 Found 39 hits with Last Name = 'williams' and Initial = 'de'
Found 39 hits with Last Name = 'williams' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163646

(Montbretin A (MbA))Show SMILES CC1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2cc(O)c(O)c(O)c2)[C@@H](O[C@@H]2OC(COC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H](O)C(O)[C@@H]2O)C(O)[C@H]1O |r| Show InChI InChI=1S/C36H36O20/c1-12-25(44)30(49)34(56-35-31(50)29(48)27(46)22(54-35)11-51-23(43)5-3-13-2-4-16(38)17(39)6-13)36(52-12)55-33-28(47)24-18(40)9-15(37)10-21(24)53-32(33)14-7-19(41)26(45)20(42)8-14/h2-10,12,22,25,27,29-31,34-42,44-46,48-50H,11H2,1H3/b5-3+/t12?,22?,25-,27+,29?,30?,31-,34-,35-,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | -47.0 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163647

(MbA-G (1))Show SMILES C[C@@H]1O[C@@H](O[C@@H]2CO[C@@H](Oc3c(O)cc(cc3O)-c3oc4cc(O)cc(O)c4c(=O)c3O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](O)[C@H]3O[C@@H]3O[C@H](COC(=O)\C=C\c4ccc(O)c(O)c4)[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C47H54O28/c1-14-29(55)34(60)38(64)45(68-14)71-26-13-67-44(37(63)32(26)58)73-41-22(52)8-17(9-23(41)53)40-42(33(59)28-21(51)10-18(48)11-24(28)70-40)74-47-43(36(62)30(56)15(2)69-47)75-46-39(65)35(61)31(57)25(72-46)12-66-27(54)6-4-16-3-5-19(49)20(50)7-16/h3-11,14-15,25-26,29-32,34-39,43-53,55-58,60-65H,12-13H2,1-2H3/b6-4+/t14-,15-,25+,26+,29-,30-,31+,32-,34+,35-,36+,37+,38+,39+,43+,44-,45-,46-,47-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | -46.7 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50201794

(CHEMBL3897445)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)NCCCCNC(N)=N)C(=O)N[C@H]1CCCN(C1O)C(N)=N |r| Show InChI InChI=1S/C20H40N10O5/c1-11(2)14(16(33)27-12-6-5-9-30(17(12)34)19(23)24)29-15(32)13(10-31)28-20(35)26-8-4-3-7-25-18(21)22/h11-14,17,31,34H,3-10H2,1-2H3,(H3,23,24)(H,27,33)(H,29,32)(H4,21,22,25)(H2,26,28,35)/t12-,13-,14-,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin K using Z-FR-MCA fluorogenic substrate by Handerson plot analysis |
J Nat Prod 79: 1962-70 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00215
BindingDB Entry DOI: 10.7270/Q2F76FHF |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163648

(MbA-R (2))Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2cc(O)c(O[C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)c(O)c2)[C@H](O[C@@H]2O[C@H](COC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H](O)[C@H](O)[C@H]2O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C47H54O29/c1-14-29(57)35(63)42(76-47-43(75-45-38(66)34(62)31(59)25(11-48)71-45)36(64)32(60)26(72-47)13-67-27(56)5-3-15-2-4-18(50)19(51)6-15)46(69-14)74-41-33(61)28-20(52)9-17(49)10-24(28)70-39(41)16-7-21(53)40(22(54)8-16)73-44-37(65)30(58)23(55)12-68-44/h2-10,14,23,25-26,29-32,34-38,42-55,57-60,62-66H,11-13H2,1H3/b5-3+/t14-,23+,25+,26+,29-,30-,31+,32+,34-,35+,36-,37+,38+,42+,43+,44-,45-,46-,47-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21.3 | -44.5 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50213272
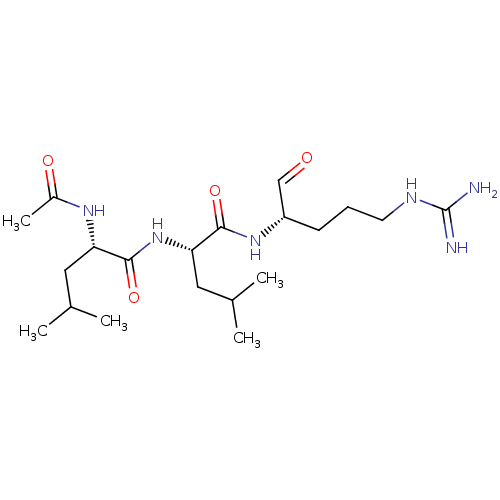
(CHEBI:6426 | Leupeptin)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C=O |r| Show InChI InChI=1S/C20H38N6O4/c1-12(2)9-16(24-14(5)28)19(30)26-17(10-13(3)4)18(29)25-15(11-27)7-6-8-23-20(21)22/h11-13,15-17H,6-10H2,1-5H3,(H,24,28)(H,25,29)(H,26,30)(H4,21,22,23)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human cathepsin K expressed in Pichia pastoris using Z-FR-MCA as substrate by Dixon plot method |
Bioorg Med Chem Lett 27: 1397-1400 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.007
BindingDB Entry DOI: 10.7270/Q2H70J26 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50201793

