 Found 10961 hits with Last Name = 'wen' and Initial = 'c'
Found 10961 hits with Last Name = 'wen' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from recombinant human D2 long receptor stably expressed in HEK293 cell membranes measured after 60 mins by scintillation ... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]ABN from human dopamine D2 long receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting method |
J Med Chem 60: 9905-9910 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01248
BindingDB Entry DOI: 10.7270/Q21838X7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50250803
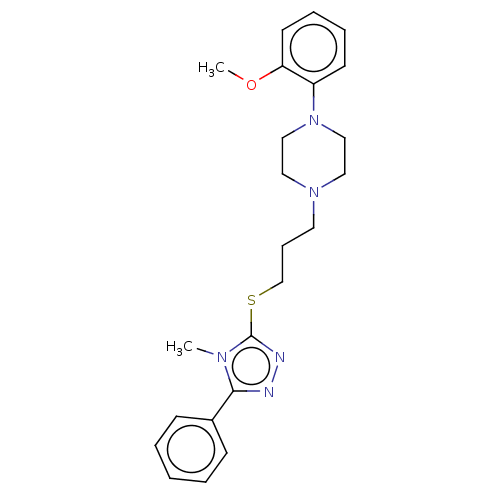
(CHEMBL4083252)Show SMILES COc1ccccc1N1CCN(CCCSc2nnc(-c3ccccc3)n2C)CC1 Show InChI InChI=1S/C23H29N5OS/c1-26-22(19-9-4-3-5-10-19)24-25-23(26)30-18-8-13-27-14-16-28(17-15-27)20-11-6-7-12-21(20)29-2/h3-7,9-12H,8,13-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting method |
J Med Chem 60: 9905-9910 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01248
BindingDB Entry DOI: 10.7270/Q21838X7 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]ABN from human dopamine D3 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting method |
J Med Chem 60: 9905-9910 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01248
BindingDB Entry DOI: 10.7270/Q21838X7 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from recombinant human D3 receptor stably expressed in HEK293 cell membranes measured after 60 mins by scintillation count... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM21842
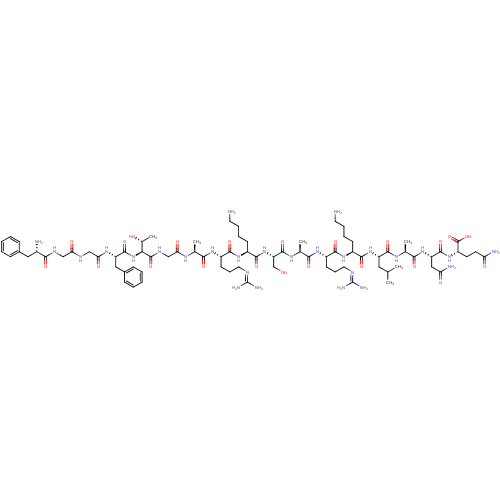
((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by PDSP Ki Database
| |
J Chem Neuroanat 25: 233-47 (2003)
Article DOI: 10.1016/s0891-0618(03)00032-2
BindingDB Entry DOI: 10.7270/Q2XP73G2 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D4 receptor (unknown origin) |
J Med Chem 60: 9905-9910 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01248
BindingDB Entry DOI: 10.7270/Q21838X7 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of D4 receptor (unknown origin) |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DVanderbilt Program in Drug Discovery
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat recombinant muscarinic M4 receptor expressed in CHO cells after 2 hrs by microplate scintillation counting |
Nat Chem Biol 4: 42-50 (2007)
Article DOI: 10.1038/nchembio.2007.55
BindingDB Entry DOI: 10.7270/Q2D50N55 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DVanderbilt Program in Drug Discovery
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat recombinant muscarinic M4 receptor expressed in CHO cells after 2 hrs by microplate scintillation counting |
Nat Chem Biol 4: 42-50 (2007)
Article DOI: 10.1038/nchembio.2007.55
BindingDB Entry DOI: 10.7270/Q2D50N55 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat muscarinic M4 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 885-90 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.051
BindingDB Entry DOI: 10.7270/Q2DZ095Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50240552
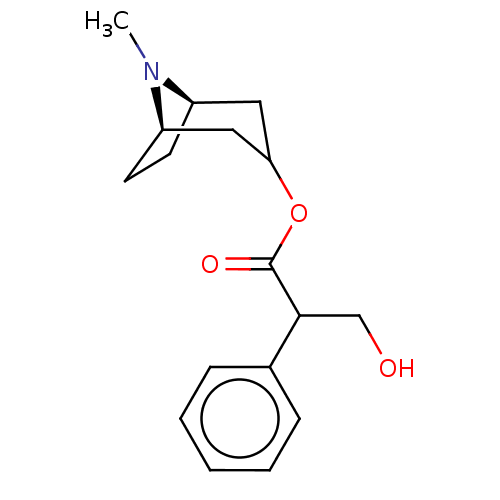
(CHEMBL195)Show SMILES [H][C@@]12CC[C@]([H])(CC(C1)OC(=O)C(CO)c1ccccc1)N2C |r,@@:7,TLB:9:7:21:2.3| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14-,16?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic M4 receptor expressed in CHO cell membranes |
J Med Chem 34: 1879-84 (1991)
Article DOI: 10.1016/j.bmcl.2017.05.042
BindingDB Entry DOI: 10.7270/Q29G5KR1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DVanderbilt Program in Drug Discovery
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat recombinant muscarinic M5 receptor expressed in CHO cells after 2 hrs by microplate scintillation counting |
Nat Chem Biol 4: 42-50 (2007)
Article DOI: 10.1038/nchembio.2007.55
BindingDB Entry DOI: 10.7270/Q2D50N55 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50250803

