 Found 383 hits with Last Name = 'wang' and Initial = 'hc'
Found 383 hits with Last Name = 'wang' and Initial = 'hc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321894
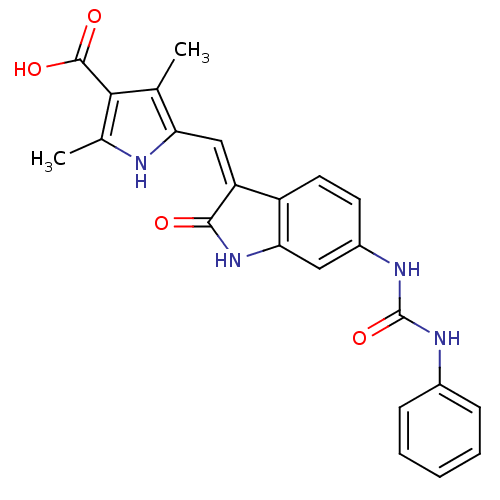
((Z)-2,4-Dimethyl-5-[2-oxo-6-(3-phenylureido)-1,2-d...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)Nc4ccccc4)ccc23)c(C)c1C(O)=O Show InChI InChI=1S/C23H20N4O4/c1-12-18(24-13(2)20(12)22(29)30)11-17-16-9-8-15(10-19(16)27-21(17)28)26-23(31)25-14-6-4-3-5-7-14/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056188

(CHEMBL3329654)Show SMILES CN(CCO)CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)n[nH]3)ncnc2c1 Show InChI InChI=1S/C25H28FN7O3/c1-33(9-10-34)8-3-11-36-20-6-7-21-22(15-20)27-16-28-25(21)30-23-13-19(31-32-23)14-24(35)29-18-5-2-4-17(26)12-18/h2,4-7,12-13,15-16,34H,3,8-11,14H2,1H3,(H,29,35)(H2,27,28,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056199
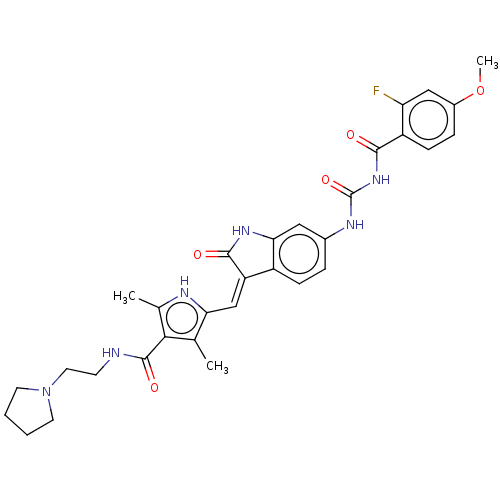
(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50321892

((Z)-5-{6-[3-(4-Methoxyphenyl)-ureido]-2-oxo-1,2-di...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4[nH]cc(C(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C23H20N4O5/c1-12-18(22(29)30)11-24-19(12)10-17-16-8-5-14(9-20(16)27-21(17)28)26-23(31)25-13-3-6-15(32-2)7-4-13/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321892

((Z)-5-{6-[3-(4-Methoxyphenyl)-ureido]-2-oxo-1,2-di...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4[nH]cc(C(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C23H20N4O5/c1-12-18(22(29)30)11-24-19(12)10-17-16-8-5-14(9-20(16)27-21(17)28)26-23(31)25-13-3-6-15(32-2)7-4-13/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321909
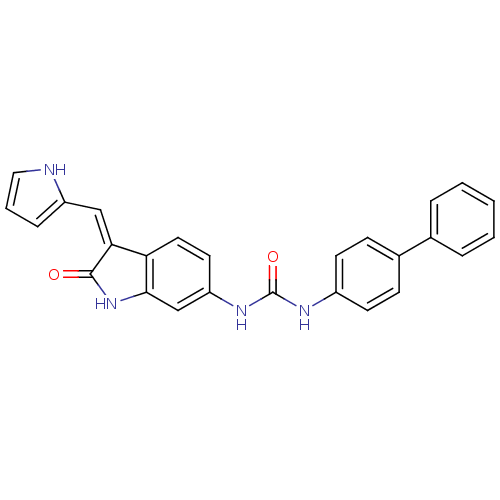
((Z)-1-Biphenyl-4-yl-3-[2-oxo-3-(1H-pyrrol-2-ylmeth...)Show SMILES O=C(Nc1ccc(cc1)-c1ccccc1)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C26H20N4O2/c31-25-23(15-20-7-4-14-27-20)22-13-12-21(16-24(22)30-25)29-26(32)28-19-10-8-18(9-11-19)17-5-2-1-3-6-17/h1-16,27H,(H,30,31)(H2,28,29,32)/b23-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321908
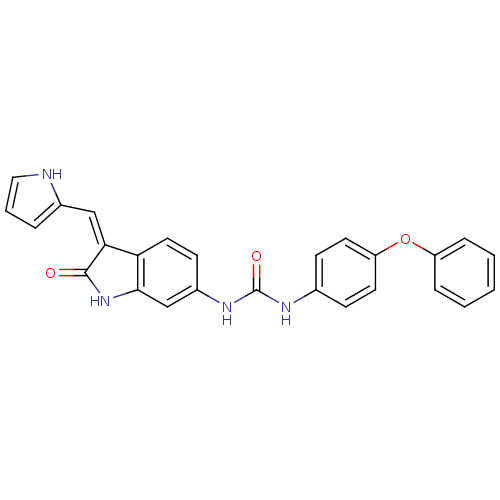
((Z)-1-[2-Oxo-3-(1H-pyrrol-2-ylmethylene)-2,3-dihyd...)Show SMILES O=C(Nc1ccc(Oc2ccccc2)cc1)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C26H20N4O3/c31-25-23(15-18-5-4-14-27-18)22-13-10-19(16-24(22)30-25)29-26(32)28-17-8-11-21(12-9-17)33-20-6-2-1-3-7-20/h1-16,27H,(H,30,31)(H2,28,29,32)/b23-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50321892

