 Found 2320 hits with Last Name = 'wagner' and Initial = 'j'
Found 2320 hits with Last Name = 'wagner' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153971
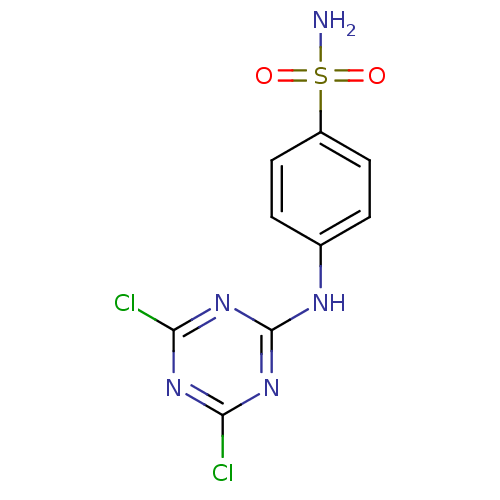
(4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenes...)Show InChI InChI=1S/C9H7Cl2N5O2S/c10-7-14-8(11)16-9(15-7)13-5-1-3-6(4-2-5)19(12,17)18/h1-4H,(H2,12,17,18)(H,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50441372
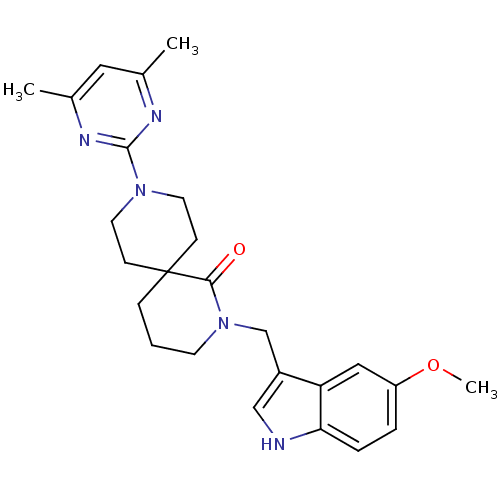
(CHEMBL2435400)Show SMILES COc1ccc2[nH]cc(CN3CCCC4(CCN(CC4)c4nc(C)cc(C)n4)C3=O)c2c1 Show InChI InChI=1S/C25H31N5O2/c1-17-13-18(2)28-24(27-17)29-11-8-25(9-12-29)7-4-10-30(23(25)31)16-19-15-26-22-6-5-20(32-3)14-21(19)22/h5-6,13-15,26H,4,7-12,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... |
J Med Chem 56: 7590-607 (2013)
Article DOI: 10.1021/jm4007627
BindingDB Entry DOI: 10.7270/Q2M90B3S |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11625
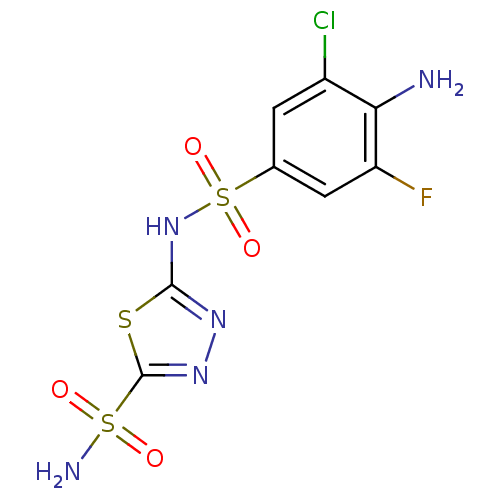
(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50153971

(4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenes...)Show InChI InChI=1S/C9H7Cl2N5O2S/c10-7-14-8(11)16-9(15-7)13-5-1-3-6(4-2-5)19(12,17)18/h1-4H,(H2,12,17,18)(H,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM102168

(US8530648, 75)Show SMILES O=C1CCCC2(CCN(CC2)c2cnc3ccccc3n2)N1Cc1cccc2[nH]ccc12 Show InChI InChI=1S/C26H27N5O/c32-25-9-4-11-26(31(25)18-19-5-3-8-21-20(19)10-14-27-21)12-15-30(16-13-26)24-17-28-22-6-1-2-7-23(22)29-24/h1-3,5-8,10,14,17,27H,4,9,11-13,15-16,18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... |
J Med Chem 56: 7590-607 (2013)
Article DOI: 10.1021/jm4007627
BindingDB Entry DOI: 10.7270/Q2M90B3S |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235282