(CHEMBL4083252)Show SMILES COc1ccccc1N1CCN(CCCSc2nnc(-c3ccccc3)n2C)CC1 Show InChI InChI=1S/C23H29N5OS/c1-26-22(19-9-4-3-5-10-19)24-25-23(26)30-18-8-13-27-14-16-28(17-15-27)20-11-6-7-12-21(20)29-2/h3-7,9-12H,8,13-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Way100635 from recombinant human 5HT1A receptor transiently expressed in CHO cell membranes measured after 90 mins by microbeta ... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat muscarinic M1 receptor expressed in CHO cells after 3 hrs |
Bioorg Med Chem Lett 23: 223-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.132
BindingDB Entry DOI: 10.7270/Q2TM7CF9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat muscarinic M1 receptor expressed in CHO cells after 3 hrs |
Bioorg Med Chem Lett 23: 223-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.132
BindingDB Entry DOI: 10.7270/Q2TM7CF9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic acetylcholine receptor subtype 5 expressed in CHO cell membranes by scintillation counting method |
J Med Chem 57: 7804-10 (2014)
Article DOI: 10.1021/jm500995y
BindingDB Entry DOI: 10.7270/Q2ST7RFM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50240552

(CHEMBL195)Show SMILES [H][C@@]12CC[C@]([H])(CC(C1)OC(=O)C(CO)c1ccccc1)N2C |r,@@:7,TLB:9:7:21:2.3| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14-,16?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from recombinant human M4 receptor expressed in CHO cell membranes |
Bioorg Med Chem Lett 27: 2479-2483 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.009
BindingDB Entry DOI: 10.7270/Q2VT1V7H |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372
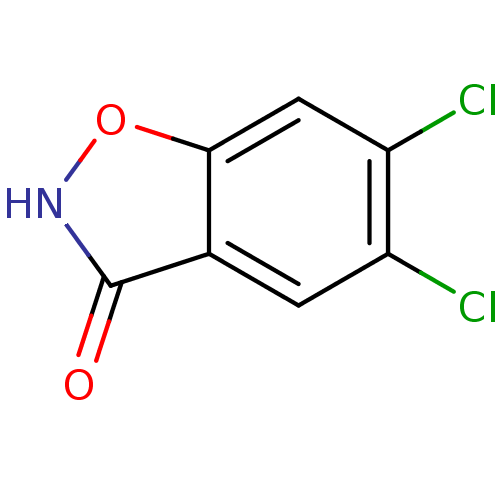
(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat muscarinic M5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 885-90 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.051
BindingDB Entry DOI: 10.7270/Q2DZ095Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50045114
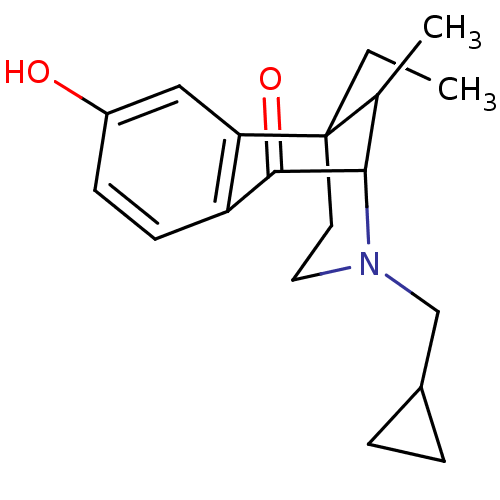
(3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-methyl-3,...)Show SMILES CCC12CCN(CC3CC3)C(C1C)C(=O)c1ccc(O)cc21 |TLB:6:5:11:15.21.13,14:13:11:5.4.3,THB:20:21:11:5.4.3| Show InChI InChI=1S/C19H25NO2/c1-3-19-8-9-20(11-13-4-5-13)17(12(19)2)18(22)15-7-6-14(21)10-16(15)19/h6-7,10,12-13,17,21H,3-5,8-9,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by PDSP Ki Database
| |
J Chem Neuroanat 25: 233-47 (2003)
Article DOI: 10.1016/s0891-0618(03)00032-2
BindingDB Entry DOI: 10.7270/Q2XP73G2 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50161275