((Z)-5-{6-[3-(4-Methoxyphenyl)-ureido]-2-oxo-1,2-di...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4[nH]cc(C(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C23H20N4O5/c1-12-18(22(29)30)11-24-19(12)10-17-16-8-5-14(9-20(16)27-21(17)28)26-23(31)25-13-3-6-15(32-2)7-4-13/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-10- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321906
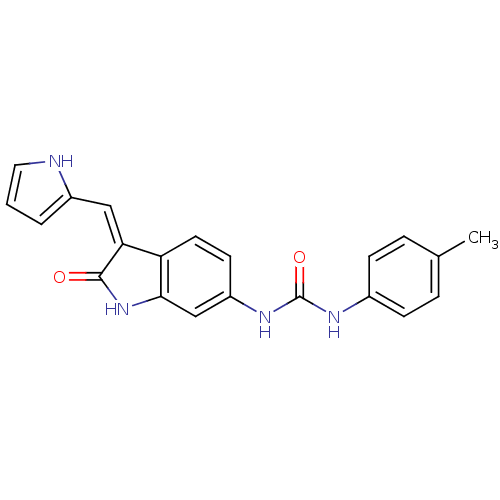
((Z)-1-[2-Oxo-3-(1H-pyrrol-2-ylmethylene)-2,3-dihyd...)Show SMILES Cc1ccc(NC(=O)Nc2ccc3\C(=C\c4ccc[nH]4)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C21H18N4O2/c1-13-4-6-14(7-5-13)23-21(27)24-16-8-9-17-18(11-15-3-2-10-22-15)20(26)25-19(17)12-16/h2-12,22H,1H3,(H,25,26)(H2,23,24,27)/b18-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50321898

((Z)-{2,4-Dimethyl-5-[2-oxo-6-(3-phenylureido)-1,2-...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)Nc4ccccc4)ccc23)c(C)c1CC(O)=O Show InChI InChI=1S/C24H22N4O4/c1-13-18(12-22(29)30)14(2)25-20(13)11-19-17-9-8-16(10-21(17)28-23(19)31)27-24(32)26-15-6-4-3-5-7-15/h3-11,25H,12H2,1-2H3,(H,28,31)(H,29,30)(H2,26,27,32)/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50321892

((Z)-5-{6-[3-(4-Methoxyphenyl)-ureido]-2-oxo-1,2-di...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4[nH]cc(C(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C23H20N4O5/c1-12-18(22(29)30)11-24-19(12)10-17-16-8-5-14(9-20(16)27-21(17)28)26-23(31)25-13-3-6-15(32-2)7-4-13/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-10- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50321893

((Z)-1-[2-Oxo-3-(1H-pyrrol-2-ylmethylene)-2,3-dihyd...)Show SMILES O=C(Nc1ccccc1)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C20H16N4O2/c25-19-17(11-14-7-4-10-21-14)16-9-8-15(12-18(16)24-19)23-20(26)22-13-5-2-1-3-6-13/h1-12,21H,(H,24,25)(H2,22,23,26)/b17-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50321893