(CHEMBL4077967)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-9-16-10-11-20(27)22(18(16)12-15)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-17(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Reversible competitive inhibition of PKCbeta1 (unknown origin) |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Homo sapiens (Human)) | BDBM11625

(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 13 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50349850

(CHEMBL1738787)Show InChI InChI=1S/C11H13ClN6O3S/c12-9-16-10(14-5-6-19)18-11(17-9)15-7-1-3-8(4-2-7)22(13,20)21/h1-4,19H,5-6H2,(H2,13,20,21)(H2,14,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349847

(CHEMBL1813208)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(NCCC(O)=O)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C15H19N7O6S/c16-29(27,28)10-3-1-9(2-4-10)19-15-21-13(17-7-5-11(23)24)20-14(22-15)18-8-6-12(25)26/h1-4H,5-8H2,(H,23,24)(H,25,26)(H2,16,27,28)(H3,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50441378

(CHEMBL2435410)Show SMILES Cc1cc(C)nc(n1)N1CCC2(CCCC(=O)N2Cc2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C24H29N5O/c1-17-15-18(2)27-23(26-17)28-13-10-24(11-14-28)9-4-7-22(30)29(24)16-19-5-3-6-21-20(19)8-12-25-21/h3,5-6,8,12,15,25H,4,7,9-11,13-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... |
J Med Chem 56: 7590-607 (2013)
Article DOI: 10.1021/jm4007627
BindingDB Entry DOI: 10.7270/Q2M90B3S |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM11625

(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50349847

(CHEMBL1813208)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(NCCC(O)=O)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C15H19N7O6S/c16-29(27,28)10-3-1-9(2-4-10)19-15-21-13(17-7-5-11(23)24)20-14(22-15)18-8-6-12(25)26/h1-4H,5-8H2,(H,23,24)(H,25,26)(H2,16,27,28)(H3,17,18,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50349848
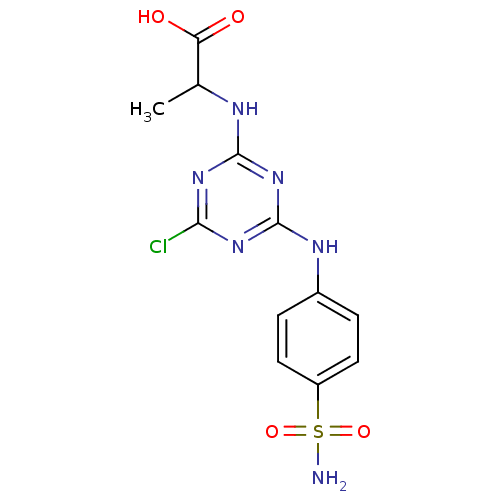
(CHEMBL1813209)Show SMILES CC(Nc1nc(Cl)nc(Nc2ccc(cc2)S(N)(=O)=O)n1)C(O)=O Show InChI InChI=1S/C12H13ClN6O4S/c1-6(9(20)21)15-11-17-10(13)18-12(19-11)16-7-2-4-8(5-3-7)24(14,22)23/h2-6H,1H3,(H,20,21)(H2,14,22,23)(H2,15,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM102168

(US8530648, 75)Show SMILES O=C1CCCC2(CCN(CC2)c2cnc3ccccc3n2)N1Cc1cccc2[nH]ccc12 Show InChI InChI=1S/C26H27N5O/c32-25-9-4-11-26(31(25)18-19-5-3-8-21-20(19)10-14-27-21)12-15-30(16-13-26)24-17-28-22-6-1-2-7-23(22)29-24/h1-3,5-8,10,14,17,27H,4,9,11-13,15-16,18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
FLIPR assay using orexin receptor. |
US Patent US8530648 (2013)
BindingDB Entry DOI: 10.7270/Q2SB44DR |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235282

