 Found 1616 hits with Last Name = 'wilson' and Initial = 'l'
Found 1616 hits with Last Name = 'wilson' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50160855
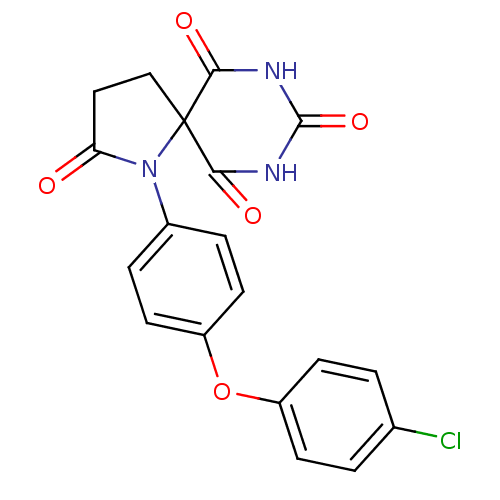
(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11551
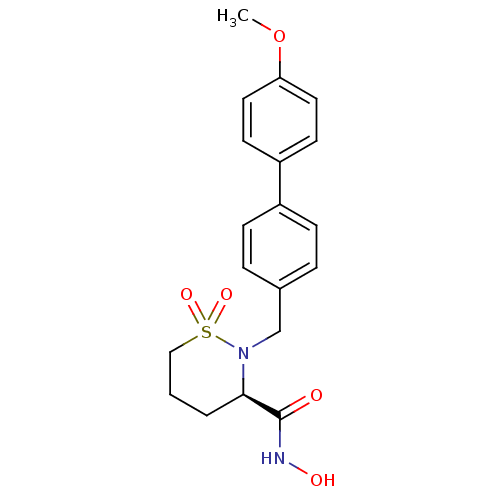
((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50160855

(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11551

((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50160855

(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11551

((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50580195
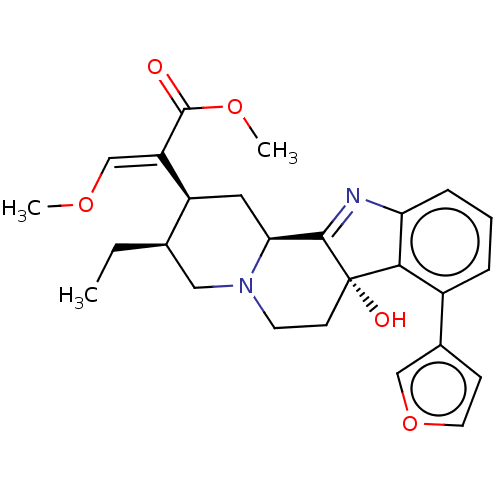
(CHEMBL5071286)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@@]1(O)C2=Nc2cccc(-c3ccoc3)c12)C(=C/OC)\C(=O)OC |r,t:15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor (unknown origin) expressed in CHO cells at 10 uM |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01273
BindingDB Entry DOI: 10.7270/Q25D8WQH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50067734
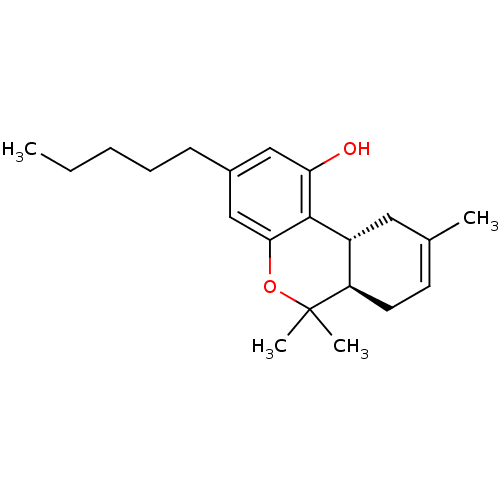
((-)-(6aR-trans)-6,6,9-trimethyl-3-pentyl-6a,7,10,1...)Show SMILES CCCCCc1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9,12-13,16-17,22H,5-8,10-11H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50304090
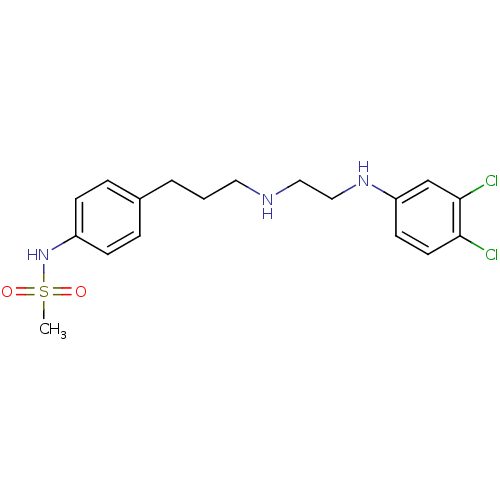
(CHEMBL594615 | N-(4-(3-(2-(3,4-Dichlorophenylamino...)Show SMILES CS(=O)(=O)Nc1ccc(CCCNCCNc2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C18H23Cl2N3O2S/c1-26(24,25)23-15-6-4-14(5-7-15)3-2-10-21-11-12-22-16-8-9-17(19)18(20)13-16/h4-9,13,21-23H,2-3,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells |
Bioorg Med Chem 17: 6463-80 (2009)
Article DOI: 10.1016/j.bmc.2009.05.085
BindingDB Entry DOI: 10.7270/Q2M61KB5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50580193