((Z)-1-[2-Oxo-3-(1H-pyrrol-2-ylmethylene)-2,3-dihyd...)Show SMILES O=C(Nc1ccccc1)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C20H16N4O2/c25-19-17(11-14-7-4-10-21-14)16-9-8-15(12-18(16)24-19)23-20(26)22-13-5-2-1-3-6-13/h1-12,21H,(H,24,25)(H2,22,23,26)/b17-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation in HUVEC cells after 24 hrs by Western blotting |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50051957
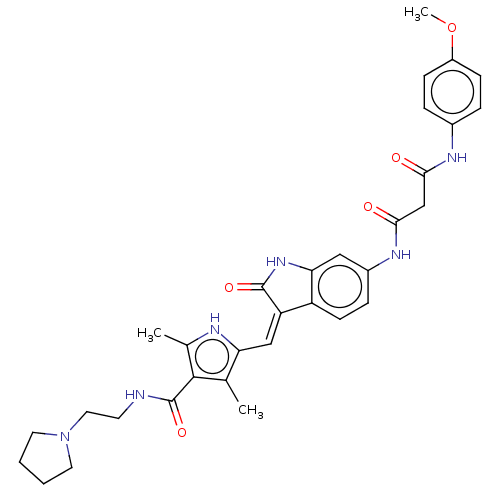
(CHEMBL3322566)Show SMILES COc1ccc(NC(=O)CC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C32H36N6O5/c1-19-26(34-20(2)30(19)32(42)33-12-15-38-13-4-5-14-38)17-25-24-11-8-22(16-27(24)37-31(25)41)36-29(40)18-28(39)35-21-6-9-23(43-3)10-7-21/h6-11,16-17,34H,4-5,12-15,18H2,1-3H3,(H,33,42)(H,35,39)(H,36,40)(H,37,41)/b25-17- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha (unknown origin) using EAIYAAPFAKKK substrate by radioisotope-based P81 filter-binding assay |
Eur J Med Chem 84: 312-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.033
BindingDB Entry DOI: 10.7270/Q2RV0QB7 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50051941
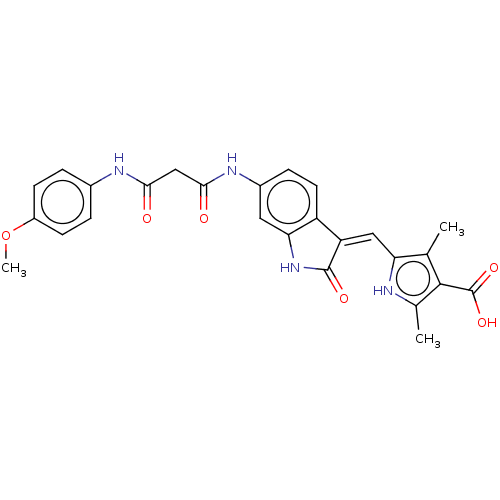
(CHEMBL3322565)Show SMILES COc1ccc(NC(=O)CC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C26H24N4O6/c1-13-20(27-14(2)24(13)26(34)35)11-19-18-9-6-16(10-21(18)30-25(19)33)29-23(32)12-22(31)28-15-4-7-17(36-3)8-5-15/h4-11,27H,12H2,1-3H3,(H,28,31)(H,29,32)(H,30,33)(H,34,35)/b19-11- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha (unknown origin) using EAIYAAPFAKKK substrate by radioisotope-based P81 filter-binding assay |
Eur J Med Chem 84: 312-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.033
BindingDB Entry DOI: 10.7270/Q2RV0QB7 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321910
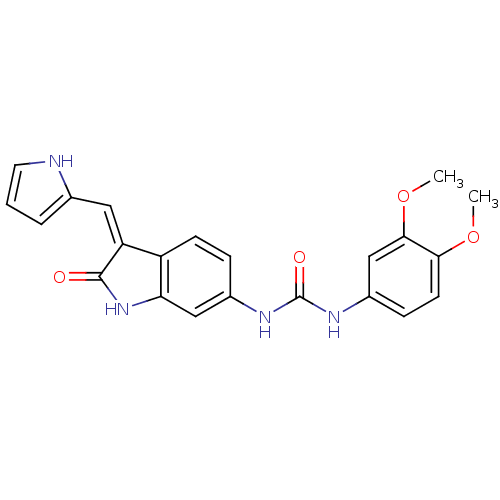
((Z)-1-(3,4-Dimethoxyphenyl)-3-[2-oxo-3-(1H-pyrrol-...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4ccc[nH]4)C(=O)Nc3c2)cc1OC Show InChI InChI=1S/C22H20N4O4/c1-29-19-8-6-15(12-20(19)30-2)25-22(28)24-14-5-7-16-17(10-13-4-3-9-23-13)21(27)26-18(16)11-14/h3-12,23H,1-2H3,(H,26,27)(H2,24,25,28)/b17-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50321892

((Z)-5-{6-[3-(4-Methoxyphenyl)-ureido]-2-oxo-1,2-di...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4[nH]cc(C(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C23H20N4O5/c1-12-18(22(29)30)11-24-19(12)10-17-16-8-5-14(9-20(16)27-21(17)28)26-23(31)25-13-3-6-15(32-2)7-4-13/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of AuroraB |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056146
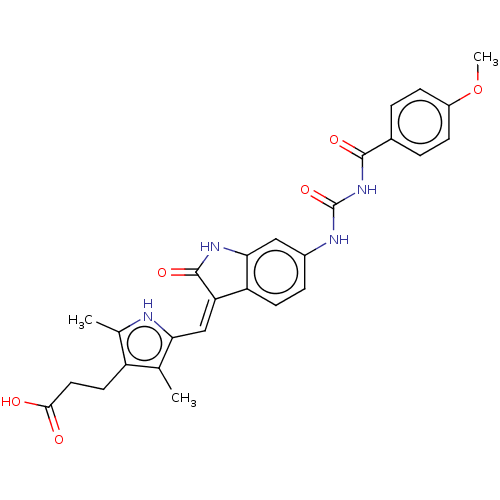
(CHEMBL3329669)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(CCC(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C27H26N4O6/c1-14-19(10-11-24(32)33)15(2)28-22(14)13-21-20-9-6-17(12-23(20)30-26(21)35)29-27(36)31-25(34)16-4-7-18(37-3)8-5-16/h4-9,12-13,28H,10-11H2,1-3H3,(H,30,35)(H,32,33)(H2,29,31,34,36)/b21-13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056142

