 Found 1237 hits with Last Name = 'wilcox' and Initial = 'd'
Found 1237 hits with Last Name = 'wilcox' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucocorticoid receptor
(RAT) | BDBM86689
(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151078
((4R)-4-[(1S,2S,5S,7R,9R,10R,11S,14R,15R,16S)-5-[3-...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@@](O)(CCCN(C)c5ccc(cc5)[C@H]5C[C@@]6(C)[C@@H](CC[C@@]6(O)C#C)[C@@H]6CCC7=CC(=O)CCC7=C56)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C |t:46,53| Show InChI InChI=1S/C54H75NO7/c1-7-54(62)23-21-42-39-16-12-34-27-37(56)15-17-38(34)48(39)40(31-51(42,54)4)33-10-13-36(14-11-33)55(6)26-8-22-53(61)25-24-50(3)35(30-53)28-45(57)49-43-19-18-41(32(2)9-20-47(59)60)52(43,5)46(58)29-44(49)50/h1,10-11,13-14,27,32,35,39-46,49,57-58,61-62H,8-9,12,15-26,28-31H2,2-6H3,(H,59,60)/t32-,35-,39+,40-,41-,42+,43+,44+,45-,46+,49+,50+,51+,52-,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151076
((4R)-4-[(1S,2S,7R,10R,11S,14R,15R,16S)-5-[2-({4-[(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-23-44-41-16-11-34-28-37(55)15-18-39(34)49(41)42(31-51(44,53)4)33-9-13-36(14-10-33)54(6)26-27-60-38-22-24-50(3)35(29-38)12-17-40-45-20-19-43(32(2)8-21-48(57)58)52(45,5)47(56)30-46(40)50/h1,9-10,13-14,28,32,35,38,40-47,56,59H,8,11-12,15-27,29-31H2,2-6H3,(H,57,58)/t32-,35-,38?,40+,41+,42-,43-,44+,45+,46+,47+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50410188
(CHEMBL2096708)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39+,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151077
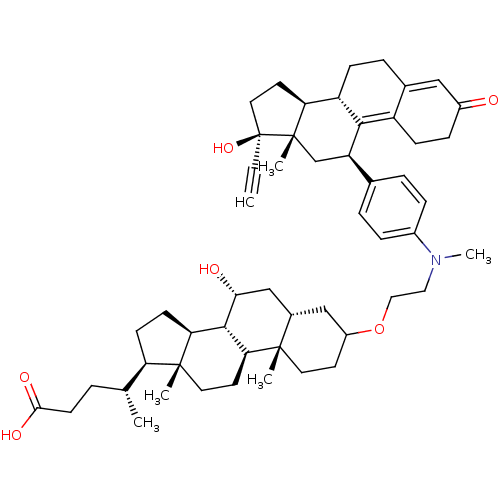
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R)-5-[2-({4-[(1...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3CC[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-22-43-40-15-11-34-28-37(55)14-16-39(34)48(40)41(31-52(43,53)5)33-9-12-36(13-10-33)54(6)26-27-60-38-20-23-50(3)35(29-38)30-46(56)49-44-18-17-42(32(2)8-19-47(57)58)51(44,4)24-21-45(49)50/h1,9-10,12-13,28,32,35,38,40-46,49,56,59H,8,11,14-27,29-31H2,2-6H3,(H,57,58)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,49+,50+,51-,52+,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50410187
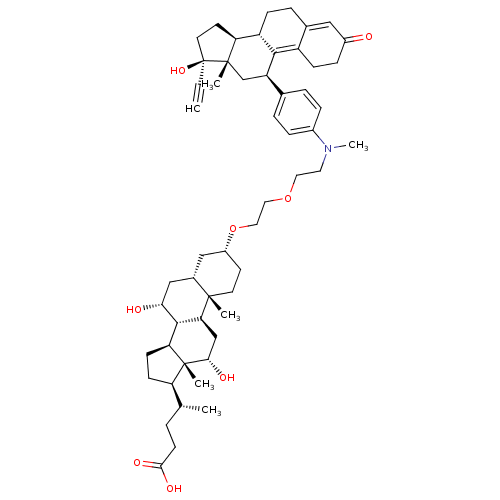
(CHEMBL2096804)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39-,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151064
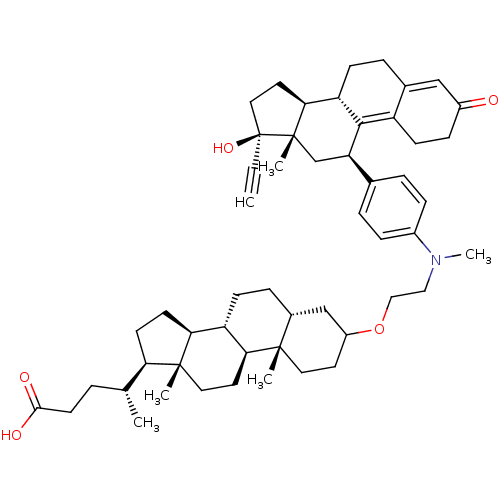