(CHEMBL4077967)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-9-16-10-11-20(27)22(18(16)12-15)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-17(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Reversible competitive inhibition of PKCalpha (unknown origin) |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167355
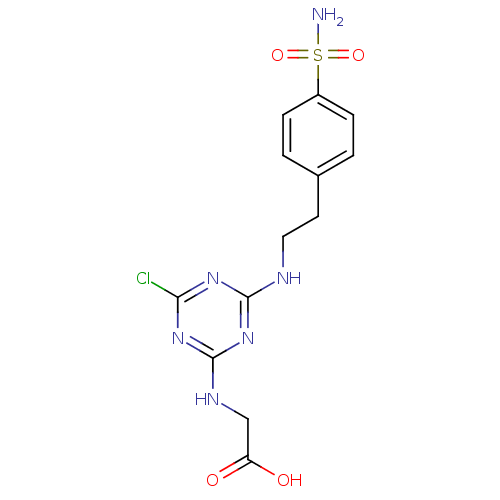
(CHEMBL189526 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCC(O)=O)n2)cc1 Show InChI InChI=1S/C13H15ClN6O4S/c14-11-18-12(20-13(19-11)17-7-10(21)22)16-6-5-8-1-3-9(4-2-8)25(15,23)24/h1-4H,5-7H2,(H,21,22)(H2,15,23,24)(H2,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50441368
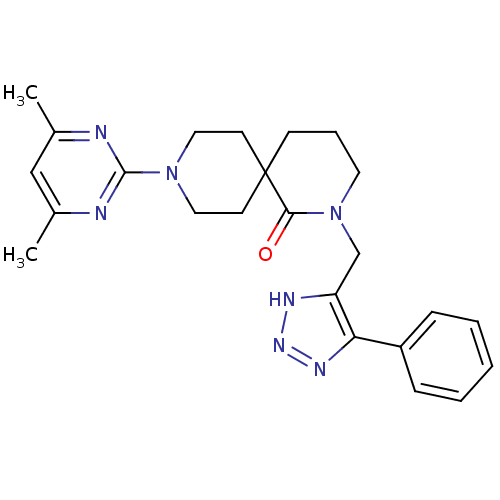
(CHEMBL2435396)Show SMILES Cc1cc(C)nc(n1)N1CCC2(CCCN(Cc3[nH]nnc3-c3ccccc3)C2=O)CC1 Show InChI InChI=1S/C24H29N7O/c1-17-15-18(2)26-23(25-17)30-13-10-24(11-14-30)9-6-12-31(22(24)32)16-20-21(28-29-27-20)19-7-4-3-5-8-19/h3-5,7-8,15H,6,9-14,16H2,1-2H3,(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... |
J Med Chem 56: 7590-607 (2013)
Article DOI: 10.1021/jm4007627
BindingDB Entry DOI: 10.7270/Q2M90B3S |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50349858
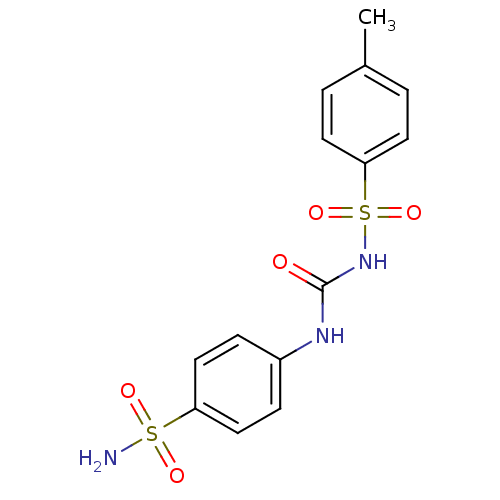
(CHEMBL78755)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H15N3O5S2/c1-10-2-6-13(7-3-10)24(21,22)17-14(18)16-11-4-8-12(9-5-11)23(15,19)20/h2-9H,1H3,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167330
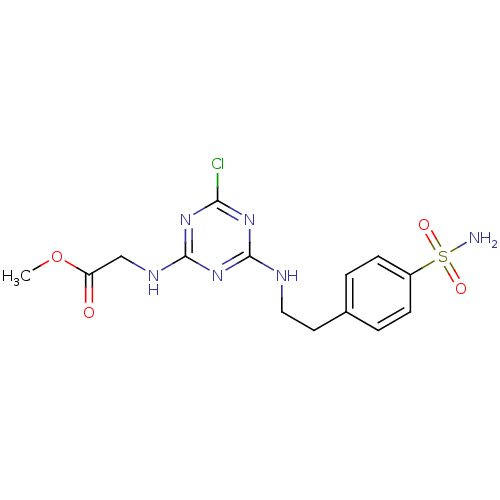
(CHEMBL190733 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES COC(=O)CNc1nc(Cl)nc(NCCc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C14H17ClN6O4S/c1-25-11(22)8-18-14-20-12(15)19-13(21-14)17-7-6-9-2-4-10(5-3-9)26(16,23)24/h2-5H,6-8H2,1H3,(H2,16,23,24)(H2,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM11625

