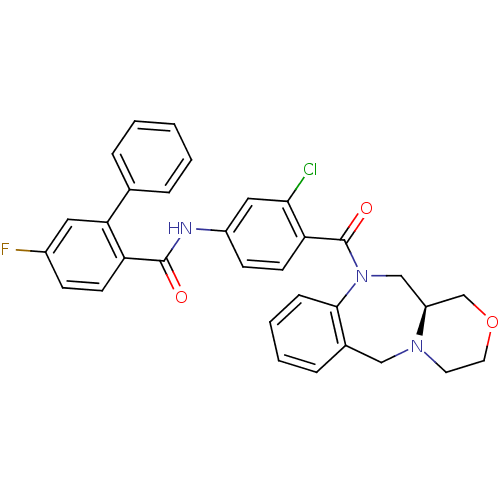 (5-Fluoro-biphenyl-2-carboxylic acid [3-chloro-4-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 626 total ) | Next | Last >> |