(CHEMBL3899320)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)NC1CCCN(C1O)C(N)=N |r| Show InChI InChI=1S/C27H44N10O6/c1-15(2)20(22(39)33-18-11-7-13-37(23(18)40)26(30)31)36-21(38)17(10-6-12-32-25(28)29)34-27(43)35-19(24(41)42)14-16-8-4-3-5-9-16/h3-5,8-9,15,17-20,23,40H,6-7,10-14H2,1-2H3,(H3,30,31)(H,33,39)(H,36,38)(H,41,42)(H4,28,29,32)(H2,34,35,43)/t17-,18?,19-,20-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin K using Z-FR-MCA fluorogenic substrate by Handerson plot analysis |
J Nat Prod 79: 1962-70 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00215
BindingDB Entry DOI: 10.7270/Q2F76FHF |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163649
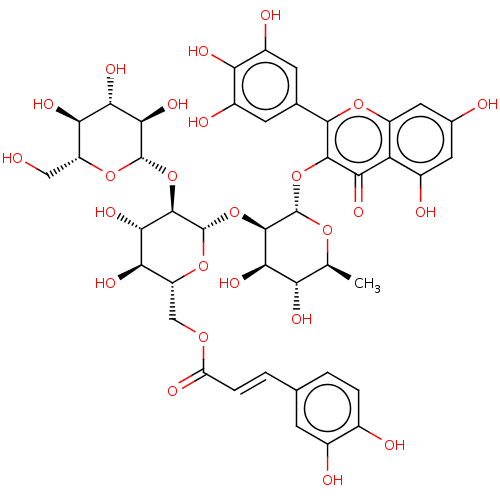
(MbA-RX (3))Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2cc(O)c(O)c(O)c2)[C@H](O[C@@H]2O[C@H](COC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H](O)[C@H](O)[C@H]2O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C42H46O25/c1-13-27(51)33(57)38(41(61-13)65-37-31(55)26-19(47)9-16(44)10-22(26)62-36(37)15-7-20(48)28(52)21(49)8-15)67-42-39(66-40-35(59)32(56)29(53)23(11-43)63-40)34(58)30(54)24(64-42)12-60-25(50)5-3-14-2-4-17(45)18(46)6-14/h2-10,13,23-24,27,29-30,32-35,38-49,51-54,56-59H,11-12H2,1H3/b5-3+/t13-,23+,24+,27-,29+,30+,32-,33+,34-,35+,38+,39+,40-,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42.4 | -42.8 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163650

(MbA-GR (4))Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2cc(O)c(O[C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)c(O)c2)[C@H](O[C@@H]2O[C@H](COC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C41H44O24/c1-13-27(50)32(55)38(65-40-34(57)31(54)29(52)24(62-40)12-58-25(49)5-3-14-2-4-17(43)18(44)6-14)41(60-13)64-37-30(53)26-19(45)9-16(42)10-23(26)61-35(37)15-7-20(46)36(21(47)8-15)63-39-33(56)28(51)22(48)11-59-39/h2-10,13,22,24,27-29,31-34,38-48,50-52,54-57H,11-12H2,1H3/b5-3+/t13-,22+,24+,27-,28-,29+,31-,32+,33+,34+,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.3 | -41.2 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163651
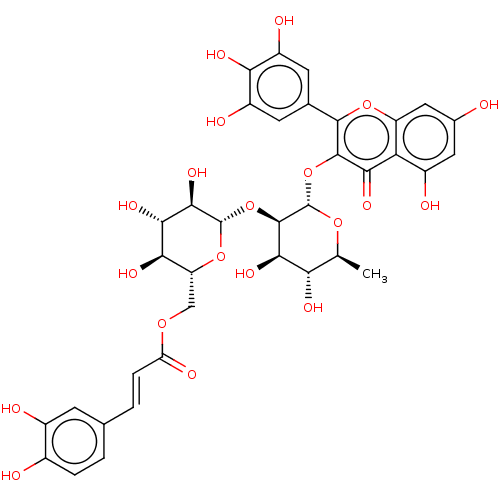
(MbA-GRX (5) | mini-MbA)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2cc(O)c(O)c(O)c2)[C@H](O[C@@H]2O[C@H](COC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C36H36O20/c1-12-25(44)30(49)34(56-35-31(50)29(48)27(46)22(54-35)11-51-23(43)5-3-13-2-4-16(38)17(39)6-13)36(52-12)55-33-28(47)24-18(40)9-15(37)10-21(24)53-32(33)14-7-19(41)26(45)20(42)8-14/h2-10,12,22,25,27,29-31,34-42,44-46,48-50H,11H2,1H3/b5-3+/t12-,22+,25-,27+,29-,30+,31+,34+,35-,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 93.3 | -40.8 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50201793