(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 1 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349858

(CHEMBL78755)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H15N3O5S2/c1-10-2-6-13(7-3-10)24(21,22)17-14(18)16-11-4-8-12(9-5-11)23(15,19)20/h2-9H,1H3,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349850

(CHEMBL1738787)Show InChI InChI=1S/C11H13ClN6O3S/c12-9-16-10(14-5-6-19)18-11(17-9)15-7-1-3-8(4-2-7)22(13,20)21/h1-4,19H,5-6H2,(H2,13,20,21)(H2,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Mus musculus) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse OX1 receptor expressed in CHO cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... |
J Med Chem 56: 7590-607 (2013)
Article DOI: 10.1021/jm4007627
BindingDB Entry DOI: 10.7270/Q2M90B3S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167337
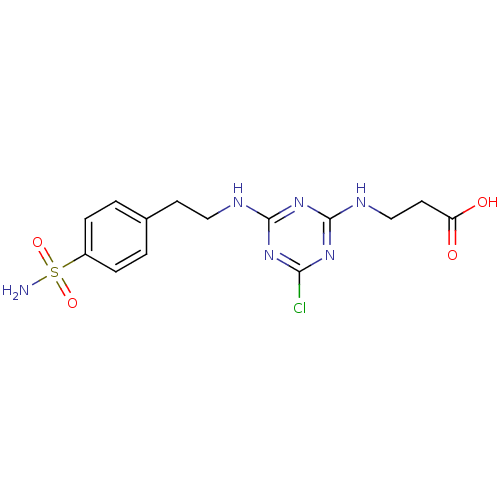
(3-{4-Chloro-6-[2-(4-sulfamoyl-phenyl)-ethylamino]-...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C14H17ClN6O4S/c15-12-19-13(21-14(20-12)18-8-6-11(22)23)17-7-5-9-1-3-10(4-2-9)26(16,24)25/h1-4H,5-8H2,(H,22,23)(H2,16,24,25)(H2,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 7 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 7 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 catalytic domain preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 4873-8 (2010)
Article DOI: 10.1016/j.bmc.2010.06.028
BindingDB Entry DOI: 10.7270/Q2D21ZKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167355

(CHEMBL189526 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCC(O)=O)n2)cc1 Show InChI InChI=1S/C13H15ClN6O4S/c14-11-18-12(20-13(19-11)17-7-10(21)22)16-6-5-8-1-3-9(4-2-8)25(15,23)24/h1-4H,5-7H2,(H,21,22)(H2,15,23,24)(H2,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50108563
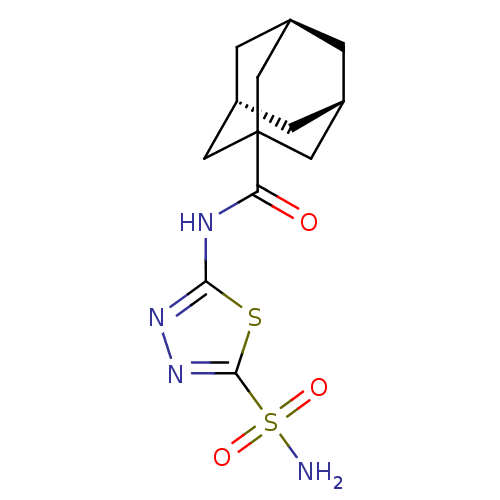
(Adamantane-1-carboxylic acid (5-sulfamoyl-[1,3,4]t...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)C23C[C@H]4C[C@H](C[C@H](C4)C2)C3)s1 |TLB:18:17:20:12.13.14,18:13:20:19.17.16| Show InChI InChI=1S/C13H18N4O3S2/c14-22(19,20)12-17-16-11(21-12)15-10(18)13-4-7-1-8(5-13)3-9(2-7)6-13/h7-9H,1-6H2,(H2,14,19,20)(H,15,16,18)/t7-,8+,9-,13? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM11625

