

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480056 (CHEMBL468083) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480058 (CHEMBL495009) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480051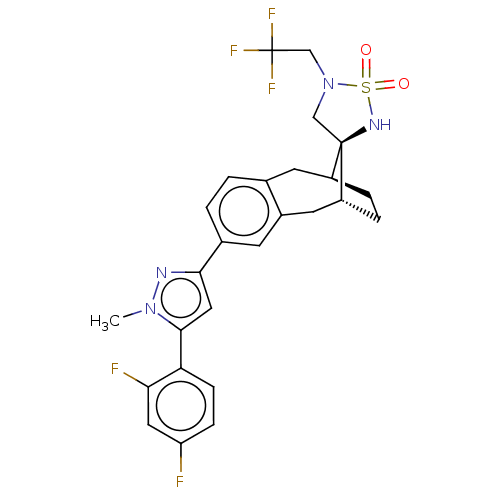 (CHEMBL512282) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480052 (CHEMBL467457) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480057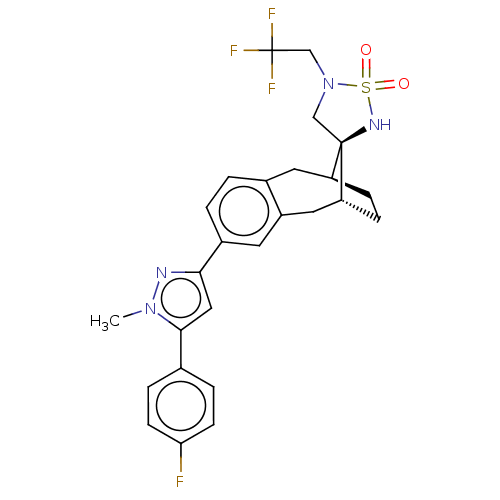 (CHEMBL523832) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480062 (CHEMBL511572) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480065 (CHEMBL495185) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480059 (CHEMBL523883) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480063 (CHEMBL511928) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480066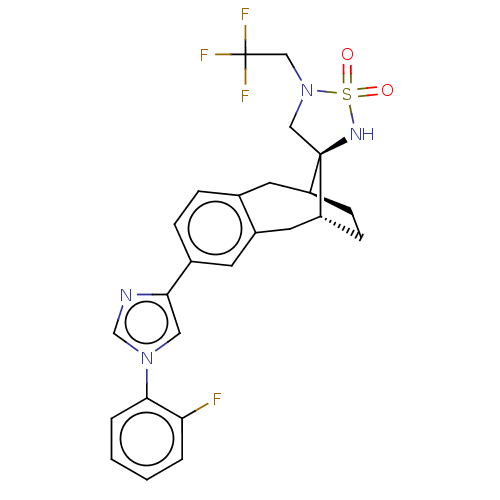 (CHEMBL494588) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480050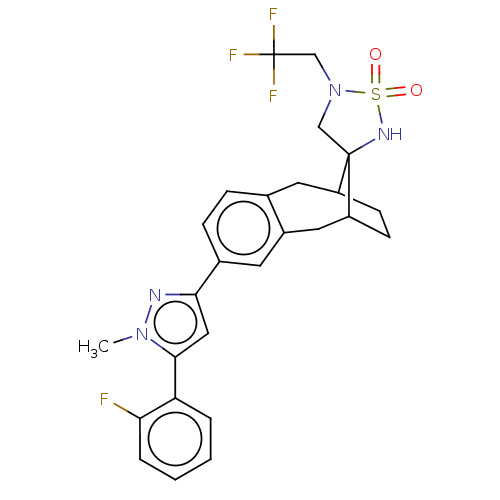 (CHEMBL511375) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50171224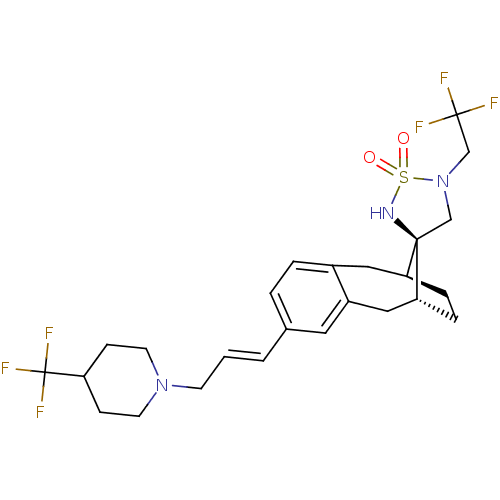 (CHEMBL196215 | Cyclic sulfamide) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480054 (CHEMBL506323) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480053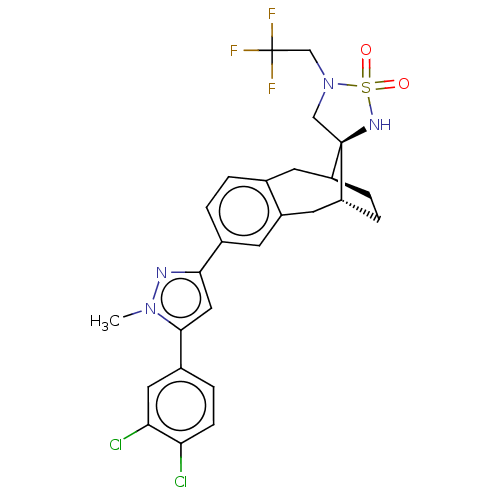 (CHEMBL466421) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480055 (CHEMBL513674) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480060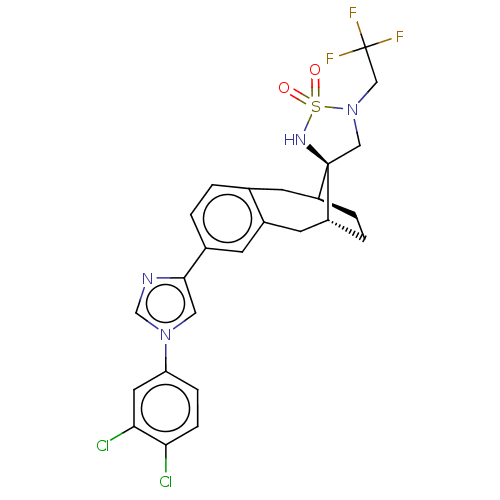 (CHEMBL466256) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480061 (CHEMBL512596) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50381918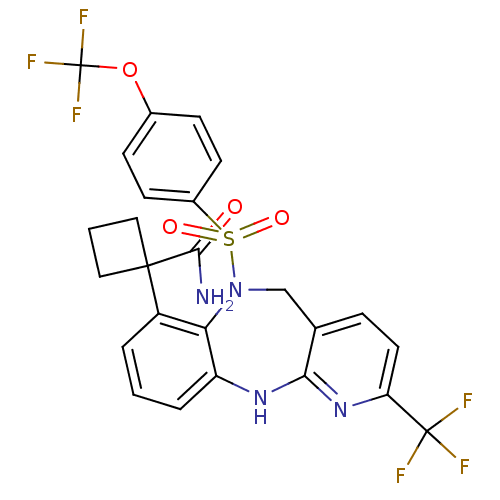 (CHEMBL2023113) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2845-9 (2012) Article DOI: 10.1016/j.bmc.2012.03.029 BindingDB Entry DOI: 10.7270/Q2F190R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50381917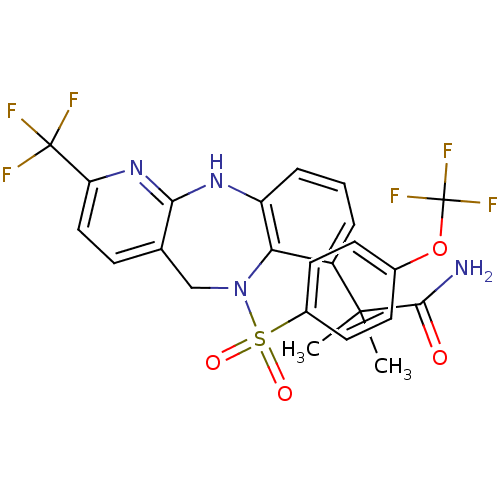 (CHEMBL2023112) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2845-9 (2012) Article DOI: 10.1016/j.bmc.2012.03.029 BindingDB Entry DOI: 10.7270/Q2F190R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50381916 (CHEMBL2023111) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2845-9 (2012) Article DOI: 10.1016/j.bmc.2012.03.029 BindingDB Entry DOI: 10.7270/Q2F190R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400247 (CHEMBL2179918) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480064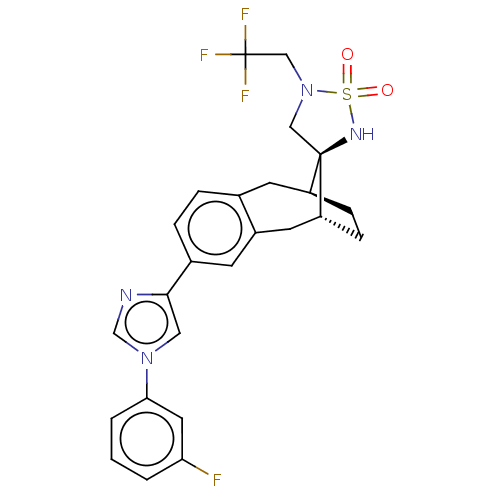 (CHEMBL494769) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400254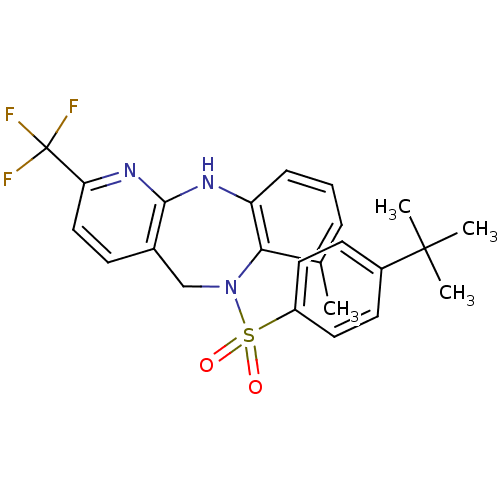 (CHEMBL2179911) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228308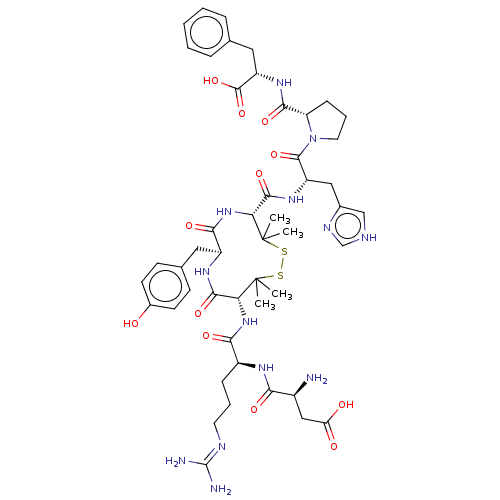 (CHEMBL413740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400257 (CHEMBL2179908) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50381915 (CHEMBL2023110) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2845-9 (2012) Article DOI: 10.1016/j.bmc.2012.03.029 BindingDB Entry DOI: 10.7270/Q2F190R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400246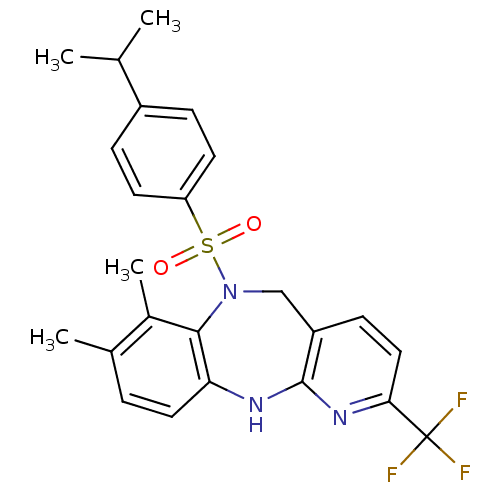 (CHEMBL2179473) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50480067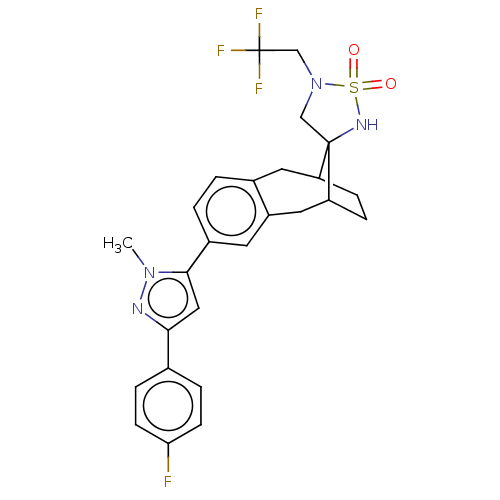 (CHEMBL492394) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma secretase in human SH-SY5Y cells by HTRF assay | J Med Chem 52: 3441-4 (2009) Article DOI: 10.1021/jm900056p BindingDB Entry DOI: 10.7270/Q2B85BZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228272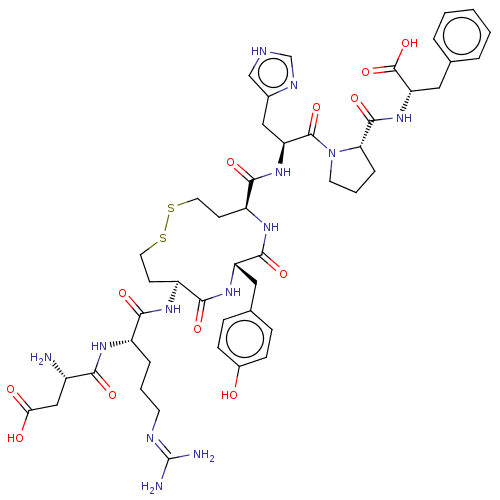 (CHEMBL411997) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471553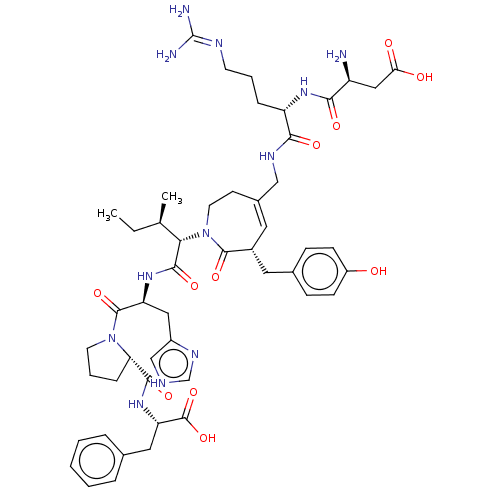 (CHEMBL1791261) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228195 (Angiotensin Ii | CHEBI:2719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228272 (CHEMBL411997) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against specific binding of [125 I]Ang II to rat uterine membranes (Angiotensin II receptor, type 1) | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50381919 (CHEMBL2023114) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2845-9 (2012) Article DOI: 10.1016/j.bmc.2012.03.029 BindingDB Entry DOI: 10.7270/Q2F190R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400249 (CHEMBL2179916) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228308 (CHEMBL413740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against specific binding of [125 I]Ang II to rat uterine membranes (Angiotensin II receptor, type 1) | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471554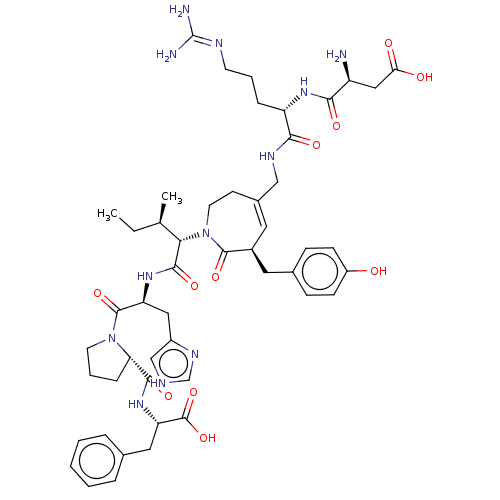 (CHEMBL1791267) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228196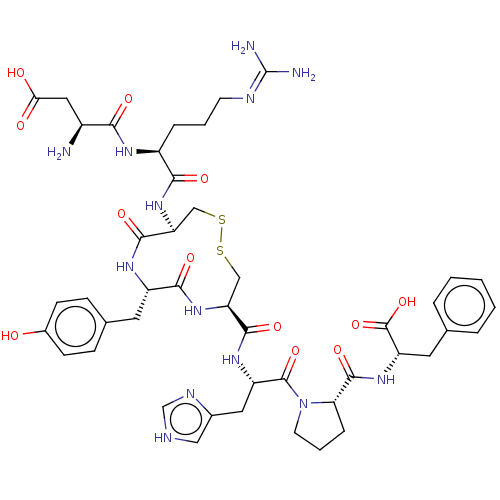 (CHEMBL405464) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400253 (CHEMBL2179912) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400243 (CHEMBL2179476) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50381914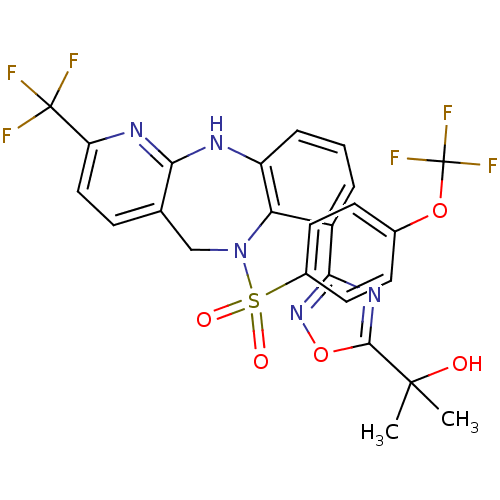 (CHEMBL2023109 | MK-7725) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting | Bioorg Med Chem 20: 2845-9 (2012) Article DOI: 10.1016/j.bmc.2012.03.029 BindingDB Entry DOI: 10.7270/Q2F190R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228308 (CHEMBL413740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against specific binding of [125 I]Ang II to rat pituitary membranes (Angiotensin II receptor, type 