((4R)-4-[(1S,2S,7R,10R,11S,14R,15R)-5-[2-({4-[(10S,...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4CC(CC[C@]4(C)[C@H]3CC[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:57,64| Show InChI InChI=1S/C53H73NO5/c1-7-53(58)27-24-47-42-16-11-35-30-38(55)15-18-40(35)49(42)43(32-52(47,53)5)34-9-13-37(14-10-34)54(6)28-29-59-39-22-25-50(3)36(31-39)12-17-41-45-20-19-44(33(2)8-21-48(56)57)51(45,4)26-23-46(41)50/h1,9-10,13-14,30,33,36,39,41-47,58H,8,11-12,15-29,31-32H2,2-6H3,(H,56,57)/t33-,36-,39?,41+,42+,43-,44-,45+,46+,47+,50+,51-,52+,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151068
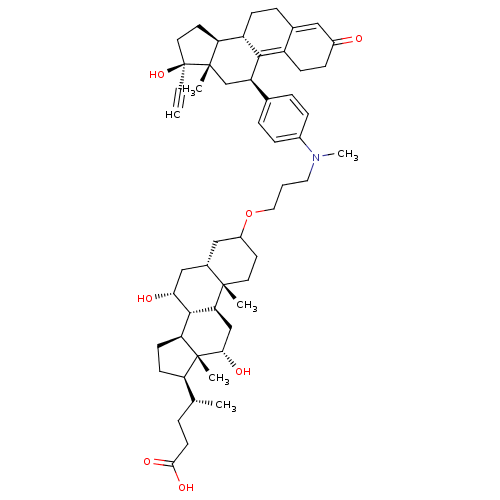
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[3-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:60,67| Show InChI InChI=1S/C54H75NO7/c1-7-54(61)24-22-43-40-16-12-34-27-37(56)15-17-39(34)49(40)41(31-52(43,54)4)33-10-13-36(14-11-33)55(6)25-8-26-62-38-21-23-51(3)35(28-38)29-46(57)50-44-19-18-42(32(2)9-20-48(59)60)53(44,5)47(58)30-45(50)51/h1,10-11,13-14,27,32,35,38,40-47,50,57-58,61H,8-9,12,15-26,28-31H2,2-6H3,(H,59,60)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,47+,50+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151059
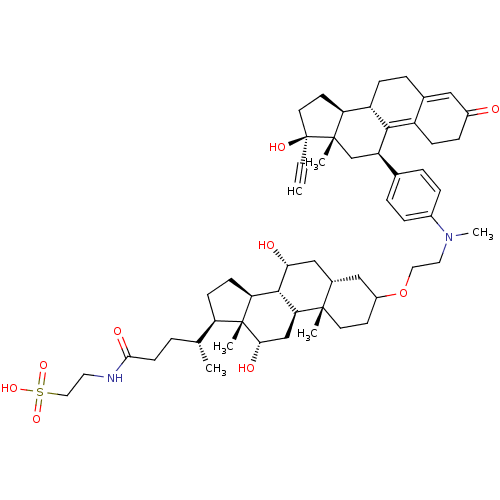
(2-[(4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-...)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:65,72| Show InChI InChI=1S/C55H78N2O9S/c1-7-55(62)23-21-44-41-15-11-35-28-38(58)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)57(6)25-26-66-39-20-22-52(3)36(29-39)30-47(59)51-45-18-17-43(54(45,5)48(60)31-46(51)52)33(2)8-19-49(61)56-24-27-67(63,64)65/h1,9-10,12-13,28,33,36,39,41-48,51,59-60,62H,8,11,14-27,29-32H2,2-6H3,(H,56,61)(H,63,64,65)/t33-,36+,39?,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151067
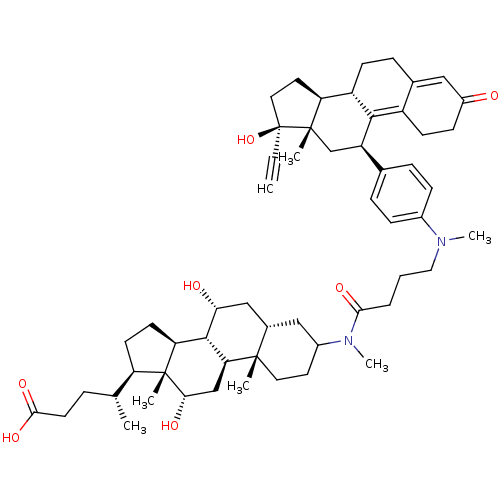
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-5-[4-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)C(=O)CCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C56H78N2O7/c1-8-56(65)26-24-44-41-18-14-35-28-39(59)17-19-40(35)51(41)42(32-54(44,56)4)34-12-15-37(16-13-34)57(6)27-9-10-49(62)58(7)38-23-25-53(3)36(29-38)30-47(60)52-45-21-20-43(33(2)11-22-50(63)64)55(45,5)48(61)31-46(52)53/h1,12-13,15-16,28,33,36,38,41-48,52,60-61,65H,9-11,14,17-27,29-32H2,2-7H3,(H,63,64)/t33-,36+,38?,41+,42-,43-,44+,45+,46+,47-,48+,52+,53+,54+,55-,56+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151060
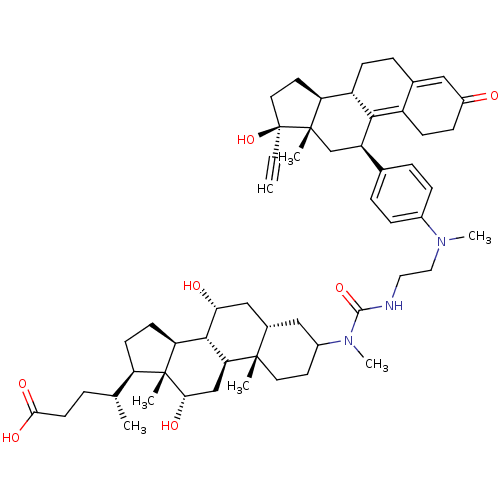
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)C(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C55H77N3O7/c1-8-55(65)24-22-43-40-16-12-34-27-38(59)15-17-39(34)49(40)41(31-53(43,55)4)33-10-13-36(14-11-33)57(6)26-25-56-51(64)58(7)37-21-23-52(3)35(28-37)29-46(60)50-44-19-18-42(32(2)9-20-48(62)63)54(44,5)47(61)30-45(50)52/h1,10-11,13-14,27,32,35,37,40-47,50,60-61,65H,9,12,15-26,28-31H2,2-7H3,(H,56,64)(H,62,63)/t32-,35+,37?,40+,41-,42-,43+,44+,45+,46-,47+,50+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151076