(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 7 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167330

(CHEMBL190733 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES COC(=O)CNc1nc(Cl)nc(NCCc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C14H17ClN6O4S/c1-25-11(22)8-18-14-20-12(15)19-13(21-14)17-7-6-9-2-4-10(5-3-9)26(16,23)24/h2-5H,6-8H2,1H3,(H2,16,23,24)(H2,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM102172
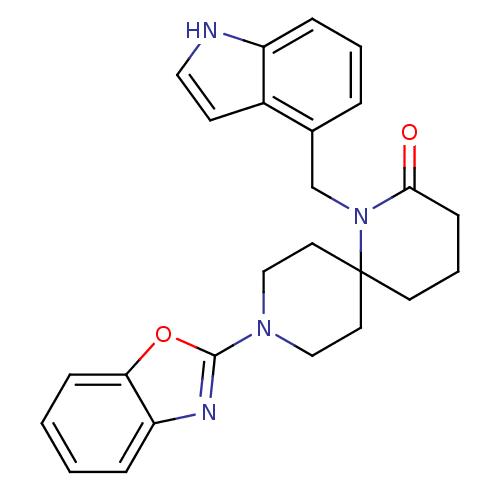
(US8530648, 94)Show SMILES O=C1CCCC2(CCN(CC2)c2nc3ccccc3o2)N1Cc1cccc2[nH]ccc12 Show InChI InChI=1S/C25H26N4O2/c30-23-9-4-11-25(29(23)17-18-5-3-7-20-19(18)10-14-26-20)12-15-28(16-13-25)24-27-21-6-1-2-8-22(21)31-24/h1-3,5-8,10,14,26H,4,9,11-13,15-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
FLIPR assay using orexin receptor. |
US Patent US8530648 (2013)
BindingDB Entry DOI: 10.7270/Q2SB44DR |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM102172

(US8530648, 94)Show SMILES O=C1CCCC2(CCN(CC2)c2nc3ccccc3o2)N1Cc1cccc2[nH]ccc12 Show InChI InChI=1S/C25H26N4O2/c30-23-9-4-11-25(29(23)17-18-5-3-7-20-19(18)10-14-26-20)12-15-28(16-13-25)24-27-21-6-1-2-8-22(21)31-24/h1-3,5-8,10,14,26H,4,9,11-13,15-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... |
J Med Chem 56: 7590-607 (2013)
Article DOI: 10.1021/jm4007627
BindingDB Entry DOI: 10.7270/Q2M90B3S |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167337

(3-{4-Chloro-6-[2-(4-sulfamoyl-phenyl)-ethylamino]-...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C14H17ClN6O4S/c15-12-19-13(21-14(20-12)18-8-6-11(22)23)17-7-5-9-1-3-10(4-2-9)26(16,24)25/h1-4H,5-8H2,(H,22,23)(H2,16,24,25)(H2,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50349850

(CHEMBL1738787)Show InChI InChI=1S/C11H13ClN6O3S/c12-9-16-10(14-5-6-19)18-11(17-9)15-7-1-3-8(4-2-7)22(13,20)21/h1-4,19H,5-6H2,(H2,13,20,21)(H2,14,15,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50441369

(CHEMBL2435397)Show SMILES Cc1cc(C)nc(n1)N1CCC2(CCCN(Cc3[nH]ncc3-c3ccccc3)C2=O)CC1 Show InChI InChI=1S/C25H30N6O/c1-18-15-19(2)28-24(27-18)30-13-10-25(11-14-30)9-6-12-31(23(25)32)17-22-21(16-26-29-22)20-7-4-3-5-8-20/h3-5,7-8,15-16H,6,9-14,17H2,1-2H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... |
J Med Chem 56: 7590-607 (2013)
Article DOI: 10.1021/jm4007627
BindingDB Entry DOI: 10.7270/Q2M90B3S |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50349858