(CHEMBL5086786)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@@]1(O)C2=Nc2cccc(c12)-c1ccccc1)C(=C/OC)\C(=O)OC |r,t:15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor (unknown origin) expressed in CHO cells at 10 uM |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01273
BindingDB Entry DOI: 10.7270/Q25D8WQH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50580194
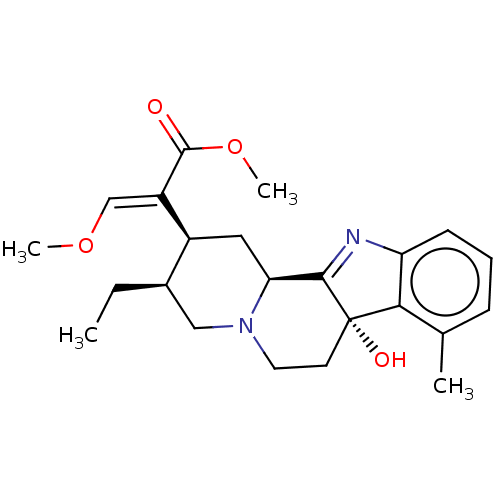
(CHEMBL5074977)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@@]1(O)C2=Nc2cccc(C)c12)C(=C/OC)\C(=O)OC |r,t:15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor (unknown origin) expressed in CHO cells at 10 uM |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01273
BindingDB Entry DOI: 10.7270/Q25D8WQH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50092347

(CHEMBL3586106)Show SMILES [H][C@@]12CC=C(C)[C@H](O)[C@@]1([H])c1c(O)cc(CCCCC)cc1OC2(C)C |r,t:3| Show InChI InChI=1S/C20H19Cl2N3O2/c1-26-19-7-6-13-15(20(19)27-12-4-2-3-5-12)9-24-25-18(13)8-14-16(21)10-23-11-17(14)22/h6-7,9-12H,2-5,8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50092347