(CHEMBL3899320)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)NC1CCCN(C1O)C(N)=N |r| Show InChI InChI=1S/C27H44N10O6/c1-15(2)20(22(39)33-18-11-7-13-37(23(18)40)26(30)31)36-21(38)17(10-6-12-32-25(28)29)34-27(43)35-19(24(41)42)14-16-8-4-3-5-9-16/h3-5,8-9,15,17-20,23,40H,6-7,10-14H2,1-2H3,(H3,30,31)(H,33,39)(H,36,38)(H,41,42)(H4,28,29,32)(H2,34,35,43)/t17-,18?,19-,20-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin K using Z-FR-MCA fluorogenic substrate by Dixon plot analysis |
J Nat Prod 79: 1962-70 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00215
BindingDB Entry DOI: 10.7270/Q2F76FHF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50201793

(CHEMBL3899320)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)NC1CCCN(C1O)C(N)=N |r| Show InChI InChI=1S/C27H44N10O6/c1-15(2)20(22(39)33-18-11-7-13-37(23(18)40)26(30)31)36-21(38)17(10-6-12-32-25(28)29)34-27(43)35-19(24(41)42)14-16-8-4-3-5-9-16/h3-5,8-9,15,17-20,23,40H,6-7,10-14H2,1-2H3,(H3,30,31)(H,33,39)(H,36,38)(H,41,42)(H4,28,29,32)(H2,34,35,43)/t17-,18?,19-,20-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin K using Z-FR-MCA fluorogenic substrate by Dixon plot analysis |
J Nat Prod 79: 1962-70 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00215
BindingDB Entry DOI: 10.7270/Q2F76FHF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50201795
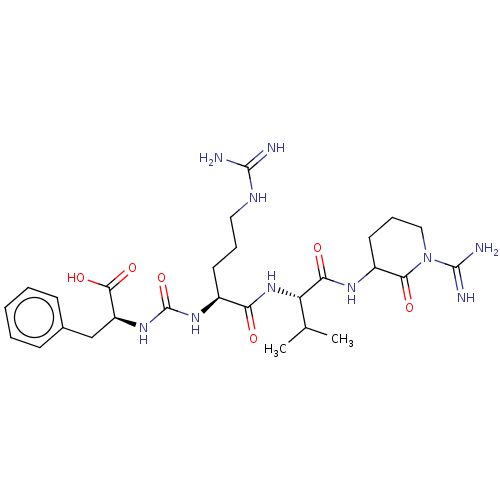
(CHEMBL3972383)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)NC1CCCN(C(N)=N)C1=O |r| Show InChI InChI=1S/C27H42N10O6/c1-15(2)20(22(39)33-18-11-7-13-37(23(18)40)26(30)31)36-21(38)17(10-6-12-32-25(28)29)34-27(43)35-19(24(41)42)14-16-8-4-3-5-9-16/h3-5,8-9,15,17-20H,6-7,10-14H2,1-2H3,(H3,30,31)(H,33,39)(H,36,38)(H,41,42)(H4,28,29,32)(H2,34,35,43)/t17-,18?,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin K using Z-FR-MCA fluorogenic substrate by Dixon plot analysis |
J Nat Prod 79: 1962-70 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00215
BindingDB Entry DOI: 10.7270/Q2F76FHF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50201792
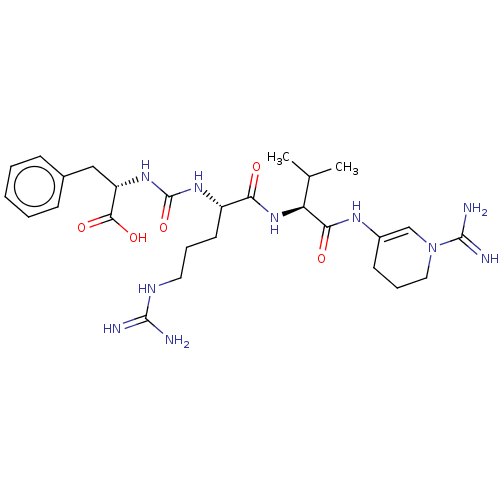
(CHEMBL3942776)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)NC1=CN(CCC1)C(N)=N |r,t:34| Show InChI InChI=1S/C27H42N10O5/c1-16(2)21(23(39)33-18-10-7-13-37(15-18)26(30)31)36-22(38)19(11-6-12-32-25(28)29)34-27(42)35-20(24(40)41)14-17-8-4-3-5-9-17/h3-5,8-9,15-16,19-21H,6-7,10-14H2,1-2H3,(H3,30,31)(H,33,39)(H,36,38)(H,40,41)(H4,28,29,32)(H2,34,35,42)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant cathepsin K using Z-FR-MCA fluorogenic substrate by Dixon plot analysis |
J Nat Prod 79: 1962-70 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00215
BindingDB Entry DOI: 10.7270/Q2F76FHF |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163652