(CHEMBL3787059)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C23H21ClN4O4/c1-2-3-8-18-25-20(24)19(22(29)30)28(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-26-23(31)32-27-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,29,30)(H,26,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Donghua University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from Angiotensin 2 type-1A receptor in rat vascular smooth muscle cells after 150 mins by gamma counting method |
Bioorg Med Chem 24: 2023-31 (2016)
Article DOI: 10.1016/j.bmc.2016.03.028
BindingDB Entry DOI: 10.7270/Q2XW4MQB |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50045114

(3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-methyl-3,...)Show SMILES CCC12CCN(CC3CC3)C(C1C)C(=O)c1ccc(O)cc21 |TLB:6:5:11:15.21.13,14:13:11:5.4.3,THB:20:21:11:5.4.3| Show InChI InChI=1S/C19H25NO2/c1-3-19-8-9-20(11-13-4-5-13)17(12(19)2)18(22)15-7-6-14(21)10-16(15)19/h6-7,10,12-13,17,21H,3-5,8-9,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by PDSP Ki Database
| |
J Chem Neuroanat 25: 233-47 (2003)
Article DOI: 10.1016/s0891-0618(03)00032-2
BindingDB Entry DOI: 10.7270/Q2XP73G2 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50045114

(3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-methyl-3,...)Show SMILES CCC12CCN(CC3CC3)C(C1C)C(=O)c1ccc(O)cc21 |TLB:6:5:11:15.21.13,14:13:11:5.4.3,THB:20:21:11:5.4.3| Show InChI InChI=1S/C19H25NO2/c1-3-19-8-9-20(11-13-4-5-13)17(12(19)2)18(22)15-7-6-14(21)10-16(15)19/h6-7,10,12-13,17,21H,3-5,8-9,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by PDSP Ki Database
| |
J Chem Neuroanat 25: 233-47 (2003)
Article DOI: 10.1016/s0891-0618(03)00032-2
BindingDB Entry DOI: 10.7270/Q2XP73G2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390424
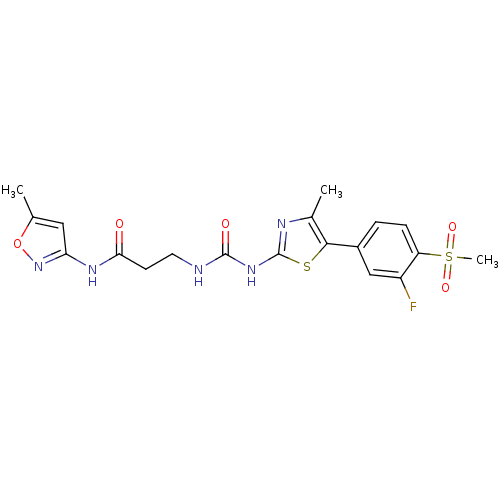