(CHEMBL3586106)Show SMILES [H][C@@]12CC=C(C)[C@H](O)[C@@]1([H])c1c(O)cc(CCCCC)cc1OC2(C)C |r,t:3| Show InChI InChI=1S/C20H19Cl2N3O2/c1-26-19-7-6-13-15(20(19)27-12-4-2-3-5-12)9-24-25-18(13)8-14-16(21)10-23-11-17(14)22/h6-7,9-12H,2-5,8H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50609010
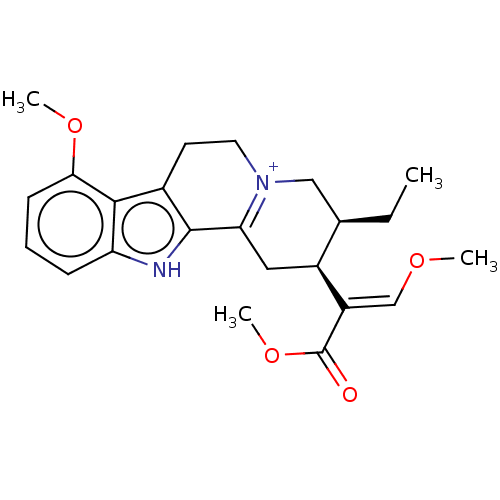
(CHEMBL5276728)Show SMILES CC[C@@H]1C[N+]2=C(C[C@@H]1\C(=C/OC)C(=O)OC)c1[nH]c3cccc(OC)c3c1CC2 |r,c:4| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50092343
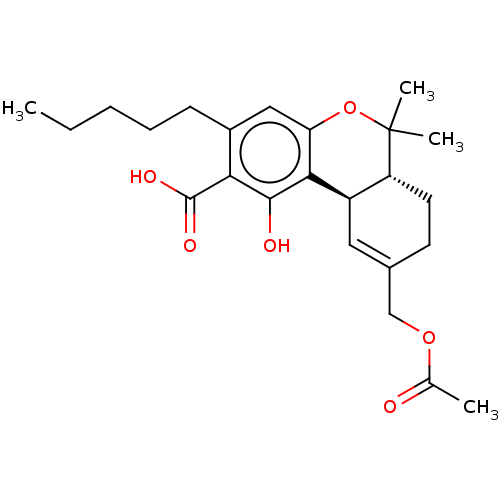
(CHEMBL3586110)Show SMILES [H][C@@]12CCC(COC(C)=O)=C[C@@]1([H])c1c(O)c(C(O)=O)c(CCCCC)cc1OC2(C)C |r,c:9| Show InChI InChI=1S/C24H32O6/c1-5-6-7-8-16-12-19-21(22(26)20(16)23(27)28)17-11-15(13-29-14(2)25)9-10-18(17)24(3,4)30-19/h11-12,17-18,26H,5-10,13H2,1-4H3,(H,27,28)/t17-,18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609010

(CHEMBL5276728)Show SMILES CC[C@@H]1C[N+]2=C(C[C@@H]1\C(=C/OC)C(=O)OC)c1[nH]c3cccc(OC)c3c1CC2 |r,c:4| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM84880
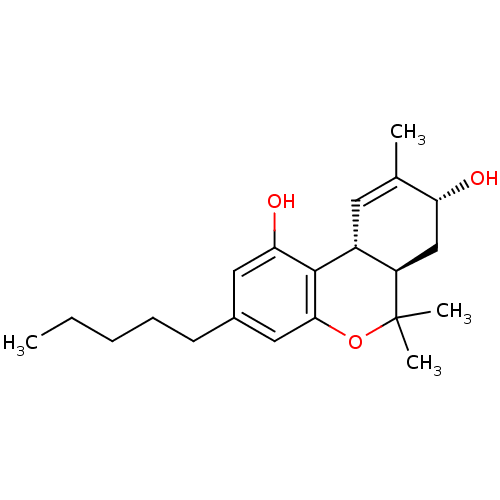
(THC, 8 beta-OH)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)[C@H](O)C[C@H]3C(C)(C)Oc2c1 |t:11| Show InChI InChI=1S/C21H30O3/c1-5-6-7-8-14-10-18(23)20-15-9-13(2)17(22)12-16(15)21(3,4)24-19(20)11-14/h9-11,15-17,22-23H,5-8,12H2,1-4H3/t15-,16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50067734

