

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117522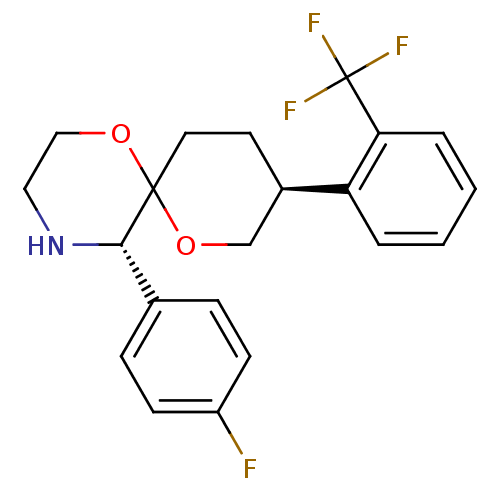 ((5S,6R,9S)-5-(4-Fluoro-phenyl)-9-(2-trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to human Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117508 ((5S,6R,9R)-5-(4-Fluoro-phenyl)-9-(2-trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to human Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50403954 (CHEMBL2112366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50290861 (5-{3-[1-(3,5-Dichloro-phenyl)-ethoxy]-2-phenyl-pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106711 (5-[2-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 | J Med Chem 44: 4296-9 (2001) BindingDB Entry DOI: 10.7270/Q2PN96BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117509 ((3S,5S,10S)-5-(4-Fluoro-phenyl)-10-[2-methoxy-5-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117523 (5-{(5S,10S)-5-(4-Fluoro-phenyl)-10-[2-methoxy-5-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118664 (5-{3-[2-Methoxy-5-(5-trifluoromethyl-tetrazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118667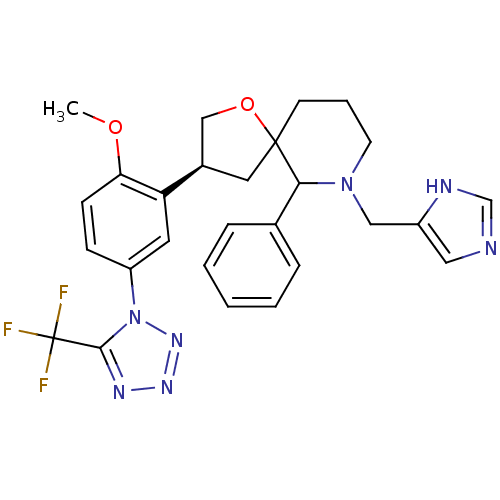 (7-(1H-Imidazol-4-ylmethyl)-3-[2-methoxy-5-(5-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118666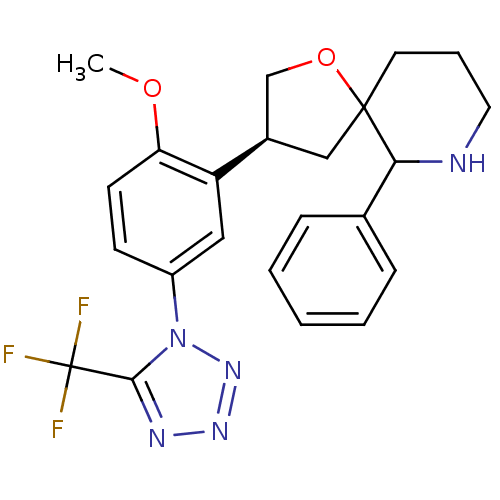 (3-[2-Methoxy-5-(5-trifluoromethyl-tetrazol-1-yl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50290865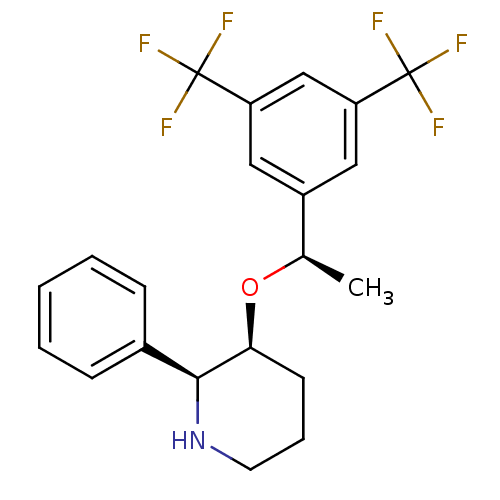 (3-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-2-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50290859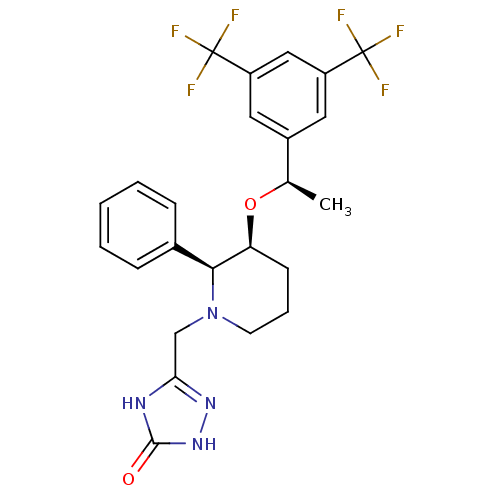 (5-{3-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50290856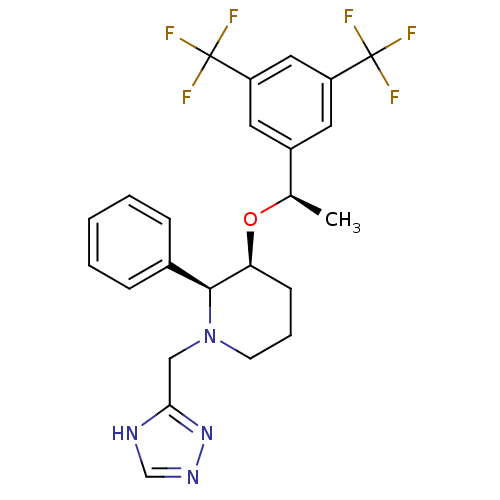 (3-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-2-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50052280 ((2S,3S)-3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of NK1 receptor in rat liver microsomes. | Bioorg Med Chem Lett 7: 2959-2962 (1997) Article DOI: 10.1016/S0960-894X(97)10118-4 BindingDB Entry DOI: 10.7270/Q2W66KRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106712 (4-(5-Azetidin-1-ylmethyl-1H-[1,2,3]triazol-4-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 | J Med Chem 44: 4296-9 (2001) BindingDB Entry DOI: 10.7270/Q2PN96BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106717 (CHEMBL553778 | {5-[2-[1-(3,5-Bis-trifluoromethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 | J Med Chem 44: 4296-9 (2001) BindingDB Entry DOI: 10.7270/Q2PN96BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50409466 (CHEMBL2112743) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 | J Med Chem 44: 4296-9 (2001) BindingDB Entry DOI: 10.7270/Q2PN96BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50409465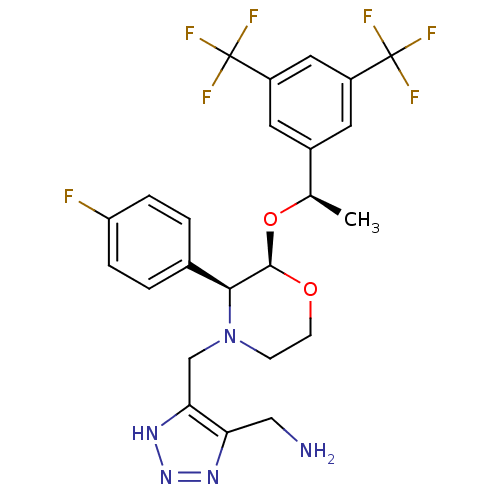 (CHEMBL2112741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 | J Med Chem 44: 4296-9 (2001) BindingDB Entry DOI: 10.7270/Q2PN96BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106716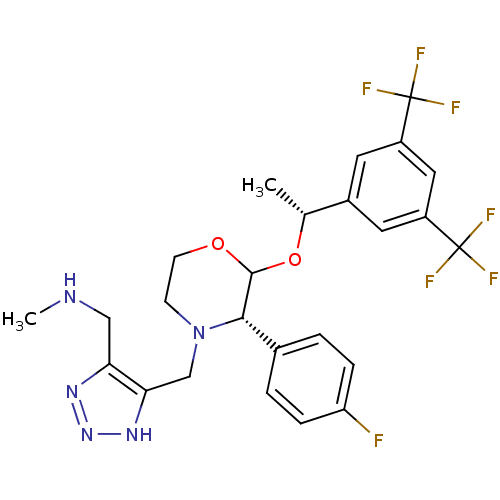 (CHEMBL133140 | {5-[2-[1-(3,5-Bis-trifluoromethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 | J Med Chem 44: 4296-9 (2001) BindingDB Entry DOI: 10.7270/Q2PN96BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286220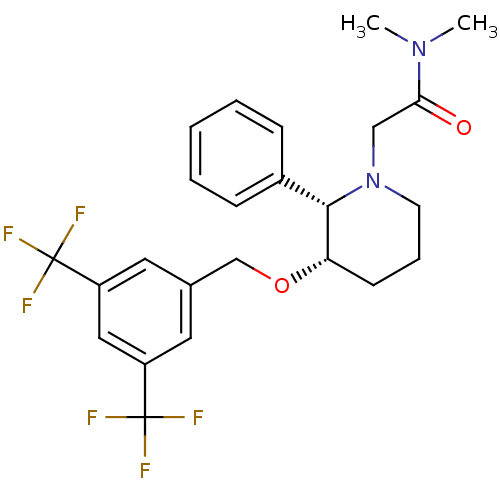 (2-[(2S,3S)-3-(3,5-Bis-trifluoromethyl-benzyloxy)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]- Substance P from human Neurokinin 1 (hNK1) receptor expressed in CHO cells | Bioorg Med Chem Lett 5: 209-212 (1995) Article DOI: 10.1016/0960-894X(95)00009-I BindingDB Entry DOI: 10.7270/Q2ZP463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50403953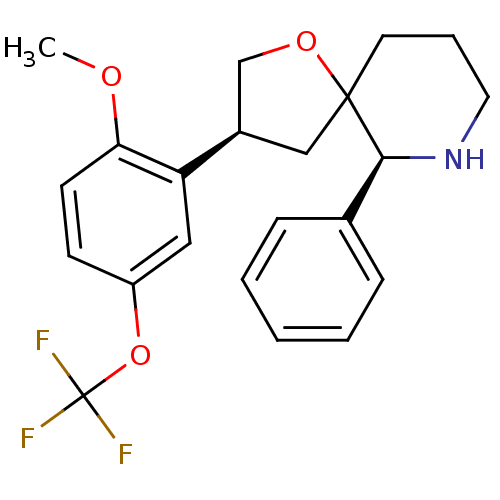 (CHEMBL2112368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118671 (3-(2-Methoxy-5-trifluoromethoxy-phenyl)-6-phenyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2719-22 (2002) BindingDB Entry DOI: 10.7270/Q2C828NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50409467 (CHEMBL2112742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 | J Med Chem 44: 4296-9 (2001) BindingDB Entry DOI: 10.7270/Q2PN96BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128502 (US8796244, 81) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.447 