((4R)-4-[(1S,2S,7R,10R,11S,14R,15R,16S)-5-[2-({4-[(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-23-44-41-16-11-34-28-37(55)15-18-39(34)49(41)42(31-51(44,53)4)33-9-13-36(14-10-33)54(6)26-27-60-38-22-24-50(3)35(29-38)12-17-40-45-20-19-43(32(2)8-21-48(57)58)52(45,5)47(56)30-46(40)50/h1,9-10,13-14,28,32,35,38,40-47,56,59H,8,11-12,15-27,29-31H2,2-6H3,(H,57,58)/t32-,35-,38?,40+,41+,42-,43-,44+,45+,46+,47+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151069
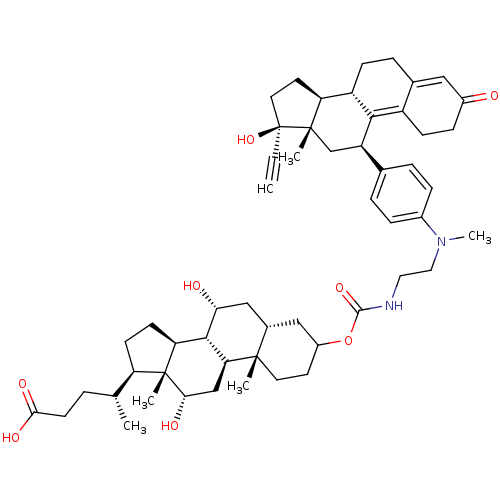
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OC(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C54H74N2O8/c1-7-54(63)23-21-42-39-15-11-33-26-36(57)14-16-38(33)48(39)40(30-52(42,54)4)32-9-12-35(13-10-32)56(6)25-24-55-50(62)64-37-20-22-51(3)34(27-37)28-45(58)49-43-18-17-41(31(2)8-19-47(60)61)53(43,5)46(59)29-44(49)51/h1,9-10,12-13,26,31,34,37,39-46,49,58-59,63H,8,11,14-25,27-30H2,2-6H3,(H,55,62)(H,60,61)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151069

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OC(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C54H74N2O8/c1-7-54(63)23-21-42-39-15-11-33-26-36(57)14-16-38(33)48(39)40(30-52(42,54)4)32-9-12-35(13-10-32)56(6)25-24-55-50(62)64-37-20-22-51(3)34(27-37)28-45(58)49-43-18-17-41(31(2)8-19-47(60)61)53(43,5)46(59)29-44(49)51/h1,9-10,12-13,26,31,34,37,39-46,49,58-59,63H,8,11,14-25,27-30H2,2-6H3,(H,55,62)(H,60,61)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151074
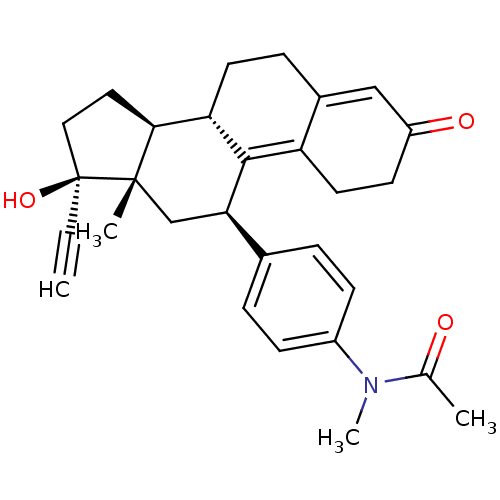
(CHEMBL364368 | N-[4-((2R,8S,13S,14S,17R)-17-Ethyny...)Show SMILES CN(C(C)=O)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:27,34| Show InChI InChI=1S/C29H33NO3/c1-5-29(33)15-14-26-24-12-8-20-16-22(32)11-13-23(20)27(24)25(17-28(26,29)3)19-6-9-21(10-7-19)30(4)18(2)31/h1,6-7,9-10,16,24-26,33H,8,11-15,17H2,2-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151063
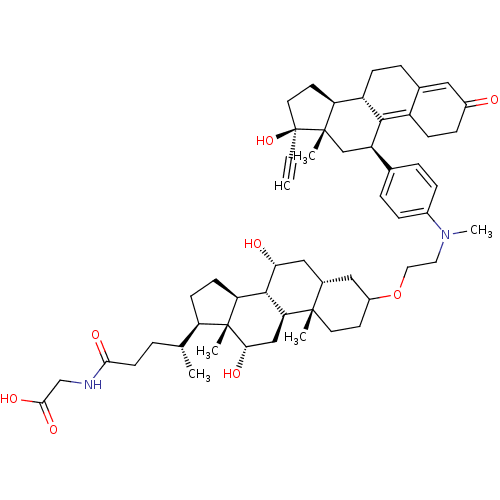
(2-[(4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-...)Show SMILES C[C@H](CCC(=O)NCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C55H76N2O8/c1-7-55(64)23-21-43-40-15-11-34-26-37(58)14-16-39(34)50(40)41(30-53(43,55)4)33-9-12-36(13-10-33)57(6)24-25-65-38-20-22-52(3)35(27-38)28-46(59)51-44-18-17-42(54(44,5)47(60)29-45(51)52)32(2)8-19-48(61)56-31-49(62)63/h1,9-10,12-13,26,32,35,38,40-47,51,59-60,64H,8,11,14-25,27-31H2,2-6H3,(H,56,61)(H,62,63)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,47+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151065
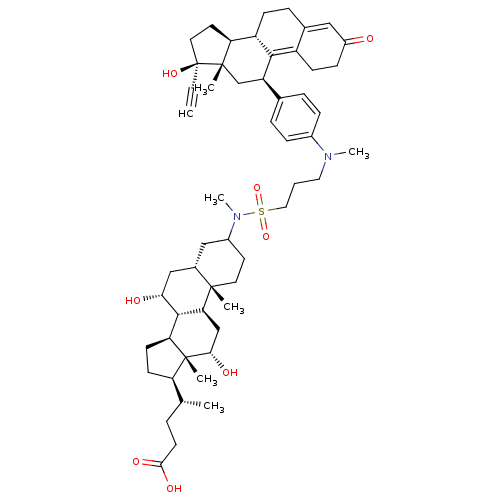