(CHEMBL3329673)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(CCC(O)=O)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C27H25FN4O6/c1-13-17(8-9-24(33)34)14(2)29-22(13)12-20-18-6-4-15(10-23(18)31-26(20)36)30-27(37)32-25(35)19-7-5-16(38-3)11-21(19)28/h4-7,10-12,29H,8-9H2,1-3H3,(H,31,36)(H,33,34)(H2,30,32,35,37)/b20-12- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321905
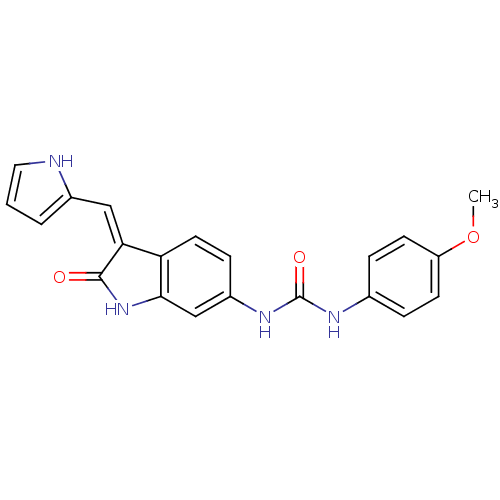
((Z)-1-(4-Methoxyphenyl)-3-[2-oxo-3-(1H-pyrrol-2-yl...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4ccc[nH]4)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C21H18N4O3/c1-28-16-7-4-13(5-8-16)23-21(27)24-15-6-9-17-18(11-14-3-2-10-22-14)20(26)25-19(17)12-15/h2-12,22H,1H3,(H,25,26)(H2,23,24,27)/b18-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50051957

(CHEMBL3322566)Show SMILES COc1ccc(NC(=O)CC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C32H36N6O5/c1-19-26(34-20(2)30(19)32(42)33-12-15-38-13-4-5-14-38)17-25-24-11-8-22(16-27(24)37-31(25)41)36-29(40)18-28(39)35-21-6-9-23(43-3)10-7-21/h6-11,16-17,34H,4-5,12-15,18H2,1-3H3,(H,33,42)(H,35,39)(H,36,40)(H,37,41)/b25-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta (unknown origin) using EAIYAAPFAKKK substrate by radioisotope-based P81 filter-binding assay |
Eur J Med Chem 84: 312-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.033
BindingDB Entry DOI: 10.7270/Q2RV0QB7 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321911

((Z)-1-Naphthalen-2-yl-3-[2-oxo-3-(1H-pyrrol-2-ylme...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc2ccccc2c1)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C30H22N4O2/c35-29-27(17-24-6-3-15-31-24)26-14-13-25(18-28(26)34-29)33-30(36)32-23-11-9-20(10-12-23)22-8-7-19-4-1-2-5-21(19)16-22/h1-18,31H,(H,34,35)(H2,32,33,36)/b27-17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056140
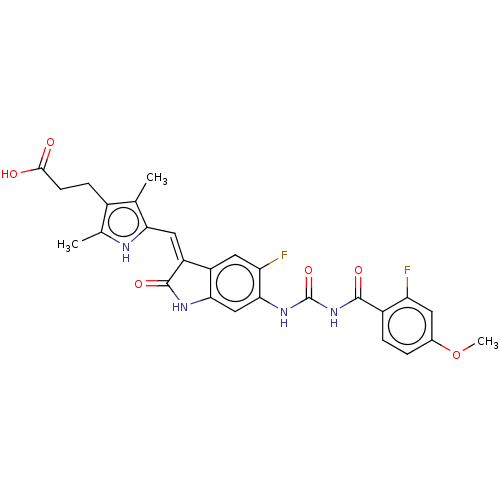
(CHEMBL3329675)Show SMILES COc1ccc(C(=O)NC(=O)Nc2cc3NC(=O)\C(=C/c4[nH]c(C)c(CCC(O)=O)c4C)c3cc2F)c(F)c1 Show InChI InChI=1S/C27H24F2N4O6/c1-12-15(6-7-24(34)35)13(2)30-21(12)10-18-17-9-20(29)23(11-22(17)31-26(18)37)32-27(38)33-25(36)16-5-4-14(39-3)8-19(16)28/h4-5,8-11,30H,6-7H2,1-3H3,(H,31,37)(H,34,35)(H2,32,33,36,38)/b18-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056145

(CHEMBL3329670)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1cc2NC(=O)\C(=C/c3[nH]c(C)c(C(O)=O)c3C)c2cc1F Show InChI InChI=1S/C25H21FN4O6/c1-11-18(27-12(2)21(11)24(33)34)9-16-15-8-17(26)20(10-19(15)28-23(16)32)29-25(35)30-22(31)13-4-6-14(36-3)7-5-13/h4-10,27H,1-3H3,(H,28,32)(H,33,34)(H2,29,30,31,35)/b16-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50321905

((Z)-1-(4-Methoxyphenyl)-3-[2-oxo-3-(1H-pyrrol-2-yl...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4ccc[nH]4)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C21H18N4O3/c1-28-16-7-4-13(5-8-16)23-21(27)24-15-6-9-17-18(11-14-3-2-10-22-14)20(26)25-19(17)12-15/h2-12,22H,1H3,(H,25,26)(H2,23,24,27)/b18-11- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of cKIT |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056151