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128624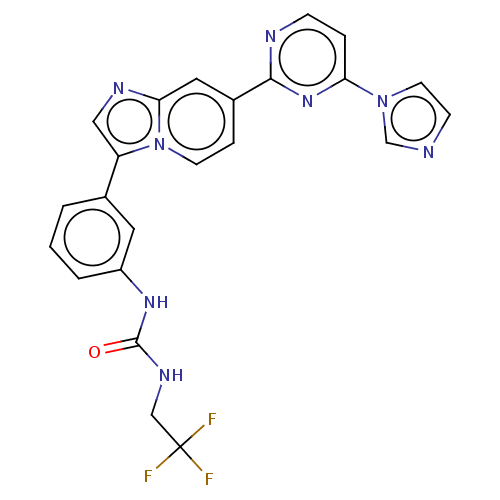 (US8796244, 213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128551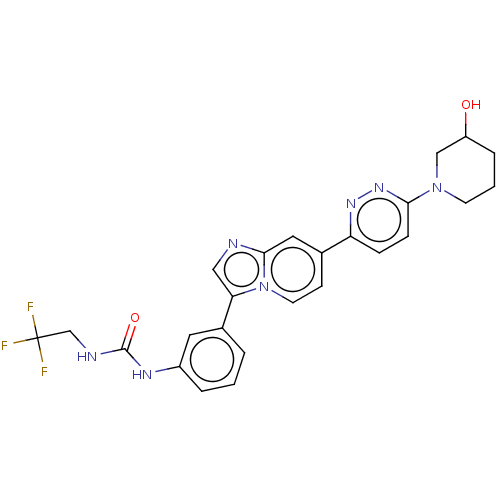 (US8796244, 133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.484 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128659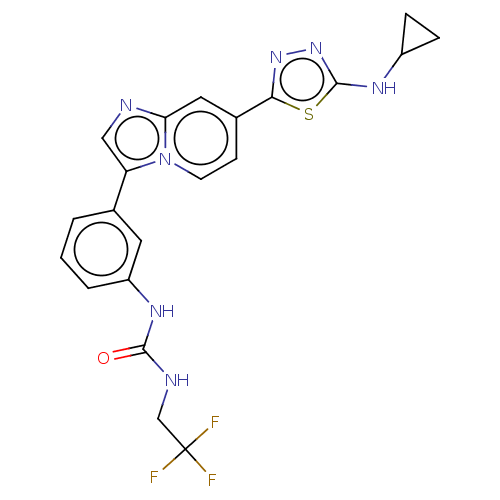 (US8796244, 250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50093095 (2-[(S)-1-(3,5-Bis-trifluoromethyl-benzyloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Tachykinin receptor 1 expressed in CHO cells by the displacement of [125 I] substance P | Bioorg Med Chem Lett 4: 1903-1908 (1994) Article DOI: 10.1016/S0960-894X(01)80531-X BindingDB Entry DOI: 10.7270/Q2NK3DZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128516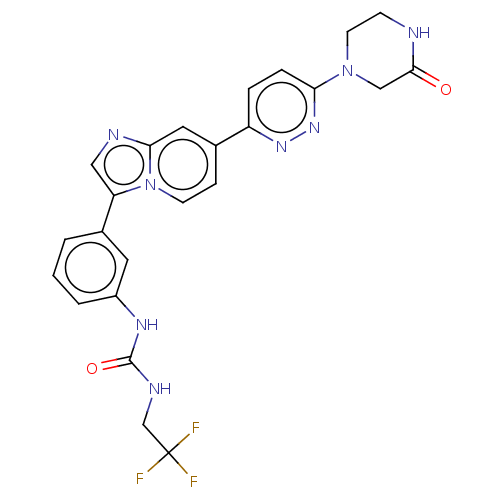 (US8796244, 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128496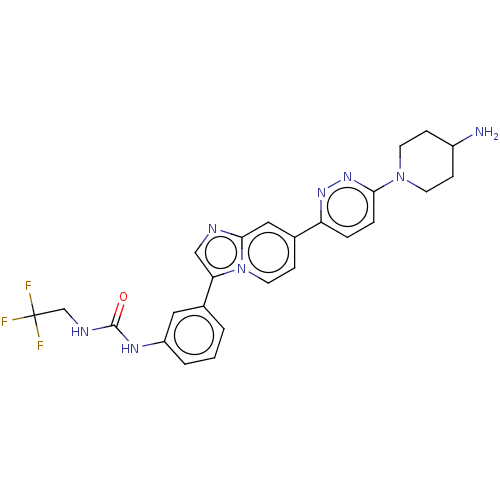 (US8796244, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.535 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128668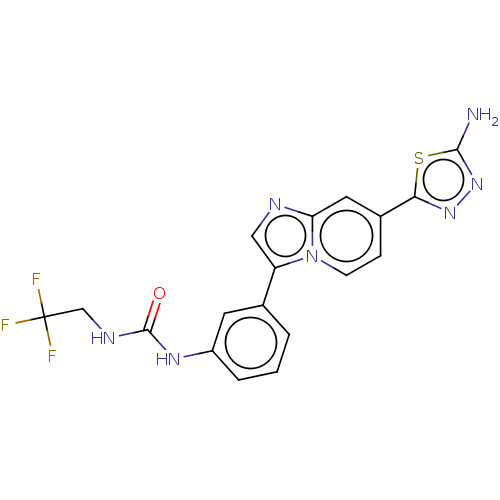 (US8796244, 261) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128468 (US8796244, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.542 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128427 (US8796244, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.545 