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-9,16-dih...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)S(=O)(=O)CCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:64,71| Show InChI InChI=1S/C55H78N2O8S/c1-8-55(63)25-23-44-41-17-13-35-28-39(58)16-18-40(35)50(41)42(32-53(44,55)4)34-11-14-37(15-12-34)56(6)26-9-27-66(64,65)57(7)38-22-24-52(3)36(29-38)30-47(59)51-45-20-19-43(33(2)10-21-49(61)62)54(45,5)48(60)31-46(51)52/h1,11-12,14-15,28,33,36,38,41-48,51,59-60,63H,9-10,13,16-27,29-32H2,2-7H3,(H,61,62)/t33-,36+,38?,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50410188

(CHEMBL2096708)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39+,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2
(Homo sapiens (Human)) | BDBM50457489
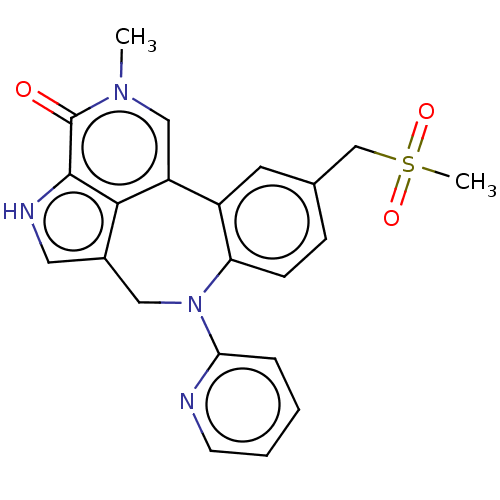
(CHEMBL4208129)Show SMILES Cn1cc2-c3cc(CS(C)(=O)=O)ccc3N(Cc3c[nH]c(c23)c1=O)c1ccccn1 Show InChI InChI=1S/C22H20N4O3S/c1-25-12-17-16-9-14(13-30(2,28)29)6-7-18(16)26(19-5-3-4-8-23-19)11-15-10-24-21(20(15)17)22(25)27/h3-10,12,24H,11,13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to BRD2 bromodomain 1 to 2 (G73 to A560 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by b... |
Bioorg Med Chem Lett 28: 1804-1810 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.020
BindingDB Entry DOI: 10.7270/Q2542R63 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50511849
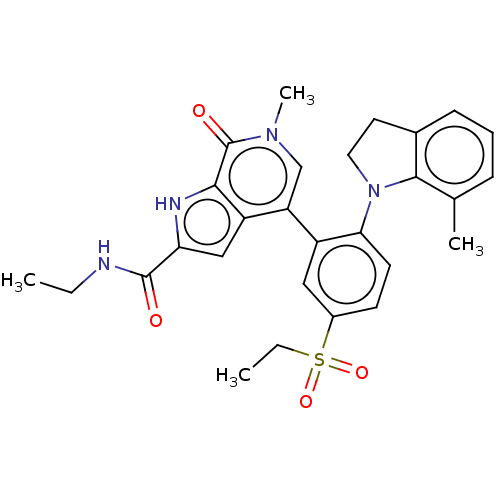
(CHEMBL4465299)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1N1CCc2cccc(C)c12)S(=O)(=O)CC Show InChI InChI=1S/C28H30N4O4S/c1-5-29-27(33)23-15-21-22(16-31(4)28(34)25(21)30-23)20-14-19(37(35,36)6-2)10-11-24(20)32-13-12-18-9-7-8-17(3)26(18)32/h7-11,14-16,30H,5-6,12-13H2,1-4H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50511850
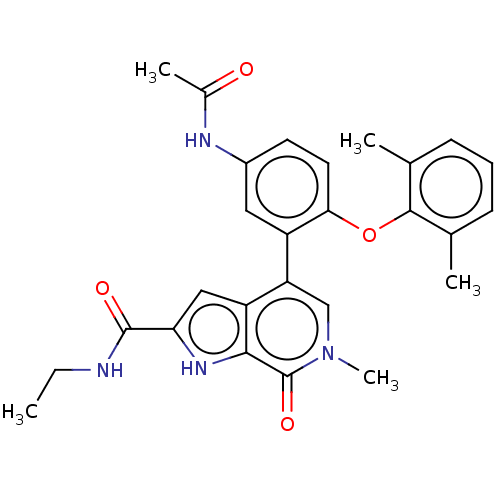
(CHEMBL4435166)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(NC(C)=O)ccc1Oc1c(C)cccc1C Show InChI InChI=1S/C27H28N4O4/c1-6-28-26(33)22-13-20-21(14-31(5)27(34)24(20)30-22)19-12-18(29-17(4)32)10-11-23(19)35-25-15(2)8-7-9-16(25)3/h7-14,30H,6H2,1-5H3,(H,28,33)(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50511875
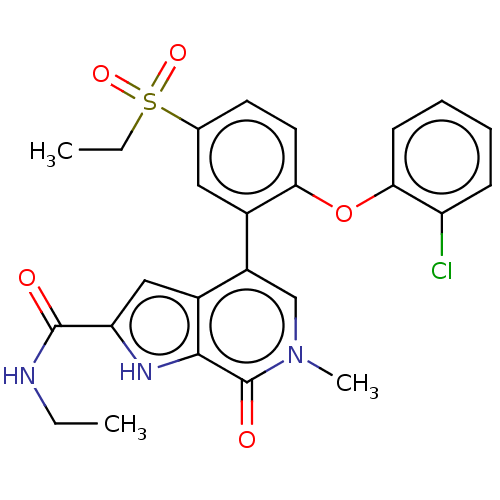
(CHEMBL4548794)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1ccccc1Cl)S(=O)(=O)CC Show InChI InChI=1S/C25H24ClN3O5S/c1-4-27-24(30)20-13-17-18(14-29(3)25(31)23(17)28-20)16-12-15(35(32,33)5-2)10-11-21(16)34-22-9-7-6-8-19(22)26/h6-14,28H,4-5H2,1-3H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM439590
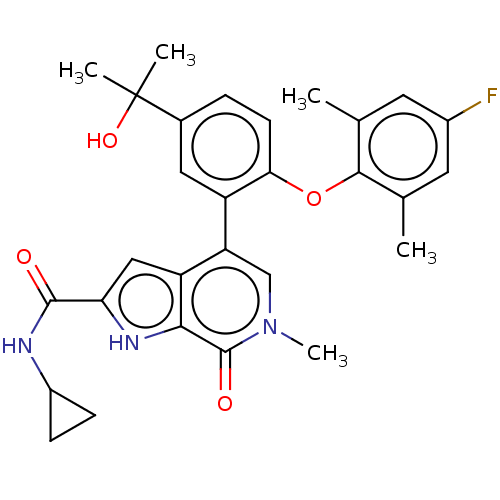