((-)-(6aR-trans)-6,6,9-trimethyl-3-pentyl-6a,7,10,1...)Show SMILES CCCCCc1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9,12-13,16-17,22H,5-8,10-11H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50519927
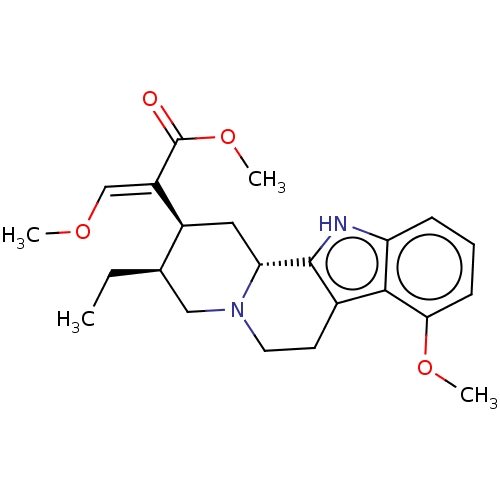
(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM84880

(THC, 8 beta-OH)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)[C@H](O)C[C@H]3C(C)(C)Oc2c1 |t:11| Show InChI InChI=1S/C21H30O3/c1-5-6-7-8-14-10-18(23)20-15-9-13(2)17(22)12-16(15)21(3,4)24-19(20)11-14/h9-11,15-17,22-23H,5-8,12H2,1-4H3/t15-,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50092345
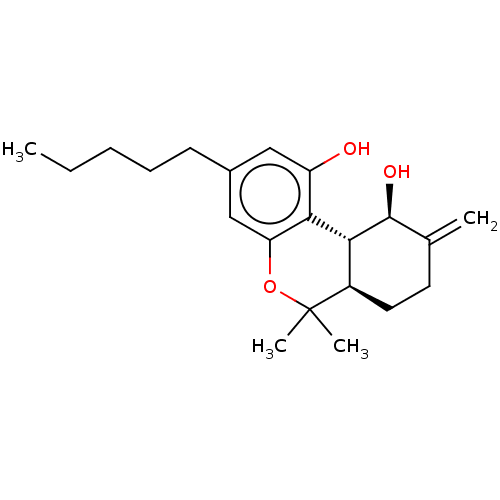
(CHEMBL3586108)Show SMILES [H][C@@]12CCC(=C)[C@H](O)[C@@]1([H])c1c(O)cc(CCCCC)cc1OC2(C)C |r| Show InChI InChI=1S/C26H25Cl2N3O2/c1-32-25-12-11-19-21(15-30-31-24(19)14-20-22(27)16-29-17-23(20)28)26(25)33-13-7-3-6-10-18-8-4-2-5-9-18/h2,4-5,8-9,11-12,15-17H,3,6-7,10,13-14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50304085
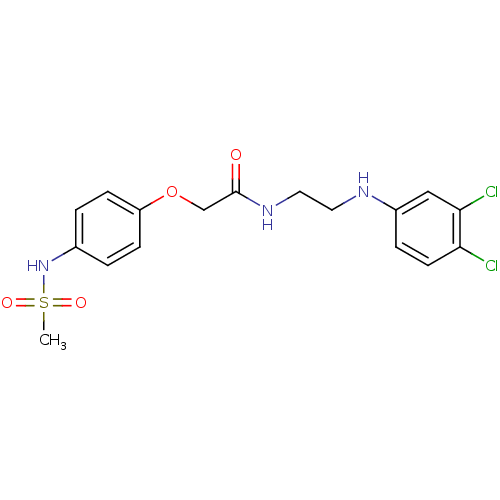
(CHEMBL607819 | N-(2-(3,4-Dichlorophenylamino)ethyl...)Show SMILES CS(=O)(=O)Nc1ccc(OCC(=O)NCCNc2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C17H19Cl2N3O4S/c1-27(24,25)22-12-2-5-14(6-3-12)26-11-17(23)21-9-8-20-13-4-7-15(18)16(19)10-13/h2-7,10,20,22H,8-9,11H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membrane |
Bioorg Med Chem 17: 6463-80 (2009)
Article DOI: 10.1016/j.bmc.2009.05.085
BindingDB Entry DOI: 10.7270/Q2M61KB5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609010

(CHEMBL5276728)Show SMILES CC[C@@H]1C[N+]2=C(C[C@@H]1\C(=C/OC)C(=O)OC)c1[nH]c3cccc(OC)c3c1CC2 |r,c:4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50092345