(MbA-C (6))Show SMILES C[C@@H]1O[C@@H](O[C@@H]2CO[C@@H](Oc3c(O)cc(cc3O)-c3oc4cc(O)cc(O)c4c(=O)c3O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](O)[C@H]3O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C44H58O30/c1-10-22(51)28(57)33(62)41(65-10)70-20-9-64-40(32(61)26(20)55)71-36-15(49)3-12(4-16(36)50)35-37(27(56)21-14(48)5-13(47)6-17(21)67-35)72-43-38(30(59)23(52)11(2)66-43)74-44-39(31(60)25(54)19(8-46)69-44)73-42-34(63)29(58)24(53)18(7-45)68-42/h3-6,10-11,18-20,22-26,28-34,38-55,57-63H,7-9H2,1-2H3/t10-,11-,18+,19+,20+,22-,23-,24+,25+,26-,28+,29-,30+,31-,32+,33+,34+,38+,39+,40-,41-,42-,43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 730 | -35.6 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163653
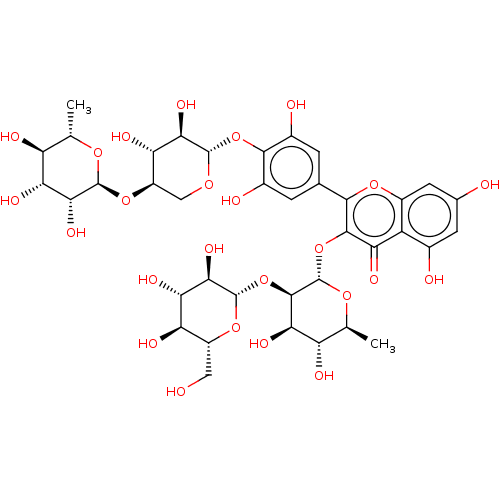
(MbA-CG (7))Show SMILES C[C@@H]1O[C@@H](O[C@@H]2CO[C@@H](Oc3c(O)cc(cc3O)-c3oc4cc(O)cc(O)c4c(=O)c3O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](O)[C@H]3O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C38H48O25/c1-9-20(44)25(49)29(53)36(56-9)60-18-8-55-35(28(52)23(18)47)61-32-14(42)3-11(4-15(32)43)31-33(24(48)19-13(41)5-12(40)6-16(19)58-31)62-38-34(27(51)21(45)10(2)57-38)63-37-30(54)26(50)22(46)17(7-39)59-37/h3-6,9-10,17-18,20-23,25-30,34-47,49-54H,7-8H2,1-2H3/t9-,10-,17+,18+,20-,21-,22+,23-,25+,26-,27+,28+,29+,30+,34+,35-,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.24E+3 | -32.8 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163656