(CHEMBL78755)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H15N3O5S2/c1-10-2-6-13(7-3-10)24(21,22)17-14(18)16-11-4-8-12(9-5-11)23(15,19)20/h2-9H,1H3,(H2,15,19,20)(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50441380

(CHEMBL2435395)Show SMILES Cc1cc(C)nc(n1)N1CCC2(CCCN(Cc3nn(C)nc3-c3ccccc3)C2=O)CC1 Show InChI InChI=1S/C25H31N7O/c1-18-16-19(2)27-24(26-18)31-14-11-25(12-15-31)10-7-13-32(23(25)33)17-21-22(29-30(3)28-21)20-8-5-4-6-9-20/h4-6,8-9,16H,7,10-15,17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... |
J Med Chem 56: 7590-607 (2013)
Article DOI: 10.1021/jm4007627
BindingDB Entry DOI: 10.7270/Q2M90B3S |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50323341
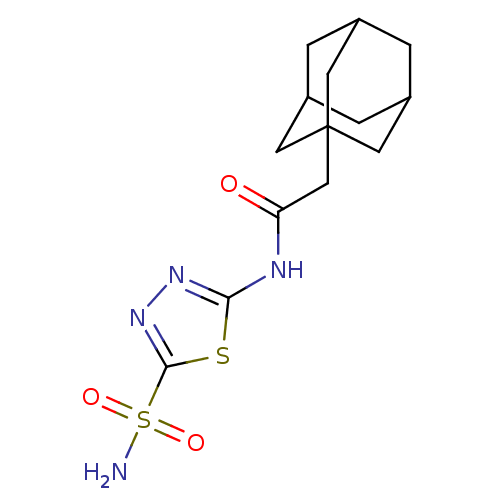
(CHEMBL1209039)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CC23CC4CC(CC(C4)C2)C3)s1 |TLB:11:12:15:19.18.17,THB:13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12| Show InChI InChI=1S/C14H20N4O3S2/c15-23(20,21)13-18-17-12(22-13)16-11(19)7-14-4-8-1-9(5-14)3-10(2-8)6-14/h8-10H,1-7H2,(H2,15,20,21)(H,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50323341

(CHEMBL1209039)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CC23CC4CC(CC(C4)C2)C3)s1 |TLB:11:12:15:19.18.17,THB:13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12| Show InChI InChI=1S/C14H20N4O3S2/c15-23(20,21)13-18-17-12(22-13)16-11(19)7-14-4-8-1-9(5-14)3-10(2-8)6-14/h8-10H,1-7H2,(H2,15,20,21)(H,16,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10868
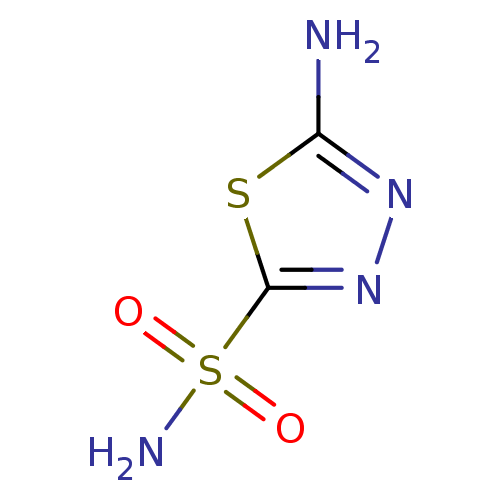
(1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...)Show InChI InChI=1S/C2H4N4O2S2/c3-1-5-6-2(9-1)10(4,7)8/h(H2,3,5)(H2,4,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 7 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 13
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 13 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349848

(CHEMBL1813209)Show SMILES CC(Nc1nc(Cl)nc(Nc2ccc(cc2)S(N)(=O)=O)n1)C(O)=O Show InChI InChI=1S/C12H13ClN6O4S/c1-6(9(20)21)15-11-17-10(13)18-12(19-11)16-7-2-4-8(5-3-7)24(14,22)23/h2-6H,1H3,(H,20,21)(H2,14,22,23)(H2,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50264007