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128428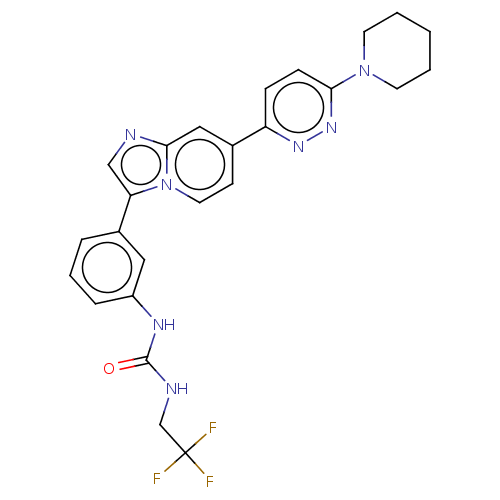 (US8796244, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128561 (US8796244, 145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50117515 (5-[(3S,5S,10S)-5-(4-Fluoro-phenyl)-10-(2-methoxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells | Bioorg Med Chem Lett 12: 2515-8 (2002) BindingDB Entry DOI: 10.7270/Q27P8XQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128488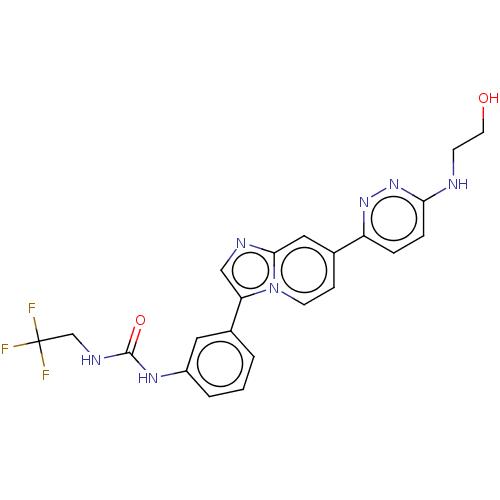 (US8796244, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.569 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128425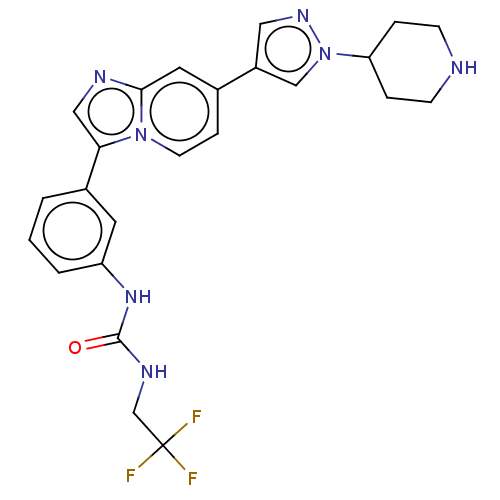 (US8796244, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128465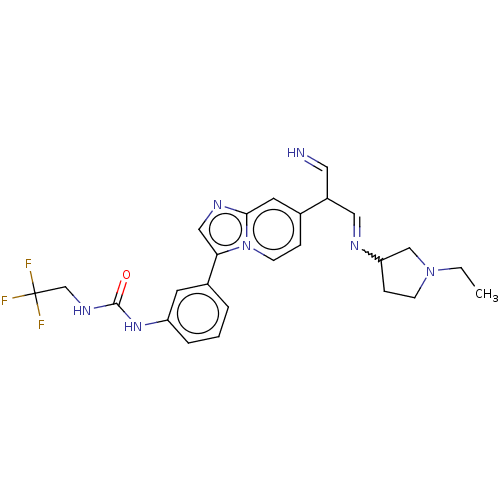 (US8796244, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.582 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]- Substance P from human Neurokinin 1 (hNK1) receptor expressed in CHO cells | Bioorg Med Chem Lett 5: 209-212 (1995) Article DOI: 10.1016/0960-894X(95)00009-I BindingDB Entry DOI: 10.7270/Q2ZP463J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128466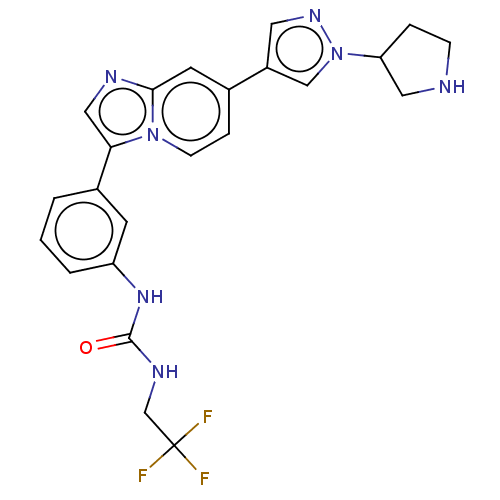 (US8796244, 45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128534 (US8796244, 113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128459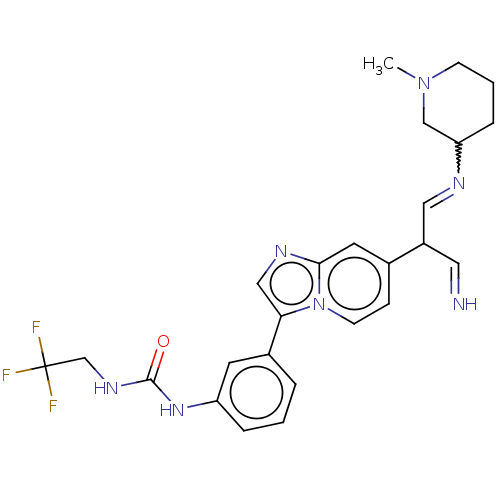 (US8796244, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128461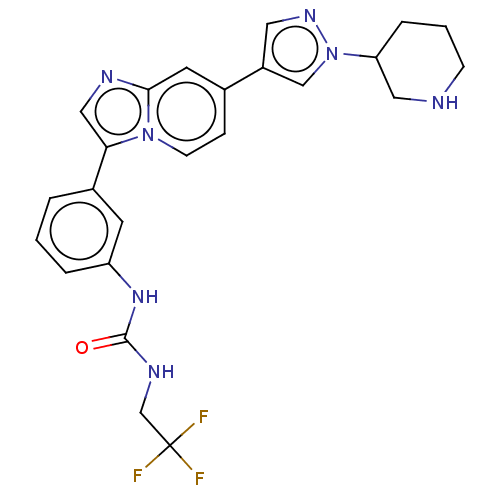 (US8796244, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.612 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128453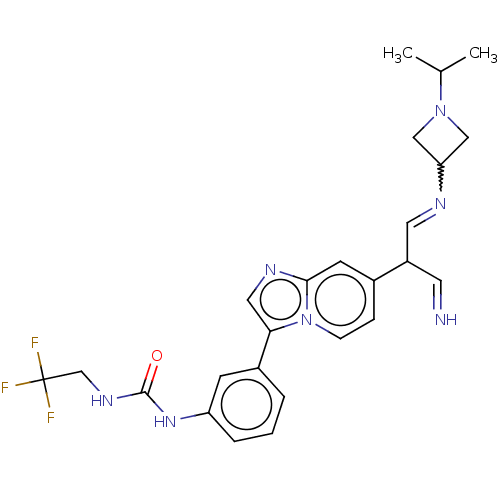 (US8796244, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.613 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286216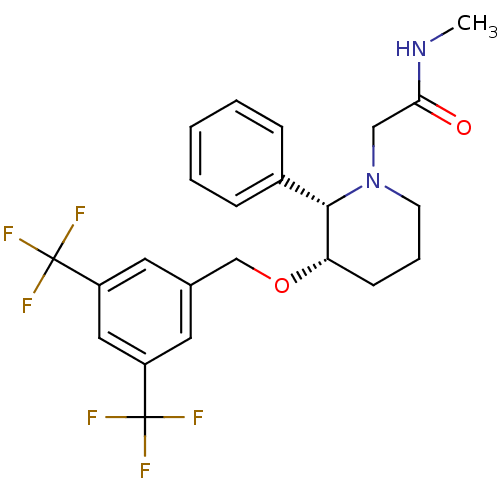 (2-[(2S,3S)-3-(3,5-Bis-trifluoromethyl-benzyloxy)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]- Substance P from human Neurokinin 1 (hNK1) receptor expressed in CHO cells | Bioorg Med Chem Lett 5: 209-212 (1995) Article DOI: 10.1016/0960-894X(95)00009-I BindingDB Entry DOI: 10.7270/Q2ZP463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128436 (US8796244, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128725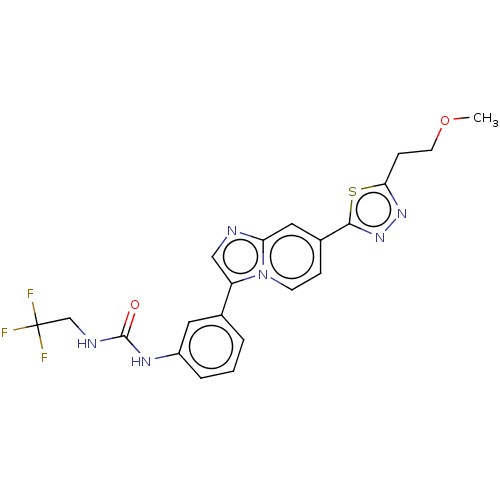 (US8796244, 319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.643 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128474 (US8796244, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.675 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the displacement of [ 1251] Substance P from hNK1 receptor in CHO cells | Bioorg Med Chem Lett 4: 2545-2550 (1994) Article DOI: 10.1016/S0960-894X(01)80280-8 BindingDB Entry DOI: 10.7270/Q2KH0N8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 753 total ) | Next | Last >> |