(US10633379, Example 121)Show SMILES Cc1cc(F)cc(C)c1Oc1ccc(cc1-c1cn(C)c(=O)c2[nH]c(cc12)C(=O)NC1CC1)C(C)(C)O Show InChI InChI=1S/C29H30FN3O4/c1-15-10-18(30)11-16(2)26(15)37-24-9-6-17(29(3,4)36)12-20(24)22-14-33(5)28(35)25-21(22)13-23(32-25)27(34)31-19-7-8-19/h6,9-14,19,32,36H,7-8H2,1-5H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50197428
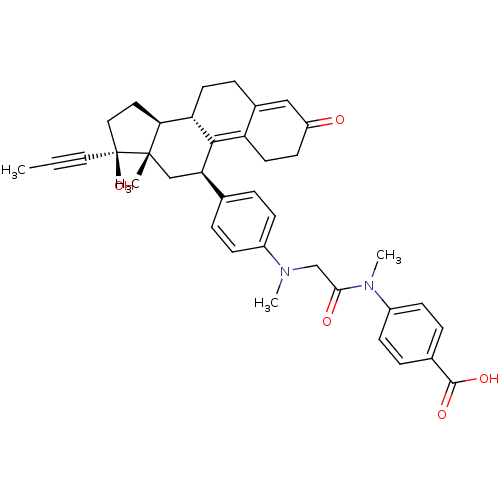
(4-[(2-{[4-((9S,11R,13S,14S,17S)-17-hydroxy-13-meth...)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CC(=O)N(C)c1ccc(cc1)C(O)=O |c:18,t:11| Show InChI InChI=1S/C38H42N2O5/c1-5-19-38(45)20-18-33-31-16-10-26-21-29(41)15-17-30(26)35(31)32(22-37(33,38)2)24-6-11-27(12-7-24)39(3)23-34(42)40(4)28-13-8-25(9-14-28)36(43)44/h6-9,11-14,21,31-33,45H,10,15-18,20,22-23H2,1-4H3,(H,43,44)/t31-,32+,33-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled Dexamethasone from human GR |
Bioorg Med Chem Lett 17: 40-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.10.001
BindingDB Entry DOI: 10.7270/Q2DR2W9G |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151059

(2-[(4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-...)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:65,72| Show InChI InChI=1S/C55H78N2O9S/c1-7-55(62)23-21-44-41-15-11-35-28-38(58)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)57(6)25-26-66-39-20-22-52(3)36(29-39)30-47(59)51-45-18-17-43(54(45,5)48(60)31-46(51)52)33(2)8-19-49(61)56-24-27-67(63,64)65/h1,9-10,12-13,28,33,36,39,41-48,51,59-60,62H,8,11,14-27,29-32H2,2-6H3,(H,56,61)(H,63,64,65)/t33-,36+,39?,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50124595

(5-Allyl-10-chloro-2,2,4-trimethyl-2,5-dihydro-1H-6...)Show SMILES CC1=CC(C)(C)Nc2ccc-3c(C(CC=C)Oc4ccc(O)c(Cl)c-34)c12 |t:1| Show InChI InChI=1S/C22H22ClNO2/c1-5-6-16-19-13(20-17(26-16)10-9-15(25)21(20)23)7-8-14-18(19)12(2)11-22(3,4)24-14/h5,7-11,16,24-25H,1,6H2,2-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human glucocorticoid receptor (GR) was determined using [3H]-Dexamethasone as radioligand in SF-1 cells |
J Med Chem 46: 1016-30 (2003)
Article DOI: 10.1021/jm020335m
BindingDB Entry DOI: 10.7270/Q2MP52NB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50197435
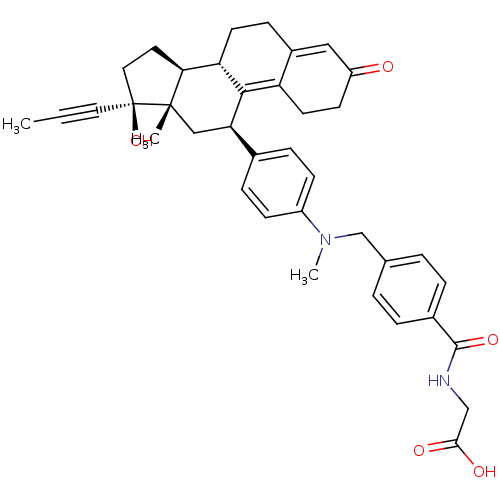
(CHEMBL387814 | [4-({[4-((9S,11R,13S,14S,17S)-17-hy...)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)Cc1ccc(cc1)C(=O)NCC(O)=O |c:18,t:11| Show InChI InChI=1S/C38H42N2O5/c1-4-18-38(45)19-17-33-31-15-11-27-20-29(41)14-16-30(27)35(31)32(21-37(33,38)2)25-9-12-28(13-10-25)40(3)23-24-5-7-26(8-6-24)36(44)39-22-34(42)43/h5-10,12-13,20,31-33,45H,11,14-17,19,21-23H2,1-3H3,(H,39,44)(H,42,43)/t31-,32+,33-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled Dexamethasone from human GR |