(CHEMBL2071340)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)no1 Show InChI InChI=1S/C19H20FN5O5S2/c1-10-8-15(25-30-10)23-16(26)6-7-21-18(27)24-19-22-11(2)17(31-19)12-4-5-14(13(20)9-12)32(3,28)29/h4-5,8-9H,6-7H2,1-3H3,(H,23,25,26)(H2,21,22,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50161276
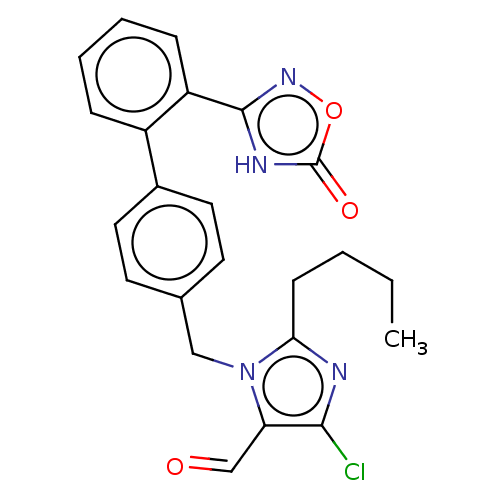
(CHEMBL3786570)Show SMILES CCCCc1nc(Cl)c(C=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C23H21ClN4O3/c1-2-3-8-20-25-21(24)19(14-29)28(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-26-23(30)31-27-22/h4-7,9-12,14H,2-3,8,13H2,1H3,(H,26,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Donghua University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from Angiotensin 2 type-1A receptor in rat vascular smooth muscle cells after 150 mins by gamma counting method |
Bioorg Med Chem 24: 2023-31 (2016)
Article DOI: 10.1016/j.bmc.2016.03.028
BindingDB Entry DOI: 10.7270/Q2XW4MQB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50525056
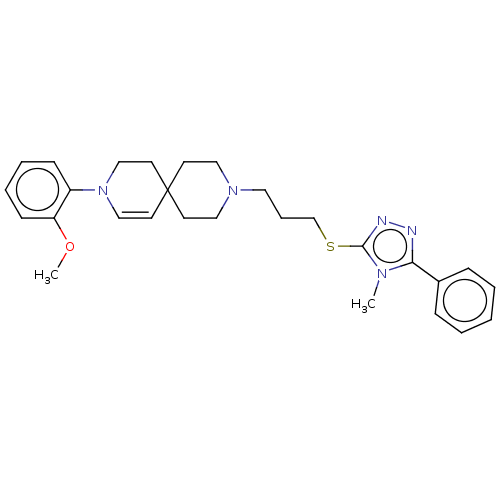
(CHEMBL4525842)Show SMILES COc1ccccc1N1CCC2(CCN(CCCSc3nnc(-c4ccccc4)n3C)CC2)C=C1 |c:37| Show InChI InChI=1S/C28H35N5OS/c1-31-26(23-9-4-3-5-10-23)29-30-27(31)35-22-8-17-32-18-13-28(14-19-32)15-20-33(21-16-28)24-11-6-7-12-25(24)34-2/h3-7,9-12,15,20H,8,13-14,16-19,21-22H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from recombinant human D3 receptor stably expressed in HEK293 cell membranes measured after 60 mins by scintillation count... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725
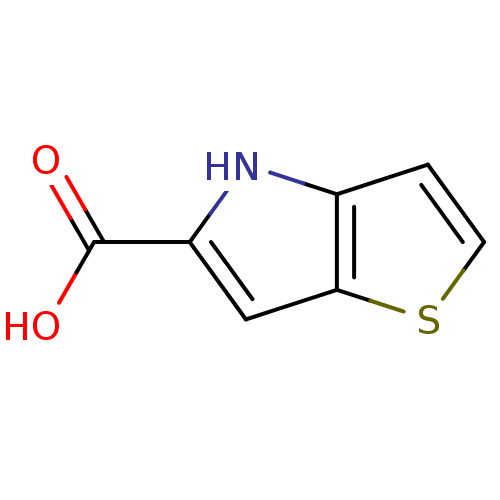
(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390409
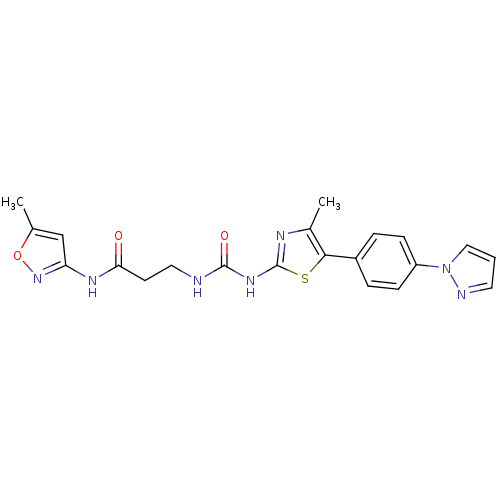
