

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM209932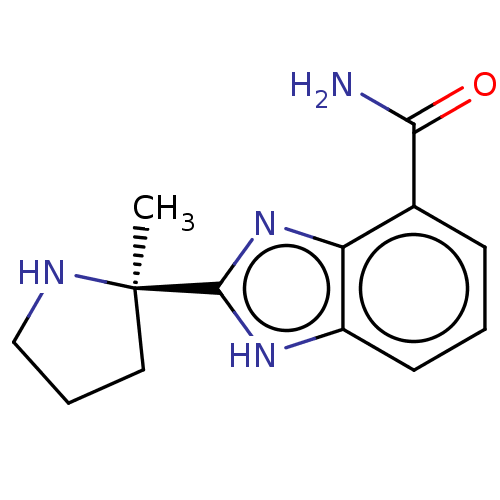 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled estrogen receptor 1 (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Chemical Diversity Center (UP-CDC) Curated by ChEMBL | Assay Description Inhibition of estrogen binding to GPR30 (unknown origin) | J Med Chem 56: 7161-76 (2013) Article DOI: 10.1021/jm400132d BindingDB Entry DOI: 10.7270/Q2V69M09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled estrogen receptor 1 (Homo sapiens (Human)) | BDBM50303803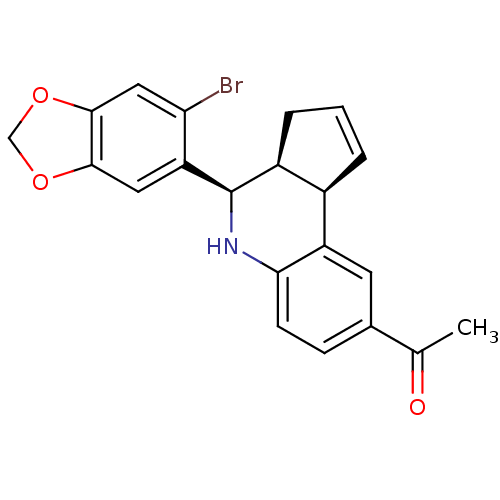 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Chemical Diversity Center (UP-CDC) Curated by ChEMBL | Assay Description Inhibition of estrogen binding to GPR30 (unknown origin) | J Med Chem 56: 7161-76 (2013) Article DOI: 10.1021/jm400132d BindingDB Entry DOI: 10.7270/Q2V69M09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242333 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260295 (2-((S)-2-(2-((S)-2-((S)-2-((S)-6-amino-2-((2S,3R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260294 ((S)-2-((S)-6-amino-2-((2S,3R)-2-((S)-2-((S)-5-guan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384440 (CHEMBL2035505 | CHEMBL2037389) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50302031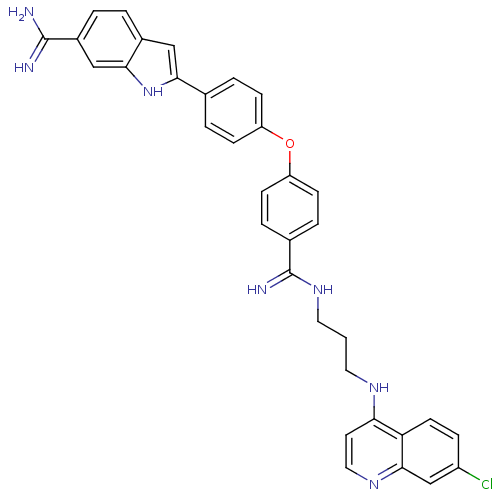 (2-(4-(4-(N'-(3-(7-chloroquinolin-4-ylamino)propyl)...