Bioorg Med Chem Lett 17: 40-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.10.001
BindingDB Entry DOI: 10.7270/Q2DR2W9G |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50511864
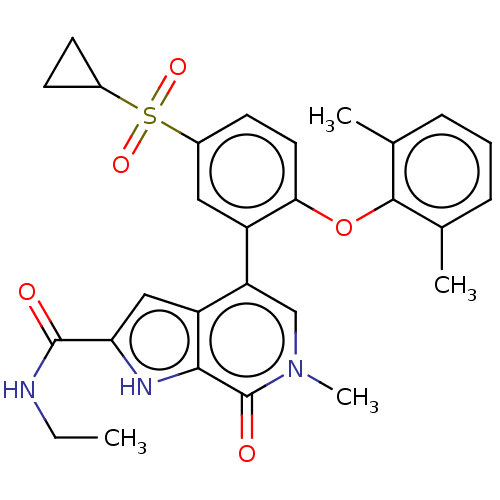
(CHEMBL4564879)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1c(C)cccc1C)S(=O)(=O)C1CC1 Show InChI InChI=1S/C28H29N3O5S/c1-5-29-27(32)23-14-21-22(15-31(4)28(33)25(21)30-23)20-13-19(37(34,35)18-9-10-18)11-12-24(20)36-26-16(2)7-6-8-17(26)3/h6-8,11-15,18,30H,5,9-10H2,1-4H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM439461
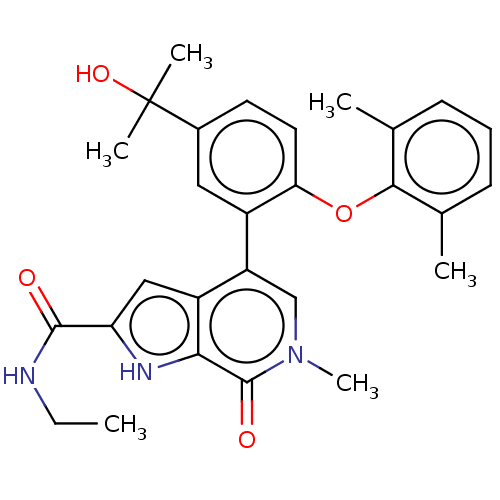
(US10633379, Example 3 | US10633379, Example 68)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1c(C)cccc1C)C(C)(C)O Show InChI InChI=1S/C28H31N3O4/c1-7-29-26(32)22-14-20-21(15-31(6)27(33)24(20)30-22)19-13-18(28(4,5)34)11-12-23(19)35-25-16(2)9-8-10-17(25)3/h8-15,30,34H,7H2,1-6H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50457489

(CHEMBL4208129)Show SMILES Cn1cc2-c3cc(CS(C)(=O)=O)ccc3N(Cc3c[nH]c(c23)c1=O)c1ccccn1 Show InChI InChI=1S/C22H20N4O3S/c1-25-12-17-16-9-14(13-30(2,28)29)6-7-18(16)26(19-5-3-4-8-23-19)11-15-10-24-21(20(15)17)22(25)27/h3-10,12,24H,11,13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to His-tagged BRD4 bromodomain 1 to 2 (K57 to K550 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent... |
Bioorg Med Chem Lett 28: 1804-1810 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.020
BindingDB Entry DOI: 10.7270/Q2542R63 |
More data for this
Ligand-Target Pair | |
Membrane-associated progesterone receptor component 1
(RAT) | BDBM86689

(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM86689

(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human androgen receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151061
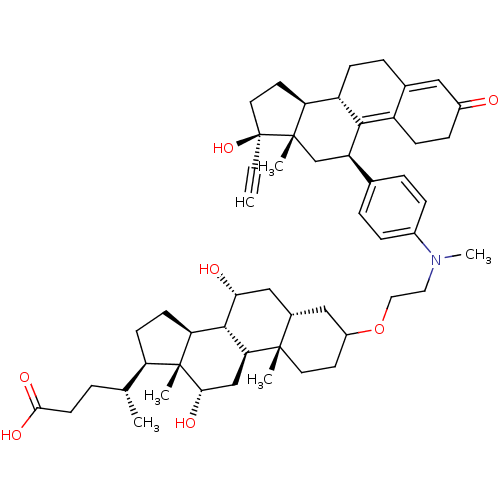
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:59,66| Show InChI InChI=1S/C53H73NO7/c1-7-53(60)23-21-42-39-15-11-33-26-36(55)14-16-38(33)48(39)40(30-51(42,53)4)32-9-12-35(13-10-32)54(6)24-25-61-37-20-22-50(3)34(27-37)28-45(56)49-43-18-17-41(31(2)8-19-47(58)59)52(43,5)46(57)29-44(49)50/h1,9-10,12-13,26,31,34,37,39-46,49,56-57,60H,8,11,14-25,27-30H2,2-6H3,(H,58,59)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151061

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:59,66| Show InChI InChI=1S/C53H73NO7/c1-7-53(60)23-21-42-39-15-11-33-26-36(55)14-16-38(33)48(39)40(30-51(42,53)4)32-9-12-35(13-10-32)54(6)24-25-61-37-20-22-50(3)34(27-37)28-45(56)49-43-18-17-41(31(2)8-19-47(58)59)52(43,5)46(57)29-44(49)50/h1,9-10,12-13,26,31,34,37,39-46,49,56-57,60H,8,11,14-25,27-30H2,2-6H3,(H,58,59)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein
(Homo sapiens (Human)) | BDBM50457489

(CHEMBL4208129)Show SMILES Cn1cc2-c3cc(CS(C)(=O)=O)ccc3N(Cc3c[nH]c(c23)c1=O)c1ccccn1 Show InChI InChI=1S/C22H20N4O3S/c1-25-12-17-16-9-14(13-30(2,28)29)6-7-18(16)26(19-5-3-4-8-23-19)11-15-10-24-21(20(15)17)22(25)27/h3-10,12,24H,11,13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to BRDT bromodomain 1 to 2 (N21to P380 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by br... |
Bioorg Med Chem Lett 28: 1804-1810 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.020
BindingDB Entry DOI: 10.7270/Q2542R63 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50511855

(CHEMBL4454597)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1ccc(F)cc1F)S(=O)(=O)CC Show InChI InChI=1S/C25H23F2N3O5S/c1-4-28-24(31)20-12-17-18(13-30(3)25(32)23(17)29-20)16-11-15(36(33,34)5-2)7-9-21(16)35-22-8-6-14(26)10-19(22)27/h6-13,29H,4-5H2,1-3H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50511870

(CHEMBL4461291)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1cc(C)cc(C)c1)S(=O)(=O)CC Show InChI InChI=1S/C27H29N3O5S/c1-6-28-26(31)23-14-21-22(15-30(5)27(32)25(21)29-23)20-13-19(36(33,34)7-2)8-9-24(20)35-18-11-16(3)10-17(4)12-18/h8-15,29H,6-7H2,1-5H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50457496
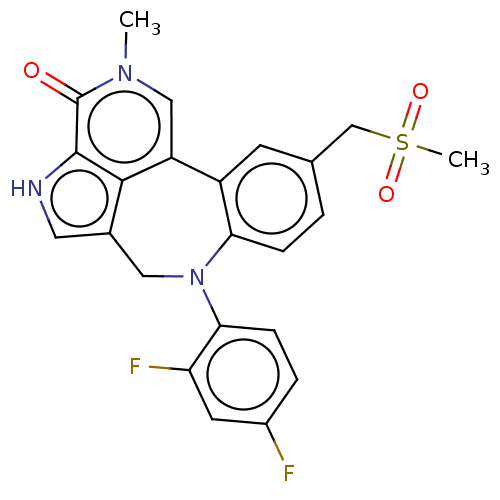
(CHEMBL4217457)Show SMILES Cn1cc2-c3cc(CS(C)(=O)=O)ccc3N(Cc3c[nH]c(c23)c1=O)c1ccc(F)cc1F Show InChI InChI=1S/C23H19F2N3O3S/c1-27-11-17-16-7-13(12-32(2,30)31)3-5-19(16)28(20-6-4-15(24)8-18(20)25)10-14-9-26-22(21(14)17)23(27)29/h3-9,11,26H,10,12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to BRD4 bromodomain 2 (E352 to M457 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromo... |
Bioorg Med Chem Lett 28: 1804-1810 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.020
BindingDB Entry DOI: 10.7270/Q2542R63 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151077

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R)-5-[2-({4-[(1...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3CC[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-22-43-40-15-11-34-28-37(55)14-16-39(34)48(40)41(31-52(43,53)5)33-9-12-36(13-10-33)54(6)26-27-60-38-20-23-50(3)35(29-38)30-46(56)49-44-18-17-42(32(2)8-19-47(57)58)51(44,4)24-21-45(49)50/h1,9-10,12-13,28,32,35,38,40-46,49,56,59H,8,11,14-27,29-31H2,2-6H3,(H,57,58)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,49+,50+,51-,52+,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151072
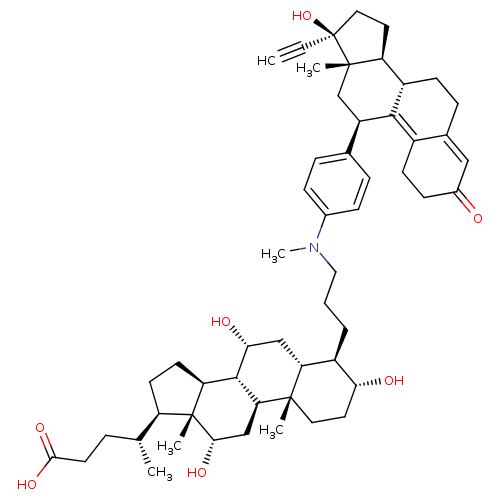