(CHEMBL1986603)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(cc2)-n2cccn2)no1 Show InChI InChI=1S/C21H21N7O3S/c1-13-12-17(27-31-13)25-18(29)8-10-22-20(30)26-21-24-14(2)19(32-21)15-4-6-16(7-5-15)28-11-3-9-23-28/h3-7,9,11-12H,8,10H2,1-2H3,(H,25,27,29)(H2,22,24,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50161277
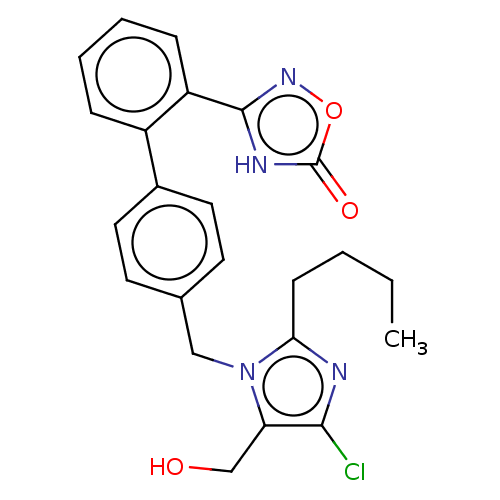
(CHEMBL3787050)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C23H23ClN4O3/c1-2-3-8-20-25-21(24)19(14-29)28(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-26-23(30)31-27-22/h4-7,9-12,29H,2-3,8,13-14H2,1H3,(H,26,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Donghua University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from Angiotensin 2 type-1A receptor in rat vascular smooth muscle cells after 150 mins by gamma counting method |
Bioorg Med Chem 24: 2023-31 (2016)
Article DOI: 10.1016/j.bmc.2016.03.028
BindingDB Entry DOI: 10.7270/Q2XW4MQB |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50257064
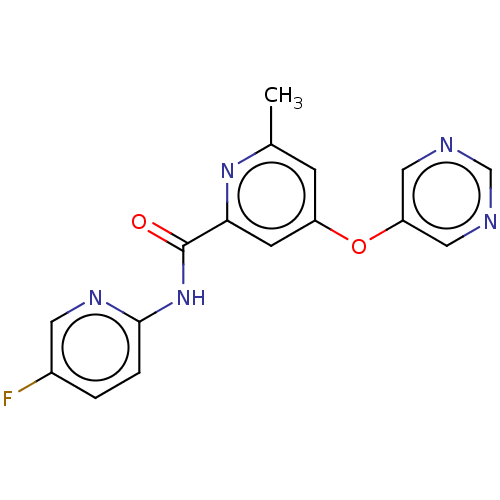
(CHEMBL2386850)Show InChI InChI=1S/C16H12FN5O2/c1-10-4-12(24-13-7-18-9-19-8-13)5-14(21-10)16(23)22-15-3-2-11(17)6-20-15/h2-9H,1H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Radiology and Radiological Sciences, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center , Nashville, Tennessee 37232, United States.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat mGlu5 receptor expressed in HEK293A cell membranes after 1 hr by scintillatio... |
J Med Chem 60: 5072-5085 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00410
BindingDB Entry DOI: 10.7270/Q2JH3PM7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390408
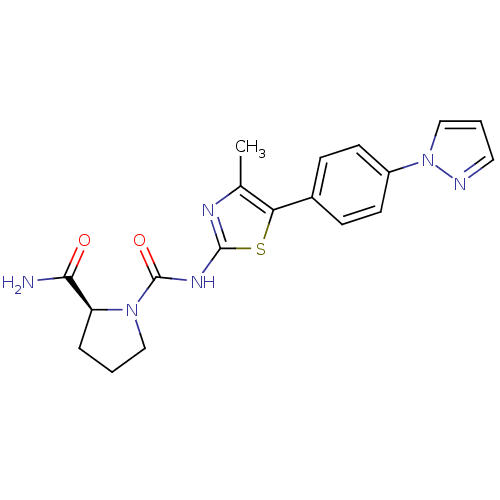
(CHEMBL2071338)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H20N6O2S/c1-12-16(13-5-7-14(8-6-13)25-11-3-9-21-25)28-18(22-12)23-19(27)24-10-2-4-15(24)17(20)26/h3,5-9,11,15H,2,4,10H2,1H3,(H2,20,26)(H,22,23,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50250803