(3-O-(6-(4-(Caffeamidomethyl)-triazolyl)hexyl)querc...)Show SMILES Oc1cc(O)c2c(c1)oc(-c1ccc(O)c(O)c1)c(OCCCCCCn1cc(CNC(=O)\C=C\c3ccc(O)c(O)c3)nn1)c2=O Show InChI InChI=1S/C33H32N4O10/c38-22-15-27(43)30-28(16-22)47-32(20-7-9-24(40)26(42)14-20)33(31(30)45)46-12-4-2-1-3-11-37-18-21(35-36-37)17-34-29(44)10-6-19-5-8-23(39)25(41)13-19/h5-10,13-16,18,38-43H,1-4,11-12,17H2,(H,34,44)/b10-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.39E+4 | -28.2 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50233350
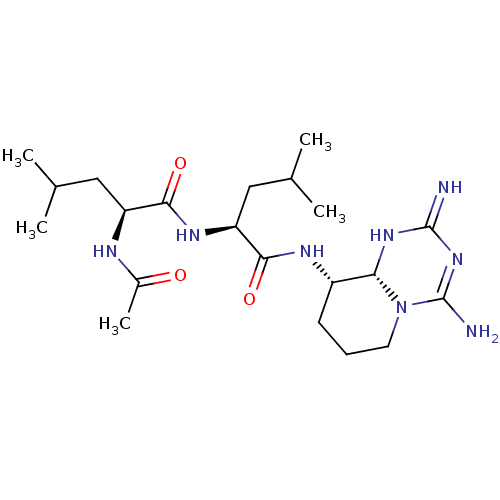
(CHEMBL4096643)Show SMILES [H][C@@]12NC(=N)N=C(N)N1CCC[C@@H]2NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(C)=O |r,t:5| Show InChI InChI=1S/C21H38N8O3/c1-11(2)9-15(24-13(5)30)18(31)26-16(10-12(3)4)19(32)25-14-7-6-8-29-17(14)27-20(22)28-21(29)23/h11-12,14-17H,6-10H2,1-5H3,(H,24,30)(H,25,32)(H,26,31)(H4,22,23,27,28)/t14-,15-,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human cathepsin K expressed in Pichia pastoris using Z-FR-MCA as substrate by Dixon plot method |
Bioorg Med Chem Lett 27: 1397-1400 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.007
BindingDB Entry DOI: 10.7270/Q2H70J26 |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163654
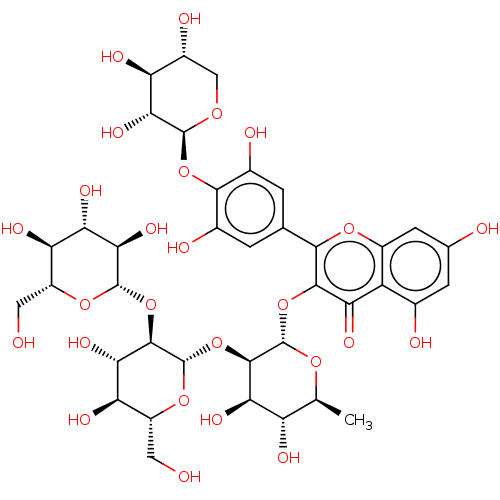
(MbA-CR (8))Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2cc(O)c(O[C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)c(O)c2)[C@H](O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C38H48O26/c1-9-20(46)26(52)33(64-38-34(27(53)23(49)18(7-40)60-38)63-36-29(55)25(51)22(48)17(6-39)59-36)37(57-9)62-32-24(50)19-12(42)4-11(41)5-16(19)58-30(32)10-2-13(43)31(14(44)3-10)61-35-28(54)21(47)15(45)8-56-35/h2-5,9,15,17-18,20-23,25-29,33-49,51-55H,6-8H2,1H3/t9-,15+,17+,18+,20-,21-,22+,23+,25-,26+,27-,28+,29+,33+,34+,35-,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.68E+4 | -25.1 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM163655
(MbA-CGR (9))Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2cc(O)c(O[C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)c(O)c2)[C@H](O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C32H38O21/c1-8-18(39)23(44)29(53-31-25(46)22(43)20(41)16(6-33)50-31)32(48-8)52-28-21(42)17-11(35)4-10(34)5-15(17)49-26(28)9-2-12(36)27(13(37)3-9)51-30-24(45)19(40)14(38)7-47-30/h2-5,8,14,16,18-20,22-25,29-41,43-46H,6-7H2,1H3/t8-,14+,16+,18-,19-,20+,22-,23+,24+,25+,29+,30-,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.28E+5 | -22.6 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia
| Assay Description
Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... |
Nat Chem Biol 11: 691-6 (2015)
Article DOI: 10.1038/nchembio.1865
BindingDB Entry DOI: 10.7270/Q2KD1WP4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50239948
(CHEMBL4071326 | US9850225, Example 1166)Show SMILES Cc1c(COc2cc(OCc3cccc(c3)C#N)c(CN3C[C@H](O)C[C@@H]3C(O)=O)cc2Cl)cccc1-c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C36H33ClN2O7/c1-22-26(6-3-7-29(22)25-8-9-32-35(14-25)44-11-10-43-32)21-46-34-16-33(45-20-24-5-2-4-23(12-24)17-38)27(13-30(34)37)18-39-19-28(40)15-31(39)36(41)42/h2-9,12-14,16,28,31,40H,10-11,15,18-21H2,1H3,(H,41,42)/t28-,31-/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50362641
(CHEMBL384120)Show SMILES Oc1ccc(cc1)[C@@H]1Oc2cc(O)cc3[C@@H]([C@H]4[C@H](c5ccc(O)cc5)c5c(O)cc(O)cc5[C@H]5[C@@H](Oc6cc(O)cc4c56)c4ccc(O)cc4)[C@H](c4ccc(O)cc4)c4c(O)cc(O)cc4[C@@H]1c23 Show InChI InChI=1S/C56H42O12/c57-29-9-1-25(2-10-29)45-47-37(17-33(61)21-41(47)65)53-49-39(19-35(63)23-43(49)67-55(53)27-5-13-31(59)14-6-27)51(45)52-40-20-36(64)24-44-50(40)54(56(68-44)28-7-15-32(60)16-8-28)38-18-34(62)22-42(66)48(38)46(52)26-3-11-30(58)12-4-26/h1-24,45-46,51-66H/t45-,46-,51-,52-,53-,54-,55+,56+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50613798
(CHEMBL5267016) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613798

(CHEMBL5267016) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50613799
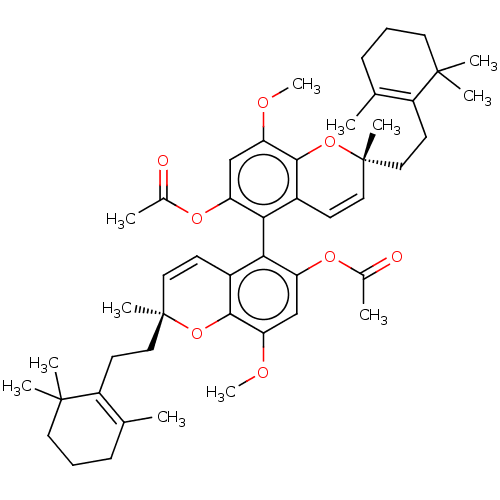
(CHEMBL5283695) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
CAAX prenyl protease 2
(Homo sapiens (Human)) | BDBM50480670