(CHEMBL489182 | N-(2,4-Disulfamoyl-5-trifluoromethy...)Show SMILES COc1ccc2c(CC(=O)Nc3cc(c(cc3S(N)(=O)=O)S(N)(=O)=O)C(F)(F)F)cc(=O)oc2c1 Show InChI InChI=1S/C19H16F3N3O8S2/c1-32-10-2-3-11-9(5-18(27)33-14(11)6-10)4-17(26)25-13-7-12(19(20,21)22)15(34(23,28)29)8-16(13)35(24,30)31/h2-3,5-8H,4H2,1H3,(H,25,26)(H2,23,28,29)(H2,24,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 catalytic domain preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 4873-8 (2010)
Article DOI: 10.1016/j.bmc.2010.06.028
BindingDB Entry DOI: 10.7270/Q2D21ZKM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349859
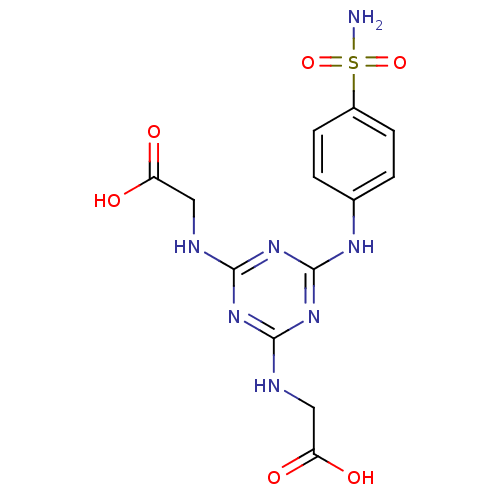
(CHEMBL1813206)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(NCC(O)=O)nc(NCC(O)=O)n2)cc1 Show InChI InChI=1S/C13H15N7O6S/c14-27(25,26)8-3-1-7(2-4-8)17-13-19-11(15-5-9(21)22)18-12(20-13)16-6-10(23)24/h1-4H,5-6H2,(H,21,22)(H,23,24)(H2,14,25,26)(H3,15,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50108563

(Adamantane-1-carboxylic acid (5-sulfamoyl-[1,3,4]t...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)C23C[C@H]4C[C@H](C[C@H](C4)C2)C3)s1 |TLB:18:17:20:12.13.14,18:13:20:19.17.16| Show InChI InChI=1S/C13H18N4O3S2/c14-22(19,20)12-17-16-11(21-12)15-10(18)13-4-7-1-8(5-13)3-9(2-7)6-13/h7-9H,1-6H2,(H2,14,19,20)(H,15,16,18)/t7-,8+,9-,13? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 after 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 20: 4376-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.082
BindingDB Entry DOI: 10.7270/Q2C24XCB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50349846
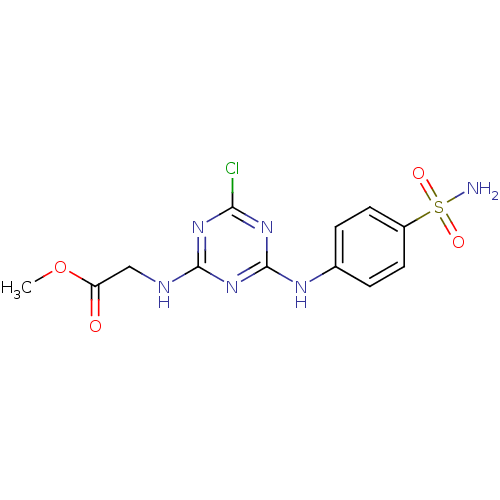
(CHEMBL1738792)Show SMILES COC(=O)CNc1nc(Cl)nc(Nc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C12H13ClN6O4S/c1-23-9(20)6-15-11-17-10(13)18-12(19-11)16-7-2-4-8(5-3-7)24(14,21)22/h2-5H,6H2,1H3,(H2,14,21,22)(H2,15,16,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data