(CHEMBL3329668)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(CC(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C26H24N4O6/c1-13-19(12-23(31)32)14(2)27-21(13)11-20-18-9-6-16(10-22(18)29-25(20)34)28-26(35)30-24(33)15-4-7-17(36-3)8-5-15/h4-11,27H,12H2,1-3H3,(H,29,34)(H,31,32)(H2,28,30,33,35)/b20-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056155

(CHEMBL3329664)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(C(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C25H22N4O6/c1-12-19(26-13(2)21(12)24(32)33)11-18-17-9-6-15(10-20(17)28-23(18)31)27-25(34)29-22(30)14-4-7-16(35-3)8-5-14/h4-11,26H,1-3H3,(H,28,31)(H,32,33)(H2,27,29,30,34)/b18-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50320036

(CHEMBL1086235 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...)Show SMILES CCOC(=O)CNC(=O)C(=O)C(COCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1cccc(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C31H38N4O9/c1-2-44-27(36)18-32-31(40)28(37)26(20-43-19-22-12-7-4-8-13-22)34-30(39)25(16-21-10-5-3-6-11-21)33-29(38)23-14-9-15-24(17-23)35(41)42/h4,7-9,12-15,17,21,25-26H,2-3,5-6,10-11,16,18-20H2,1H3,(H,32,40)(H,33,38)(H,34,39)/t25-,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay |
J Med Chem 53: 4545-9 (2010)
Article DOI: 10.1021/jm100089e
BindingDB Entry DOI: 10.7270/Q2TX3FJ2 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321907

((Z)-1-(4-Chlorophenyl)-3-[2-oxo-3-(1H-pyrrol-2-ylm...)Show SMILES Clc1ccc(NC(=O)Nc2ccc3\C(=C\c4ccc[nH]4)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C20H15ClN4O2/c21-12-3-5-13(6-4-12)23-20(27)24-15-7-8-16-17(10-14-2-1-9-22-14)19(26)25-18(16)11-15/h1-11,22H,(H,25,26)(H2,23,24,27)/b17-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM248156
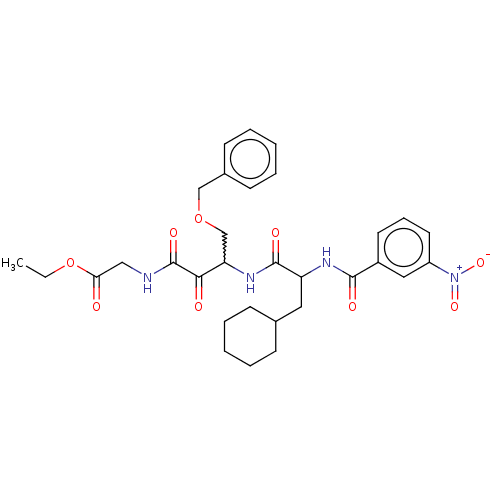
(Cathepsin S inhibitor alpha-ketoamide, 6b)Show SMILES CCOC(=O)CNC(=O)C(=O)C(COCc1ccccc1)NC(=O)C(CC1CCCCC1)NC(=O)c1cccc(c1)[N+]([O-])=O |w:11.11| Show InChI InChI=1S/C31H38N4O9/c1-2-44-27(36)18-32-31(40)28(37)26(20-43-19-22-12-7-4-8-13-22)34-30(39)25(16-21-10-5-3-6-11-21)33-29(38)23-14-9-15-24(17-23)35(41)42/h4,7-9,12-15,17,21,25-26H,2-3,5-6,10-11,16,18-20H2,1H3,(H,32,40)(H,33,38)(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Tsing Hua University
| Assay Description
A microplate-based screening procedure was used to study the effect of inhibition of re-Cat S, re-Cat L and re-Cat K on several compounds. The assays... |
J Enzyme Inhib Med Chem 29: 538-46 (2014)
Article DOI: 10.3109/14756366.2013.823957
BindingDB Entry DOI: 10.7270/Q2RN36RG |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50320040
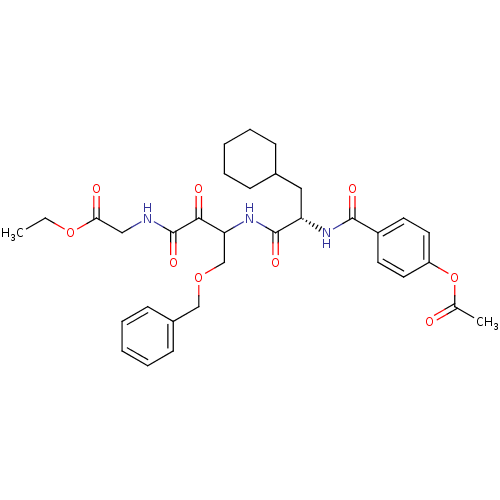
(CHEMBL1085728 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...)Show SMILES CCOC(=O)CNC(=O)C(=O)C(COCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1ccc(OC(C)=O)cc1 |r| Show InChI InChI=1S/C33H41N3O9/c1-3-44-29(38)19-34-33(42)30(39)28(21-43-20-24-12-8-5-9-13-24)36-32(41)27(18-23-10-6-4-7-11-23)35-31(40)25-14-16-26(17-15-25)45-22(2)37/h5,8-9,12-17,23,27-28H,3-4,6-7,10-11,18-21H2,1-2H3,(H,34,42)(H,35,40)(H,36,41)/t27-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay |
J Med Chem 53: 4545-9 (2010)
Article DOI: 10.1021/jm100089e
BindingDB Entry DOI: 10.7270/Q2TX3FJ2 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50320065
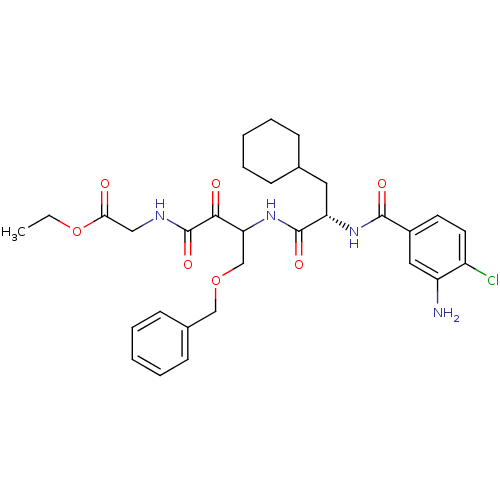
(CHEMBL1085970 | Glycine, N-[(3S)-4-Benzyloxy-3-[[[...)Show SMILES CCOC(=O)CNC(=O)C(=O)C(COCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1ccc(Cl)c(N)c1 |r| Show InChI InChI=1S/C31H39ClN4O7/c1-2-43-27(37)17-34-31(41)28(38)26(19-42-18-21-11-7-4-8-12-21)36-30(40)25(15-20-9-5-3-6-10-20)35-29(39)22-13-14-23(32)24(33)16-22/h4,7-8,11-14,16,20,25-26H,2-3,5-6,9-10,15,17-19,33H2,1H3,(H,34,41)(H,35,39)(H,36,40)/t25-,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay |
J Med Chem 53: 4545-9 (2010)
Article DOI: 10.1021/jm100089e
BindingDB Entry DOI: 10.7270/Q2TX3FJ2 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50321892