(CHEMBL553161)Show SMILES [H][C@@]12CC[C@]3(C)[C@]([H])(C[C@H](OC(=O)CC(O)CC)[C@]4(C)C5=C([C@@H](C[C@@]34[H])OCC)C(C)(O)OC5=O)[C@@]1(C)CCC[C@]2(C)CC |r,c:20| Show InChI InChI=1S/C34H54O7/c1-9-20(35)17-26(36)40-25-19-23-31(5)15-12-14-30(4,10-2)22(31)13-16-32(23,6)24-18-21(39-11-3)27-28(33(24,25)7)29(37)41-34(27,8)38/h20-25,35,38H,9-19H2,1-8H3/t20?,21-,22+,23-,24+,25+,30+,31+,32-,33-,34?/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of human RCE1 |
J Nat Prod 72: 1106-9 (2009)
Article DOI: 10.1021/np900042r
BindingDB Entry DOI: 10.7270/Q2930X0M |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50242189
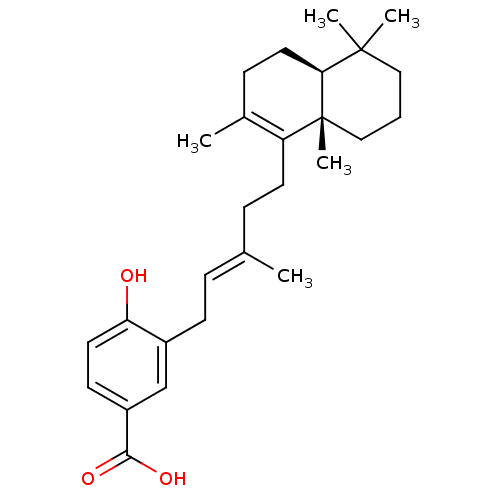
((+)-subersic acid | CHEMBL455101)Show SMILES C\C(CCC1=C(C)CC[C@H]2C(C)(C)CCC[C@]12C)=C/Cc1cc(ccc1O)C(O)=O |r,c:4| Show InChI InChI=1S/C27H38O3/c1-18(7-10-20-17-21(25(29)30)11-13-23(20)28)8-12-22-19(2)9-14-24-26(3,4)15-6-16-27(22,24)5/h7,11,13,17,24,28H,6,8-10,12,14-16H2,1-5H3,(H,29,30)/b18-7+/t24-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) |
J Nat Prod 67: 2127-9 (2004)
Article DOI: 10.1021/np049808d
BindingDB Entry DOI: 10.7270/Q2WQ03JB |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50421038

(CHEMBL2087614)Show SMILES C[C@]12CC[C@H]3[C@@](C)(CC[C@@H]4[C@]3(C)CC[C@@H]3O[C@@]5(C)CCCC(C)(C)[C@@H]5CC[C@@]43C)[C@@H]1Cc1c2c(ccc1O)C(O)C(O)=O |r| Show InChI InChI=1S/C38H56O5/c1-33(2)15-8-16-38(7)25(33)11-18-36(5)27-12-17-35(4)26(34(27,3)20-14-29(36)43-38)13-19-37(6)28(35)21-23-24(39)10-9-22(30(23)37)31(40)32(41)42/h9-10,25-29,31,39-40H,8,11-21H2,1-7H3,(H,41,42)/t25-,26+,27+,28-,29-,31?,34+,35+,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli using L-tryptophan as substrate after 60 mins by spectrophotometry |
J Nat Prod 75: 1451-8 (2012)
Article DOI: 10.1021/np300345j
BindingDB Entry DOI: 10.7270/Q2SQ91PS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50421039