(CHEMBL3586108)Show SMILES [H][C@@]12CCC(=C)[C@H](O)[C@@]1([H])c1c(O)cc(CCCCC)cc1OC2(C)C |r| Show InChI InChI=1S/C26H25Cl2N3O2/c1-32-25-12-11-19-21(15-30-31-24(19)14-20-22(27)16-29-17-23(20)28)26(25)33-13-7-3-6-10-18-8-4-2-5-9-18/h2,4-5,8-9,11-12,15-17H,3,6-7,10,13-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50092344
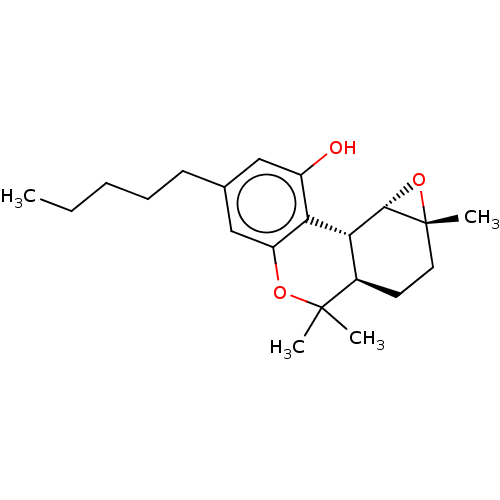
(CHEMBL3586109)Show SMILES [H][C@@]12O[C@]1(C)CC[C@]1([H])[C@]2([H])c2c(O)cc(CCCCC)cc2OC1(C)C |r| Show InChI InChI=1S/C20H19Cl2N3O3/c1-27-19-7-6-13-15(20(19)28-12-4-2-3-5-12)11-25(26)24-18(13)8-14-16(21)9-23-10-17(14)22/h6-7,9-12H,2-5,8H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50069985
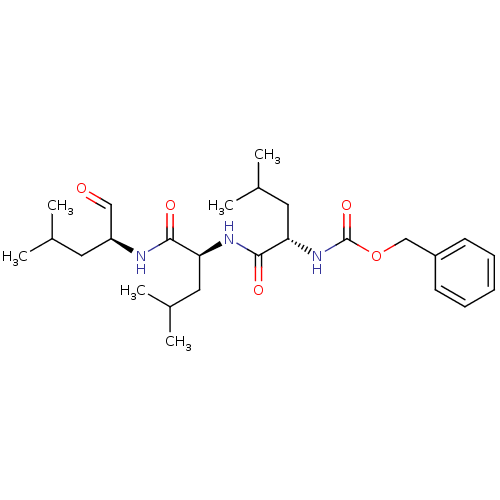
((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitman College
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Cbz-RR-AMC as substrate by fluorometric assay |
ACS Med Chem Lett 7: 250-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00401
BindingDB Entry DOI: 10.7270/Q2222WPD |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50304087
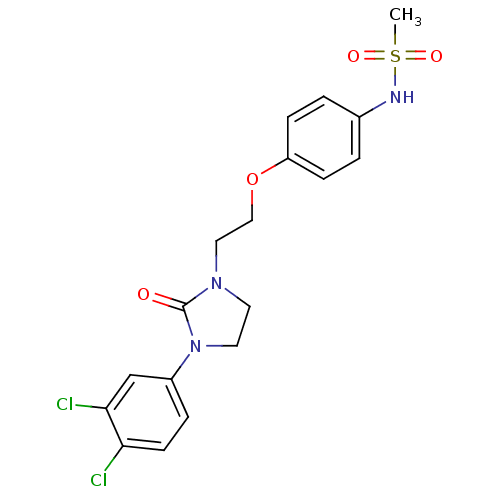
(CHEMBL596036 | N-(4-(2-(3-(3,4-Dichlorophenyl)-2-o...)Show SMILES CS(=O)(=O)Nc1ccc(OCCN2CCN(C2=O)c2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C18H19Cl2N3O4S/c1-28(25,26)21-13-2-5-15(6-3-13)27-11-10-22-8-9-23(18(22)24)14-4-7-16(19)17(20)12-14/h2-7,12,21H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membrane |
Bioorg Med Chem 17: 6463-80 (2009)
Article DOI: 10.1016/j.bmc.2009.05.085
BindingDB Entry DOI: 10.7270/Q2M61KB5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50304088