((Z)-5-{6-[3-(4-Methoxyphenyl)-ureido]-2-oxo-1,2-di...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4[nH]cc(C(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C23H20N4O5/c1-12-18(22(29)30)11-24-19(12)10-17-16-8-5-14(9-20(16)27-21(17)28)26-23(31)25-13-3-6-15(32-2)7-4-13/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50051957

(CHEMBL3322566)Show SMILES COc1ccc(NC(=O)CC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C32H36N6O5/c1-19-26(34-20(2)30(19)32(42)33-12-15-38-13-4-5-14-38)17-25-24-11-8-22(16-27(24)37-31(25)41)36-29(40)18-28(39)35-21-6-9-23(43-3)10-7-21/h6-11,16-17,34H,4-5,12-15,18H2,1-3H3,(H,33,42)(H,35,39)(H,36,40)(H,37,41)/b25-17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 (unknown origin) using EAIYAAPFAKKK substrate by radioisotope-based P81 filter-binding assay |
Eur J Med Chem 84: 312-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.033
BindingDB Entry DOI: 10.7270/Q2RV0QB7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50056199

(CHEMBL3329684)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(=O)NCCN5CCCC5)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C31H33FN6O5/c1-17-25(34-18(2)27(17)30(41)33-10-13-38-11-4-5-12-38)16-23-21-8-6-19(14-26(21)36-29(23)40)35-31(42)37-28(39)22-9-7-20(43-3)15-24(22)32/h6-9,14-16,34H,4-5,10-13H2,1-3H3,(H,33,41)(H,36,40)(H2,35,37,39,42)/b23-16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321893

((Z)-1-[2-Oxo-3-(1H-pyrrol-2-ylmethylene)-2,3-dihyd...)Show SMILES O=C(Nc1ccccc1)Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C20H16N4O2/c25-19-17(11-14-7-4-10-21-14)16-9-8-15(12-18(16)24-19)23-20(26)22-13-5-2-1-3-6-13/h1-12,21H,(H,24,25)(H2,22,23,26)/b17-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50320057
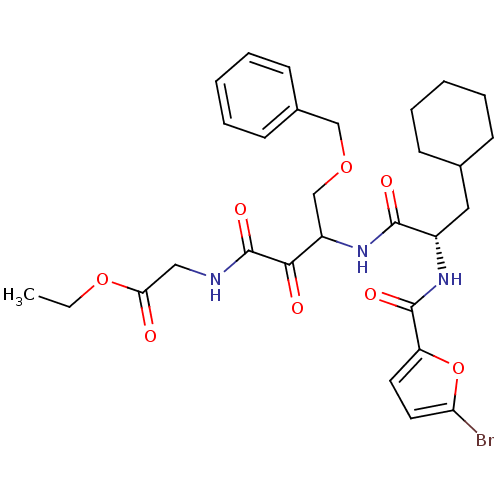
(CHEMBL1083620 | ethyl 2-(4-(benzyloxy)-3-((S)-2-(5...)Show SMILES CCOC(=O)CNC(=O)C(=O)C(COCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1ccc(Br)o1 |r| Show InChI InChI=1S/C29H36BrN3O8/c1-2-40-25(34)16-31-29(38)26(35)22(18-39-17-20-11-7-4-8-12-20)33-27(36)21(15-19-9-5-3-6-10-19)32-28(37)23-13-14-24(30)41-23/h4,7-8,11-14,19,21-22H,2-3,5-6,9-10,15-18H2,1H3,(H,31,38)(H,32,37)(H,33,36)/t21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay |
J Med Chem 53: 4545-9 (2010)
Article DOI: 10.1021/jm100089e
BindingDB Entry DOI: 10.7270/Q2TX3FJ2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056143