(CHEMBL4083252)Show SMILES COc1ccccc1N1CCN(CCCSc2nnc(-c3ccccc3)n2C)CC1 Show InChI InChI=1S/C23H29N5OS/c1-26-22(19-9-4-3-5-10-19)24-25-23(26)30-18-8-13-27-14-16-28(17-15-27)20-11-6-7-12-21(20)29-2/h3-7,9-12H,8,13-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from D3 receptor (unknown origin) measured after 90 mins by microbeta scintillation counting method relative to... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50250803

(CHEMBL4083252)Show SMILES COc1ccccc1N1CCN(CCCSc2nnc(-c3ccccc3)n2C)CC1 Show InChI InChI=1S/C23H29N5OS/c1-26-22(19-9-4-3-5-10-19)24-25-23(26)30-18-8-13-27-14-16-28(17-15-27)20-11-6-7-12-21(20)29-2/h3-7,9-12H,8,13-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from recombinant human D3 receptor stably expressed in HEK293 cell membranes measured after 60 mins by scintillation count... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50250803

(CHEMBL4083252)Show SMILES COc1ccccc1N1CCN(CCCSc2nnc(-c3ccccc3)n2C)CC1 Show InChI InChI=1S/C23H29N5OS/c1-26-22(19-9-4-3-5-10-19)24-25-23(26)30-18-8-13-27-14-16-28(17-15-27)20-11-6-7-12-21(20)29-2/h3-7,9-12H,8,13-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]ABN from human dopamine D3 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting method |
J Med Chem 60: 9905-9910 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01248
BindingDB Entry DOI: 10.7270/Q21838X7 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50442525

(CHEMBL2440659 | US8796295, Table 2: Compound: 1)Show InChI InChI=1S/C15H11FN4O2S/c1-9-7-23-15(19-9)20-14(21)10-2-11(16)4-12(3-10)22-13-5-17-8-18-6-13/h2-8H,1H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor allosteric binding site (unknown origin) |
Bioorg Med Chem Lett 23: 5779-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.001
BindingDB Entry DOI: 10.7270/Q2X92CSQ |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260722
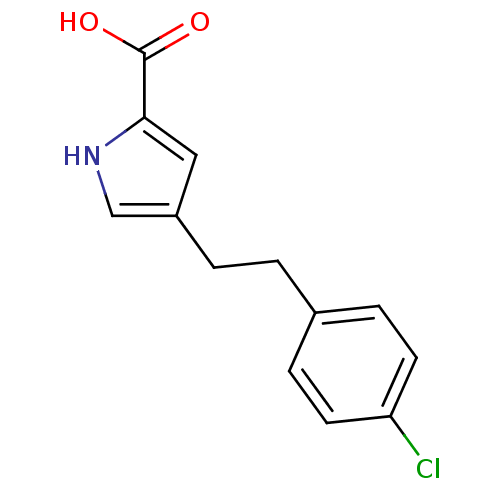
(4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...)Show InChI InChI=1S/C13H12ClNO2/c14-11-5-3-9(4-6-11)1-2-10-7-12(13(16)17)15-8-10/h3-8,15H,1-2H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50268390

(CHEMBL4071900)Show SMILES Cc1noc(C)c1S(=O)(=O)N1CCN(CC1)c1ccc(nn1)N1CCCC(C)(C)C1 Show InChI InChI=1S/C20H30N6O3S/c1-15-19(16(2)29-23-15)30(27,28)26-12-10-24(11-13-26)17-6-7-18(22-21-17)25-9-5-8-20(3,4)14-25/h6-7H,5,8-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic M4 receptor expressed in CHO cell membranes |
J Med Chem 34: 1879-84 (1991)
Article DOI: 10.1016/j.bmcl.2017.05.042
BindingDB Entry DOI: 10.7270/Q29G5KR1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50250795

(CHEMBL4084262)Show SMILES COc1ccccc1N1CCC2(CCN(CCCSc3nnc(-c4ccccc4)n3C)CC2)CC1 Show InChI InChI=1S/C28H37N5OS/c1-31-26(23-9-4-3-5-10-23)29-30-27(31)35-22-8-17-32-18-13-28(14-19-32)15-20-33(21-16-28)24-11-6-7-12-25(24)34-2/h3-7,9-12H,8,13-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pyrilamine from recombinant human H1 receptor stably expressed in HEK cell membranes measured after 90 mins by microbeta scintil... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50240548