(CHEMBL596046 | N-(4-(2-(2-(3,4-Dichlorophenylamino...)Show SMILES CS(=O)(=O)Nc1ccc(OCCNCCNc2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C17H21Cl2N3O3S/c1-26(23,24)22-13-2-5-15(6-3-13)25-11-10-20-8-9-21-14-4-7-16(18)17(19)12-14/h2-7,12,20-22H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells |
Bioorg Med Chem 17: 6463-80 (2009)
Article DOI: 10.1016/j.bmc.2009.05.085
BindingDB Entry DOI: 10.7270/Q2M61KB5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50092344

(CHEMBL3586109)Show SMILES [H][C@@]12O[C@]1(C)CC[C@]1([H])[C@]2([H])c2c(O)cc(CCCCC)cc2OC1(C)C |r| Show InChI InChI=1S/C20H19Cl2N3O3/c1-27-19-7-6-13-15(20(19)28-12-4-2-3-5-12)11-25(26)24-18(13)8-14-16(21)9-23-10-17(14)22/h6-7,9-12H,2-5,8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 335 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM168618

(US9079852, Table F, Compound 3)Show SMILES CS(=O)(=O)Nc1ccc(OC[C@@H](O)CN2CCN(CC2)c2ccc(Cl)cc2)cc1 |r| Show InChI InChI=1S/C20H26ClN3O4S/c1-29(26,27)22-17-4-8-20(9-5-17)28-15-19(25)14-23-10-12-24(13-11-23)18-6-2-16(21)3-7-18/h2-9,19,22,25H,10-15H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 553 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc.
US Patent
| Assay Description
Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... |
US Patent US9079852 (2015)
BindingDB Entry DOI: 10.7270/Q2K35SF5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 578 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50519927

(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 649 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50069985

((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 653 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitman College
Curated by ChEMBL
| Assay Description
Inhibition of m-calpain (unknown origin) using Suc-LY-AMC as substrate by fluorometric assay |
ACS Med Chem Lett 7: 250-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00401
BindingDB Entry DOI: 10.7270/Q2222WPD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50566317
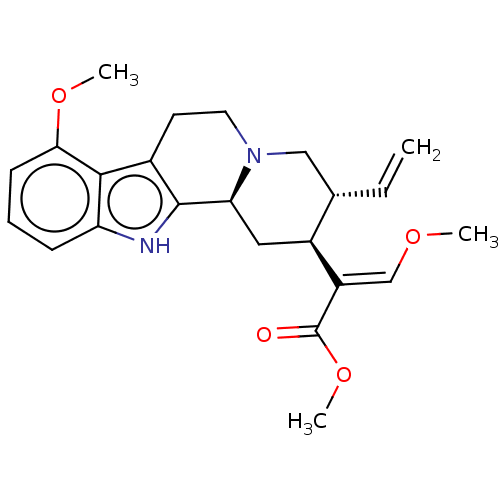
(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 666 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50304086
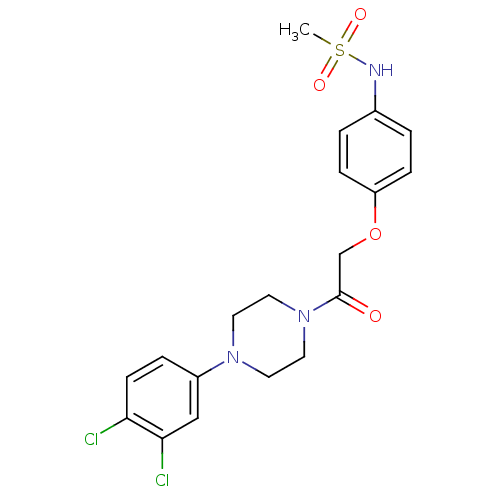
(CHEMBL594417 | N-(4-(2-(4-(3,4-Dichlorophenyl)pipe...)Show SMILES CS(=O)(=O)Nc1ccc(OCC(=O)N2CCN(CC2)c2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C19H21Cl2N3O4S/c1-29(26,27)22-14-2-5-16(6-3-14)28-13-19(25)24-10-8-23(9-11-24)15-4-7-17(20)18(21)12-15/h2-7,12,22H,8-11,13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 817 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membrane |
Bioorg Med Chem 17: 6463-80 (2009)
Article DOI: 10.1016/j.bmc.2009.05.085
BindingDB Entry DOI: 10.7270/Q2M61KB5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50092346