(CHEMBL2087615)Show SMILES C[C@@H]1CCC=C(C)[C@@]1(C)CC[C@]1(C)[C@@H](O)CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](Cc5c4c(ccc5O)C(O)C(O)=O)[C@]3(C)CC[C@@H]12 |r,t:4| Show InChI InChI=1S/C38H56O5/c1-22-9-8-10-23(2)34(22,3)19-20-37(6)28-13-16-36(5)27(35(28,4)18-15-30(37)40)14-17-38(7)29(36)21-25-26(39)12-11-24(31(25)38)32(41)33(42)43/h9,11-12,23,27-30,32,39-41H,8,10,13-21H2,1-7H3,(H,42,43)/t23-,27-,28-,29+,30+,32?,34-,35-,36-,37+,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli using L-tryptophan as substrate after 60 mins by spectrophotometry |
J Nat Prod 75: 1451-8 (2012)
Article DOI: 10.1021/np300345j
BindingDB Entry DOI: 10.7270/Q2SQ91PS |
More data for this
Ligand-Target Pair | |
CAAX prenyl protease 2
(Homo sapiens (Human)) | BDBM50480671
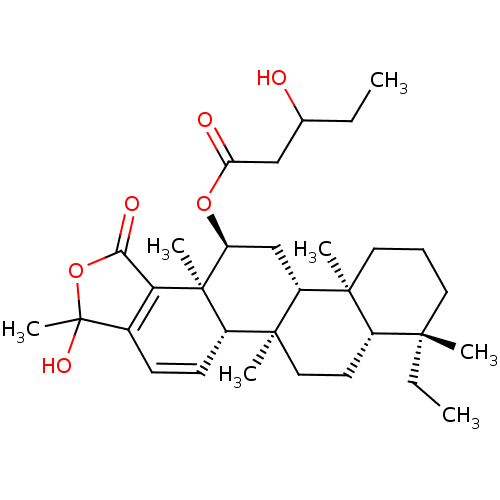
(CHEMBL555914)Show SMILES [H][C@@]12CC[C@]3(C)[C@]([H])(C[C@H](OC(=O)CC(O)CC)[C@]4(C)C5=C(C=C[C@@]34[H])C(C)(O)OC5=O)[C@@]1(C)CCC[C@]2(C)CC |r,c:20,22| Show InChI InChI=1S/C32H48O6/c1-8-19(33)17-25(34)37-24-18-23-29(4)15-10-14-28(3,9-2)21(29)13-16-30(23,5)22-12-11-20-26(31(22,24)6)27(35)38-32(20,7)36/h11-12,19,21-24,33,36H,8-10,13-18H2,1-7H3/t19?,21-,22-,23+,24-,28-,29-,30-,31+,32?/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of human RCE1 |
J Nat Prod 72: 1106-9 (2009)
Article DOI: 10.1021/np900042r
BindingDB Entry DOI: 10.7270/Q2930X0M |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50242186
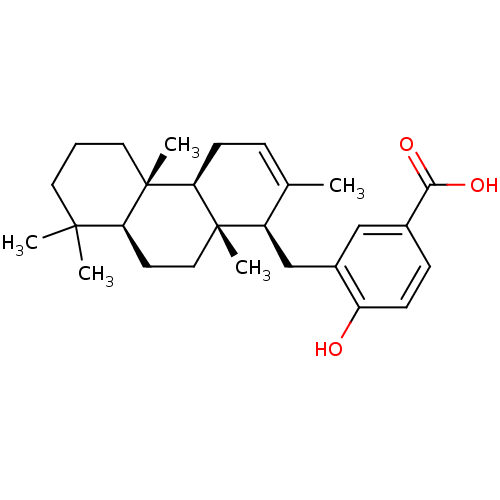
((+)-Makassaric acid | CHEMBL454582)Show SMILES CC1=CC[C@H]2[C@@](C)(CC[C@H]3C(C)(C)CCC[C@]23C)[C@@H]1Cc1cc(ccc1O)C(O)=O |r,t:1| Show InChI InChI=1S/C27H38O3/c1-17-7-10-23-26(4,14-11-22-25(2,3)12-6-13-27(22,23)5)20(17)16-19-15-18(24(29)30)8-9-21(19)28/h7-9,15,20,22-23,28H,6,10-14,16H2,1-5H3,(H,29,30)/t20-,22+,23+,26+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) |
J Nat Prod 67: 2127-9 (2004)
Article DOI: 10.1021/np049808d
BindingDB Entry DOI: 10.7270/Q2WQ03JB |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50421041
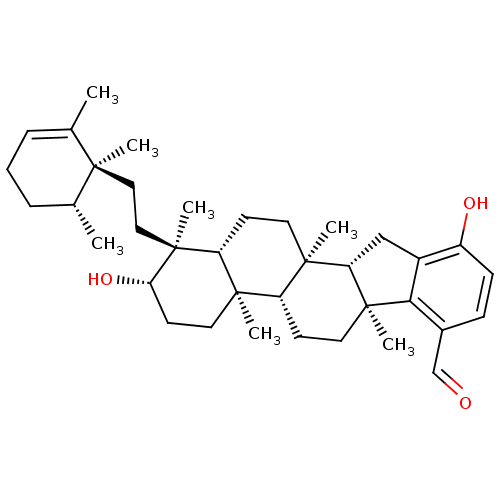
(CHEMBL2087617)Show SMILES C[C@@H]1CCC=C(C)[C@@]1(C)CC[C@]1(C)[C@@H](O)CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](Cc5c4c(C=O)ccc5O)[C@]3(C)CC[C@@H]12 |r,t:4| Show InChI InChI=1S/C37H54O3/c1-23-9-8-10-24(2)33(23,3)19-20-36(6)29-13-16-35(5)28(34(29,4)18-15-31(36)40)14-17-37(7)30(35)21-26-27(39)12-11-25(22-38)32(26)37/h9,11-12,22,24,28-31,39-40H,8,10,13-21H2,1-7H3/t24-,28-,29-,30+,31+,33-,34-,35-,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli using L-tryptophan as substrate after 60 mins by spectrophotometry |
J Nat Prod 75: 1451-8 (2012)
Article DOI: 10.1021/np300345j
BindingDB Entry DOI: 10.7270/Q2SQ91PS |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613799