(CHEMBL4059829)Show SMILES Fc1ccc(Oc2cc(ncn2)N2CCC(CC2)C(=O)N2CCc3ccccc3C2)c(F)c1 Show InChI InChI=1S/C25H24F2N4O2/c26-20-5-6-22(21(27)13-20)33-24-14-23(28-16-29-24)30-10-8-18(9-11-30)25(32)31-12-7-17-3-1-2-4-19(17)15-31/h1-6,13-14,16,18H,7-12,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from recombinant human M4 receptor expressed in CHO cell membranes |
Bioorg Med Chem Lett 27: 2479-2483 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.009
BindingDB Entry DOI: 10.7270/Q2VT1V7H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390426
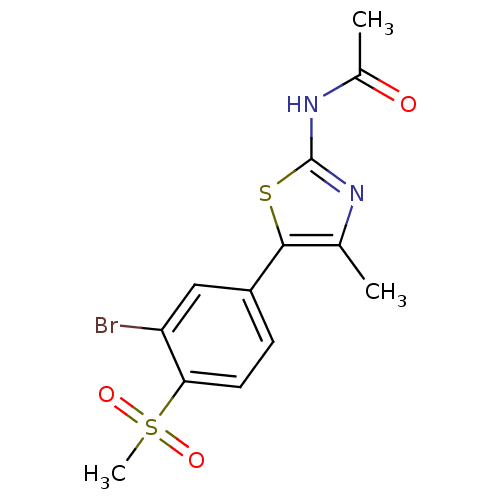
(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50250795

(CHEMBL4084262)Show SMILES COc1ccccc1N1CCC2(CCN(CCCSc3nnc(-c4ccccc4)n3C)CC2)CC1 Show InChI InChI=1S/C28H37N5OS/c1-31-26(23-9-4-3-5-10-23)29-30-27(31)35-22-8-17-32-18-13-28(14-19-32)15-20-33(21-16-28)24-11-6-7-12-25(24)34-2/h3-7,9-12H,8,13-22H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from recombinant human D3 receptor stably expressed in HEK293 cell membranes measured after 60 mins by scintillation count... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50250795

(CHEMBL4084262)Show SMILES COc1ccccc1N1CCC2(CCN(CCCSc3nnc(-c4ccccc4)n3C)CC2)CC1 Show InChI InChI=1S/C28H37N5OS/c1-31-26(23-9-4-3-5-10-23)29-30-27(31)35-22-8-17-32-18-13-28(14-19-32)15-20-33(21-16-28)24-11-6-7-12-25(24)34-2/h3-7,9-12H,8,13-22H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]ABN from human dopamine D3 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting method |
J Med Chem 60: 9905-9910 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01248
BindingDB Entry DOI: 10.7270/Q21838X7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50231845
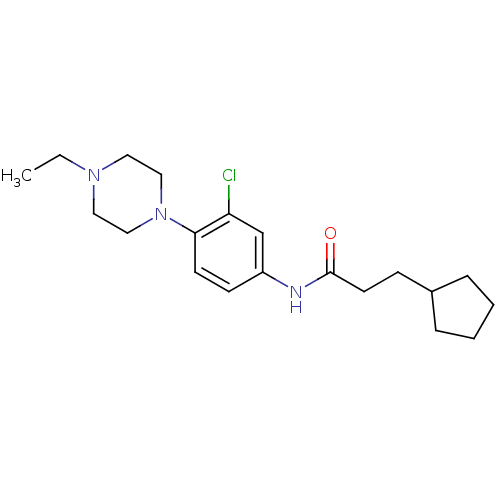
(CHEMBL255523 | N-(3-chloro-4-(4-ethylpiperazin-1-y...)Show InChI InChI=1S/C20H30ClN3O/c1-2-23-11-13-24(14-12-23)19-9-8-17(15-18(19)21)22-20(25)10-7-16-5-3-4-6-16/h8-9,15-16H,2-7,10-14H2,1H3,(H,22,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat muscarinic M1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 885-90 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.051
BindingDB Entry DOI: 10.7270/Q2DZ095Z |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50206007
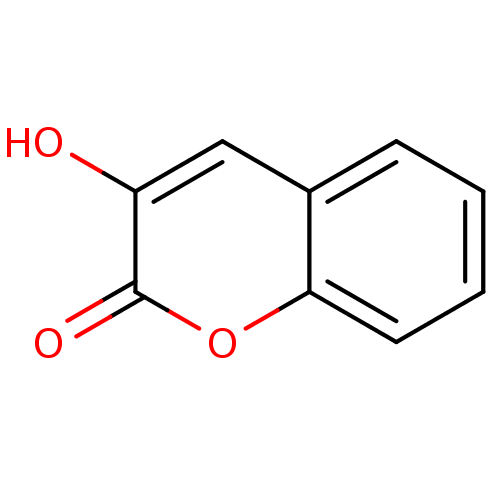
(3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...)Show InChI InChI=1S/C9H6O3/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor A
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Donghua University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from Angiotensin 2 type-1A receptor in rat vascular smooth muscle cells after 150 mins by gamma counting method |
Bioorg Med Chem 24: 2023-31 (2016)
Article DOI: 10.1016/j.bmc.2016.03.028
BindingDB Entry DOI: 10.7270/Q2XW4MQB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50512808
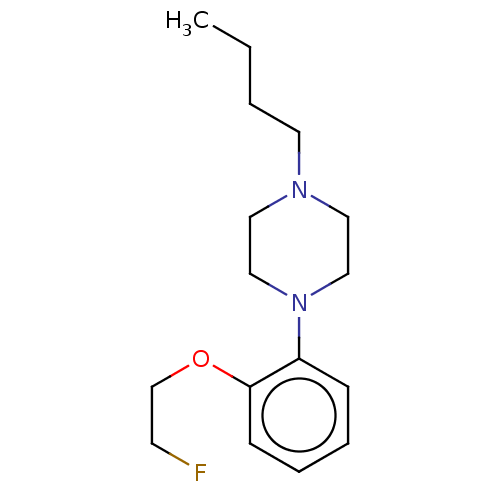
(CHEMBL4438361)Show InChI InChI=1S/C16H25FN2O/c1-2-3-9-18-10-12-19(13-11-18)15-6-4-5-7-16(15)20-14-8-17/h4-7H,2-3,8-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]IABN from recombinant human D3 receptor stably expressed in HEK293 cell membranes measured after 60 mins by scintillation count... |
J Med Chem 62: 5132-5147 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00412
BindingDB Entry DOI: 10.7270/Q2Z03CM2 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50577263