(CHEMBL3586107)Show SMILES [H][C@@]12CC=C(C)[C@@H](O)[C@@]1([H])c1c(O)cc(CCCCC)cc1OC2(C)C |r,t:3| Show InChI InChI=1S/C26H23Cl2N3O/c1-32-26-13-12-19-22(20(26)11-7-3-6-10-18-8-4-2-5-9-18)15-30-31-25(19)14-21-23(27)16-29-17-24(21)28/h2,4-5,7-9,11-13,15-17H,3,6,10,14H2,1H3/b11-7+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50566317

(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 883 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50092343

(CHEMBL3586110)Show SMILES [H][C@@]12CCC(COC(C)=O)=C[C@@]1([H])c1c(O)c(C(O)=O)c(CCCCC)cc1OC2(C)C |r,c:9| Show InChI InChI=1S/C24H32O6/c1-5-6-7-8-16-12-19-21(22(26)20(16)23(27)28)17-11-15(13-29-14(2)25)9-10-18(17)24(3,4)30-19/h11-12,17-18,26H,5-10,13H2,1-4H3,(H,27,28)/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM168620
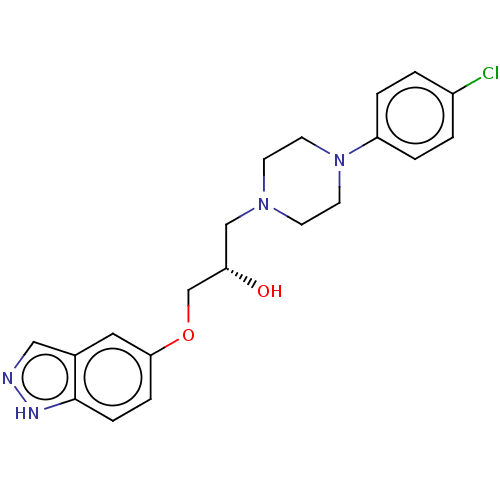
(US9079852, Table F, Compound 5)Show SMILES O[C@H](COc1ccc2[nH]ncc2c1)CN1CCN(CC1)c1ccc(Cl)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc.
US Patent
| Assay Description
Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... |
US Patent US9079852 (2015)
BindingDB Entry DOI: 10.7270/Q2K35SF5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50519927

(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609011
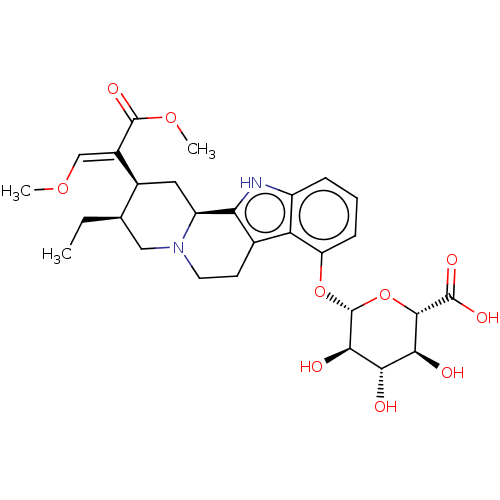
(CHEMBL5287921)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)c12)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM168617
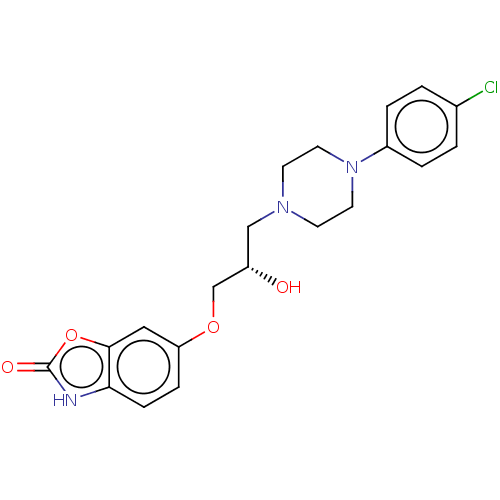
(US9079852, Table F, Compound 2)Show SMILES O[C@H](COc1ccc2[nH]c(=O)oc2c1)CN1CCN(CC1)c1ccc(Cl)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc.
US Patent
| Assay Description
Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... |
US Patent US9079852 (2015)
BindingDB Entry DOI: 10.7270/Q2K35SF5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50609011