((4R)-4-[(1S,2S,5R,6R,7R,9R,10R,11S,14R,15R,16S)-6-...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4[C@@H](CCCN(C)c5ccc(cc5)[C@H]5C[C@@]6(C)[C@@H](CC[C@@]6(O)C#C)[C@@H]6CCC7=CC(=O)CCC7=C56)[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C |t:44,51| Show InChI InChI=1S/C54H75NO7/c1-7-54(62)25-22-41-38-17-13-33-27-35(56)16-18-36(33)49(38)39(30-52(41,54)4)32-11-14-34(15-12-32)55(6)26-8-9-37-43-28-46(58)50-42-20-19-40(31(2)10-21-48(60)61)53(42,5)47(59)29-44(50)51(43,3)24-23-45(37)57/h1,11-12,14-15,27,31,37-47,50,57-59,62H,8-10,13,16-26,28-30H2,2-6H3,(H,60,61)/t31-,37-,38+,39-,40-,41+,42+,43-,44+,45-,46-,47+,50+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50511859
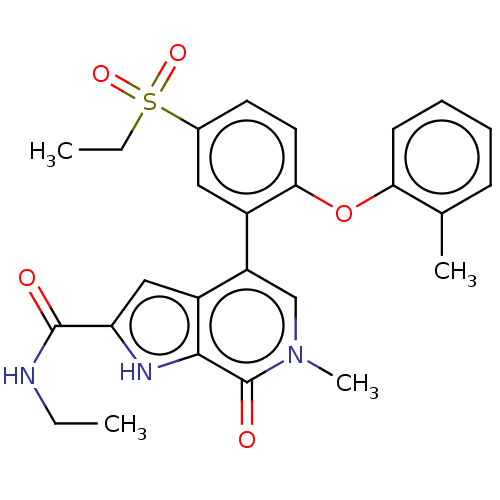
(CHEMBL4470856)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1ccccc1C)S(=O)(=O)CC Show InChI InChI=1S/C26H27N3O5S/c1-5-27-25(30)21-14-19-20(15-29(4)26(31)24(19)28-21)18-13-17(35(32,33)6-2)11-12-23(18)34-22-10-8-7-9-16(22)3/h7-15,28H,5-6H2,1-4H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50511861
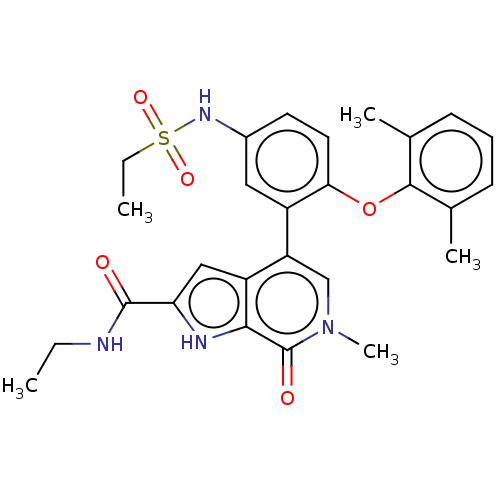
(CHEMBL4529861)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(NS(=O)(=O)CC)ccc1Oc1c(C)cccc1C Show InChI InChI=1S/C27H30N4O5S/c1-6-28-26(32)22-14-20-21(15-31(5)27(33)24(20)29-22)19-13-18(30-37(34,35)7-2)11-12-23(19)36-25-16(3)9-8-10-17(25)4/h8-15,29-30H,6-7H2,1-5H3,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
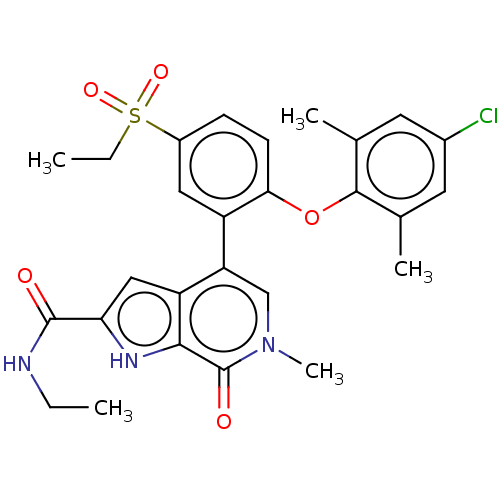
(Homo sapiens (Human)) | BDBM50511869
(CHEMBL4548127)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1c(C)cc(Cl)cc1C)S(=O)(=O)CC Show InChI InChI=1S/C27H28ClN3O5S/c1-6-29-26(32)22-13-20-21(14-31(5)27(33)24(20)30-22)19-12-18(37(34,35)7-2)8-9-23(19)36-25-15(3)10-17(28)11-16(25)4/h8-14,30H,6-7H2,1-5H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay |
J Med Chem 63: 5585-5623 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00628
BindingDB Entry DOI: 10.7270/Q2057K8M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50265666
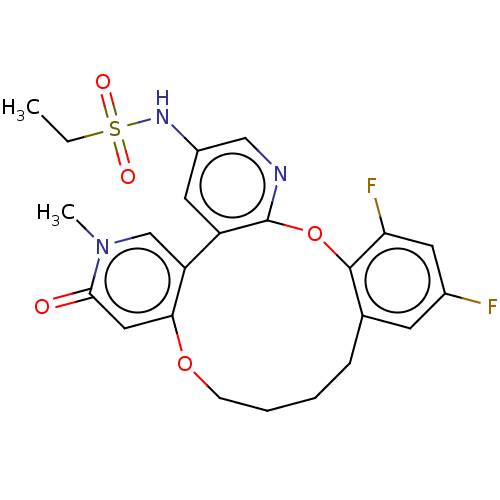
(CHEMBL4068431)Show SMILES CCS(=O)(=O)Nc1cnc2Oc3c(F)cc(F)cc3CCCCOc3cc(=O)n(C)cc3-c2c1 Show InChI InChI=1S/C23H23F2N3O5S/c1-3-34(30,31)27-16-10-17-18-13-28(2)21(29)11-20(18)32-7-5-4-6-14-8-15(24)9-19(25)22(14)33-23(17)26-12-16/h8-13,27H,3-7H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged BRD4 BD1-BD2 (57 to 550 residues) expressed in EScherichia coli BL21(DE3) after 1 hr by Alexa-647-conjugat... |
J Med Chem 60: 3828-3850 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00017
BindingDB Entry DOI: 10.7270/Q2BR8VPN |
More data for this
Ligand-Target Pair | |
Membrane-associated progesterone receptor component 1
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data