(CHEMBL3329672)Show SMILES COc1ccc(C(=O)NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(C(O)=O)c4C)C(=O)Nc3c2)c(F)c1 Show InChI InChI=1S/C25H21FN4O6/c1-11-19(27-12(2)21(11)24(33)34)10-17-15-6-4-13(8-20(15)29-23(17)32)28-25(35)30-22(31)16-7-5-14(36-3)9-18(16)26/h4-10,27H,1-3H3,(H,29,32)(H,33,34)(H2,28,30,31,35)/b17-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50056146

(CHEMBL3329669)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1ccc2\C(=C\c3[nH]c(C)c(CCC(O)=O)c3C)C(=O)Nc2c1 Show InChI InChI=1S/C27H26N4O6/c1-14-19(10-11-24(32)33)15(2)28-22(14)13-21-20-9-6-17(12-23(20)30-26(21)35)29-27(36)31-25(34)16-4-7-18(37-3)8-5-16/h4-9,12-13,28H,10-11H2,1-3H3,(H,30,35)(H,32,33)(H2,29,31,34,36)/b21-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using EAIYAAPFAKKK substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321900

((Z)-3-(5-{6-[3-(4-Methoxyphenyl)-ureido]-2-oxo-1,2...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4[nH]c(C)c(CCC(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C26H26N4O5/c1-14-19(10-11-24(31)32)15(2)27-22(14)13-21-20-9-6-17(12-23(20)30-25(21)33)29-26(34)28-16-4-7-18(35-3)8-5-16/h4-9,12-13,27H,10-11H2,1-3H3,(H,30,33)(H,31,32)(H2,28,29,34)/b21-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50052075

(CHEMBL3322595)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)c4c[nH]cc(-c5ccc(F)cc5)c4=O)ccc23)c(C)c1C(=O)N1CCOCC1 Show InChI InChI=1S/C32H28FN5O5/c1-17-26(35-18(2)28(17)32(42)38-9-11-43-12-10-38)14-23-22-8-7-21(13-27(22)37-30(23)40)36-31(41)25-16-34-15-24(29(25)39)19-3-5-20(33)6-4-19/h3-8,13-16,35H,9-12H2,1-2H3,(H,34,39)(H,36,41)(H,37,40)/b23-14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using H-LRRASLG substrate by radioisotope-based P81 filter-binding assay |
Eur J Med Chem 84: 312-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.033
BindingDB Entry DOI: 10.7270/Q2RV0QB7 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056141