(CHEMBL5287921)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50169089
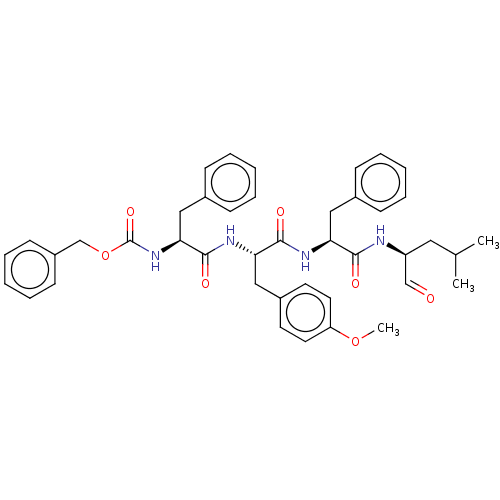
(CHEMBL3804980)Show SMILES COc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CC(C)C)C=O)cc1 |r| Show InChI InChI=1S/C42H48N4O7/c1-29(2)23-34(27-47)43-39(48)36(24-30-13-7-4-8-14-30)44-40(49)37(26-32-19-21-35(52-3)22-20-32)45-41(50)38(25-31-15-9-5-10-16-31)46-42(51)53-28-33-17-11-6-12-18-33/h4-22,27,29,34,36-38H,23-26,28H2,1-3H3,(H,43,48)(H,44,49)(H,45,50)(H,46,51)/t34-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitman College
Curated by ChEMBL
| Assay Description
Inhibition of m-calpain (unknown origin) using Suc-LY-AMC as substrate by fluorometric assay |
ACS Med Chem Lett 7: 250-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00401
BindingDB Entry DOI: 10.7270/Q2222WPD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50092348
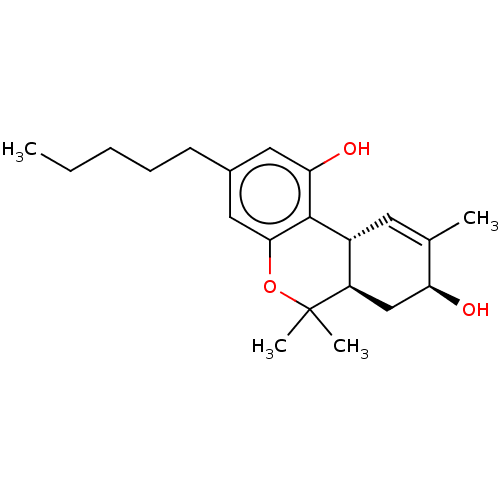
(CHEMBL3586104)Show SMILES [H][C@@]12C=C(C)[C@@H](O)C[C@@]1([H])C(C)(C)Oc1cc(CCCCC)cc(O)c21 |r,t:2| Show InChI InChI=1S/C26H21Cl2N3O/c1-32-26-13-12-19-22(20(26)11-7-3-6-10-18-8-4-2-5-9-18)15-30-31-25(19)14-21-23(27)16-29-17-24(21)28/h2,4-5,8-9,12-13,15-17H,3,6,10,14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... |
J Nat Prod 78: 1271-6 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00065
BindingDB Entry DOI: 10.7270/Q2SQ923K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data