(CHEMBL5283695) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613800
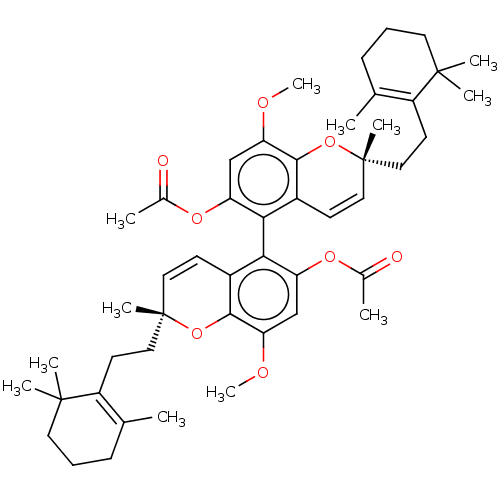
(CHEMBL5282197) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50421040

(CHEMBL2087616)Show SMILES C[C@]12CC[C@H]3[C@@](C)(CC[C@@H]4[C@]3(C)CC[C@@H]3O[C@@]5(C)CCCC(C)(C)[C@@H]5CC[C@@]43C)[C@@H]1Cc1c2c(C=O)ccc1O |r| Show InChI InChI=1S/C37H54O3/c1-32(2)15-8-16-37(7)26(32)11-18-35(5)28-12-17-34(4)27(33(28,3)20-14-30(35)40-37)13-19-36(6)29(34)21-24-25(39)10-9-23(22-38)31(24)36/h9-10,22,26-30,39H,8,11-21H2,1-7H3/t26-,27+,28+,29-,30-,33+,34+,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli using L-tryptophan as substrate after 60 mins by spectrophotometry |
J Nat Prod 75: 1451-8 (2012)
Article DOI: 10.1021/np300345j
BindingDB Entry DOI: 10.7270/Q2SQ91PS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50613801
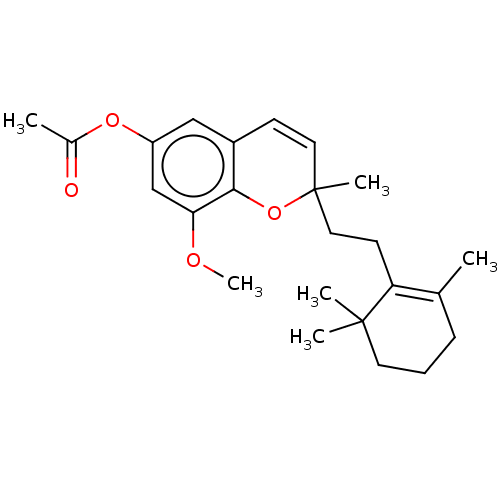
(CHEMBL5276399) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50613800

(CHEMBL5282197) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613801

(CHEMBL5276399) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
CAAX prenyl protease 2
(Homo sapiens (Human)) | BDBM50480672

(CHEMBL561185)Show SMILES [H][C@@]12CC[C@]3(C)[C@]([H])(C[C@H](OC(=O)CC(O)C(C)C)[C@]4(C)C=C([C@H](O)C[C@@]34[H])C(C)=O)[C@@]1(C)CCC[C@]2(C)CC |r,c:21| Show InChI InChI=1S/C32H52O5/c1-9-29(5)12-10-13-30(6)24(29)11-14-31(7)25-15-23(35)21(20(4)33)18-32(25,8)27(17-26(30)31)37-28(36)16-22(34)19(2)3/h18-19,22-27,34-35H,9-17H2,1-8H3/t22?,23-,24+,25+,26-,27+,29+,30+,31+,32-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of human RCE1 |
J Nat Prod 72: 1106-9 (2009)
Article DOI: 10.1021/np900042r
BindingDB Entry DOI: 10.7270/Q2930X0M |
More data for this
Ligand-Target Pair | |
CAAX prenyl protease 2
(Homo sapiens (Human)) | BDBM50480669

(CHEMBL558514)Show SMILES [H][C@@]12CC[C@]3(C)[C@]([H])(C[C@H](OC(=O)CC(O)CC)[C@]4(C)C=C([C@H](O)C[C@@]34[H])C(C)=O)[C@@]1(C)CCC[C@]2(C)CC |r,c:20| Show InChI InChI=1S/C31H50O5/c1-8-20(33)15-27(35)36-26-17-25-29(5)13-10-12-28(4,9-2)23(29)11-14-30(25,6)24-16-22(34)21(19(3)32)18-31(24,26)7/h18,20,22-26,33-34H,8-17H2,1-7H3/t20?,22-,23+,24+,25-,26+,28+,29+,30+,31-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of human RCE1 |
J Nat Prod 72: 1106-9 (2009)
Article DOI: 10.1021/np900042r
BindingDB Entry DOI: 10.7270/Q2930X0M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data