(CHEMBL4850236)Show SMILES [H][C@@]12C[C@H](C[C@]1([H])CN(CC1CCOCC1)C2)Nc1ccc(nn1)-c1cc(F)cc(F)c1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sigma1 receptor by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00363
BindingDB Entry DOI: 10.7270/Q22B92V4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390419
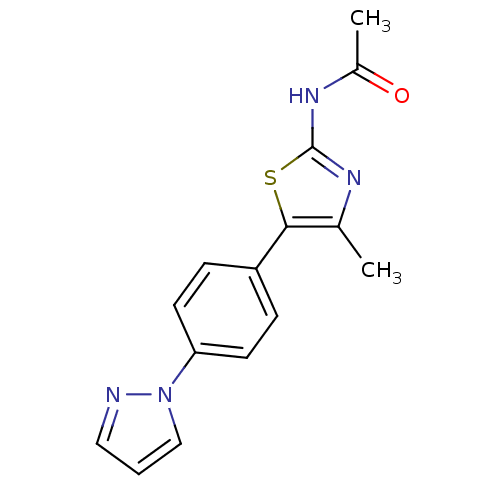
(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390423
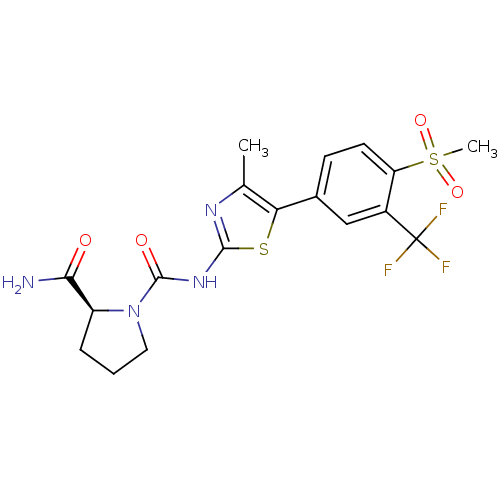
(CHEMBL2071337)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O |r| Show InChI InChI=1S/C18H19F3N4O4S2/c1-9-14(10-5-6-13(31(2,28)29)11(8-10)18(19,20)21)30-16(23-9)24-17(27)25-7-3-4-12(25)15(22)26/h5-6,8,12H,3-4,7H2,1-2H3,(H2,22,26)(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data