1) in presence of 0.2% b... | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400258 (CHEMBL2179907) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400250 (CHEMBL2179915) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400248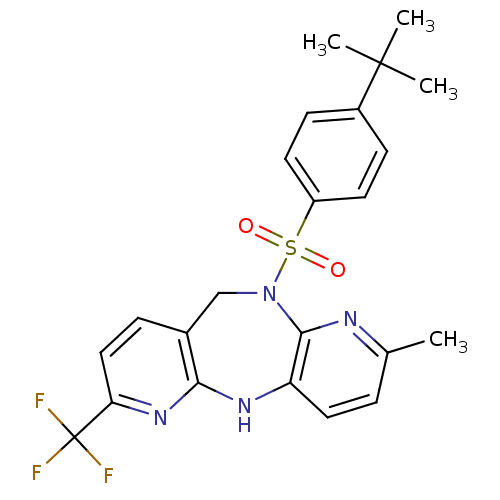 (CHEMBL2179917) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400251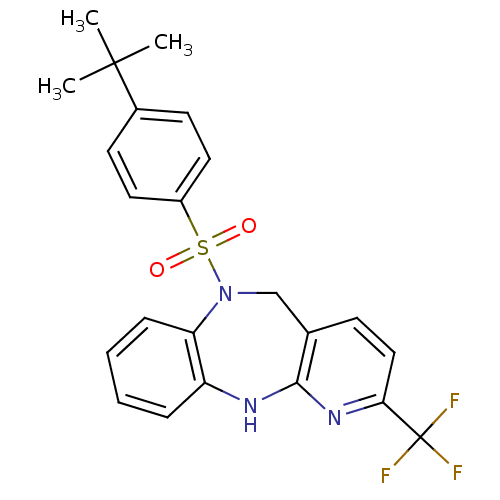 (CHEMBL2179914) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400242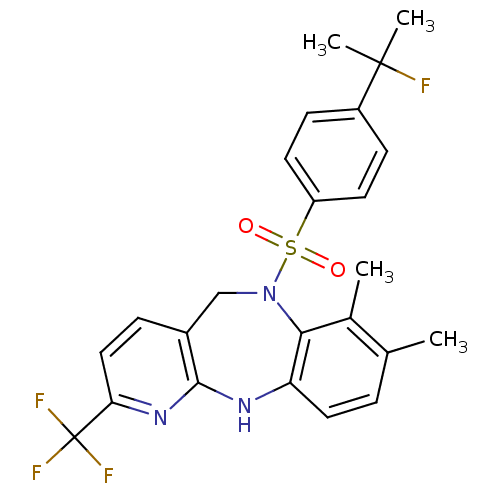 (CHEMBL2179477) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400241 (CHEMBL2179478) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400252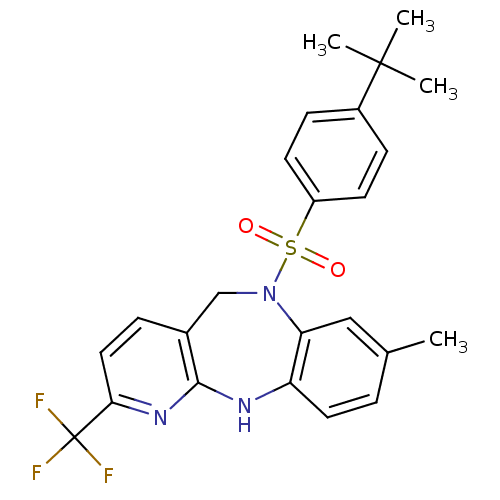 (CHEMBL2179913) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50400245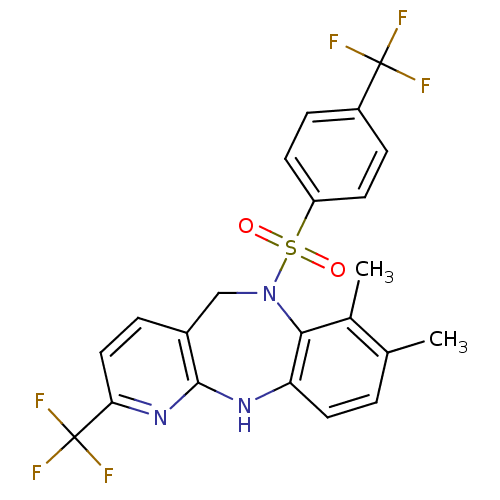 (CHEMBL2179474) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... | ACS Med Chem Lett 2: 933-937 (2011) Article DOI: 10.1021/ml200207w BindingDB Entry DOI: 10.7270/Q25Q4X77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 132 total ) | Next | Last >> |