(CHEMBL3329674)Show SMILES COc1ccc(C(=O)NC(=O)Nc2cc3NC(=O)\C(=C/c4[nH]c(C)c(C(O)=O)c4C)c3cc2F)c(F)c1 Show InChI InChI=1S/C25H20F2N4O6/c1-10-18(28-11(2)21(10)24(34)35)8-15-14-7-17(27)20(9-19(14)29-23(15)33)30-25(36)31-22(32)13-5-4-12(37-3)6-16(13)26/h4-9,28H,1-3H3,(H,29,33)(H,34,35)(H2,30,31,32,36)/b15-8- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50056144
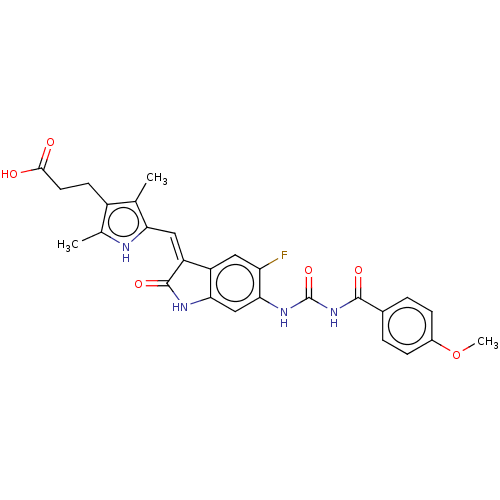
(CHEMBL3329671)Show SMILES COc1ccc(cc1)C(=O)NC(=O)Nc1cc2NC(=O)\C(=C/c3[nH]c(C)c(CCC(O)=O)c3C)c2cc1F Show InChI InChI=1S/C27H25FN4O6/c1-13-17(8-9-24(33)34)14(2)29-21(13)11-19-18-10-20(28)23(12-22(18)30-26(19)36)31-27(37)32-25(35)15-4-6-16(38-3)7-5-15/h4-7,10-12,29H,8-9H2,1-3H3,(H,30,36)(H,33,34)(H2,31,32,35,37)/b19-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) using HLRRASLG substrate |
Eur J Med Chem 85: 268-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.108
BindingDB Entry DOI: 10.7270/Q2028T6Z |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM248157
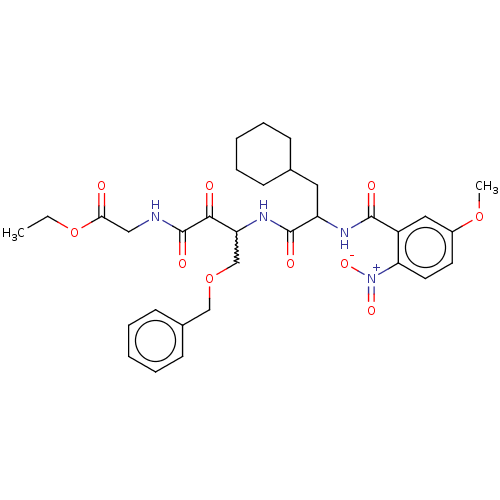
(Cathepsin S inhibitor alpha-ketoamide, 6e)Show SMILES CCOC(=O)CNC(=O)C(=O)C(COCc1ccccc1)NC(=O)C(CC1CCCCC1)NC(=O)c1cc(OC)ccc1[N+]([O-])=O |w:11.11| Show InChI InChI=1S/C32H40N4O10/c1-3-46-28(37)18-33-32(41)29(38)26(20-45-19-22-12-8-5-9-13-22)35-31(40)25(16-21-10-6-4-7-11-21)34-30(39)24-17-23(44-2)14-15-27(24)36(42)43/h5,8-9,12-15,17,21,25-26H,3-4,6-7,10-11,16,18-20H2,1-2H3,(H,33,41)(H,34,39)(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Tsing Hua University
| Assay Description
A microplate-based screening procedure was used to study the effect of inhibition of re-Cat S, re-Cat L and re-Cat K on several compounds. The assays... |
J Enzyme Inhib Med Chem 29: 538-46 (2014)
Article DOI: 10.3109/14756366.2013.823957
BindingDB Entry DOI: 10.7270/Q2RN36RG |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50320039
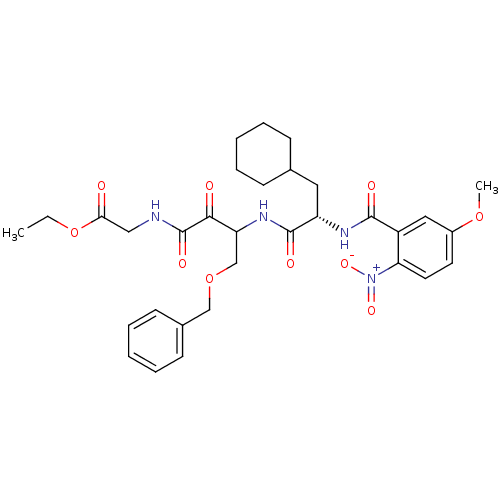
(CHEMBL1085726 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...)Show SMILES CCOC(=O)CNC(=O)C(=O)C(COCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1cc(OC)ccc1[N+]([O-])=O |r| Show InChI InChI=1S/C32H40N4O10/c1-3-46-28(37)18-33-32(41)29(38)26(20-45-19-22-12-8-5-9-13-22)35-31(40)25(16-21-10-6-4-7-11-21)34-30(39)24-17-23(44-2)14-15-27(24)36(42)43/h5,8-9,12-15,17,21,25-26H,3-4,6-7,10-11,16,18-20H2,1-2H3,(H,33,41)(H,34,39)(H,35,40)/t25-,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay |
J Med Chem 53: 4545-9 (2010)
Article DOI: 10.1021/jm100089e
BindingDB Entry DOI: 10.7270/Q2TX3FJ2 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM248161
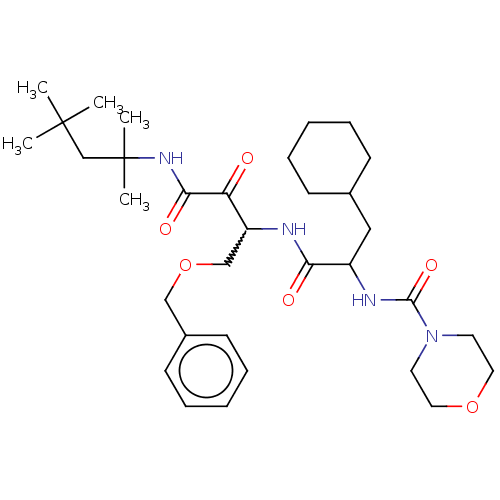
(Cathepsin S inhibitor alpha-ketoamide, 6w)Show SMILES CC(C)(C)CC(C)(C)NC(=O)C(=O)C(COCc1ccccc1)NC(=O)C(CC1CCCCC1)NC(=O)N1CCOCC1 |w:13.13| Show InChI InChI=1S/C33H52N4O6/c1-32(2,3)23-33(4,5)36-30(40)28(38)27(22-43-21-25-14-10-7-11-15-25)34-29(39)26(20-24-12-8-6-9-13-24)35-31(41)37-16-18-42-19-17-37/h7,10-11,14-15,24,26-27H,6,8-9,12-13,16-23H2,1-5H3,(H,34,39)(H,35,41)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Tsing Hua University
| Assay Description
A microplate-based screening procedure was used to study the effect of inhibition of re-Cat S, re-Cat L and re-Cat K on several compounds. The assays... |
J Enzyme Inhib Med Chem 29: 538-46 (2014)
Article DOI: 10.3109/14756366.2013.823957
BindingDB Entry DOI: 10.7270/Q2RN36RG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data