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50302032 (2-(4-(4-carbamimidoylphenoxy)phenyl)-N'-(3-(7-chlo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242337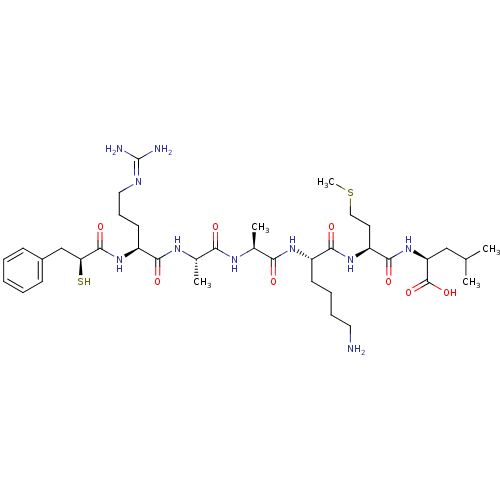 ((S)-2-{(S)-2-[(S)-6-Amino-2-((S)-2-{(S)-2-[(S)-5-g...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242339 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50558084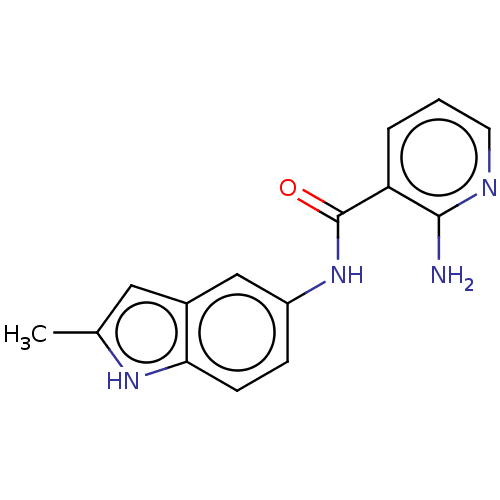 (CHEMBL4799933) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 20 uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00396 BindingDB Entry DOI: 10.7270/Q2930XVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384439 (CHEMBL2035506) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50084504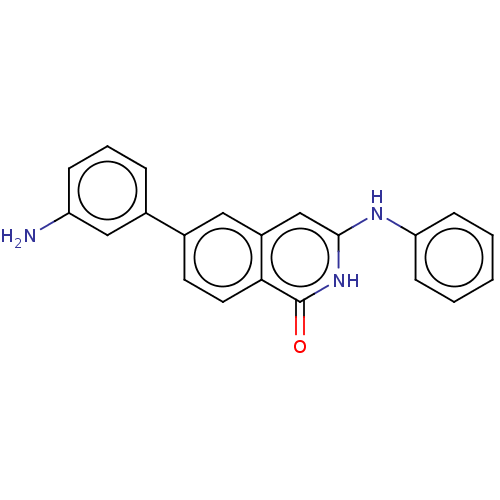 (CHEMBL3426991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Curated by ChEMBL | Assay Description Competitive inhibition of His6-tagged human recombinant Cdc25B catalytic domain (350 to 566 residues) expressed in Escherichia coli BL21 (D3) after 2... | Bioorg Med Chem 23: 2810-8 (2015) Article DOI: 10.1016/j.bmc.2015.01.043 BindingDB Entry DOI: 10.7270/Q2CJ8G62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260296 (CHEMBL501525 | CRATKML) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242336 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384441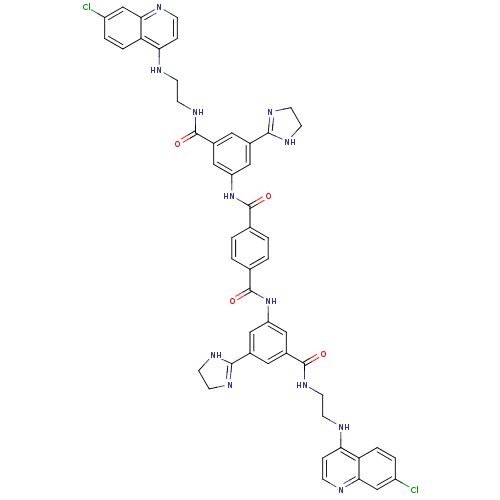 (CHEMBL2035503) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242338 ((S)-2-{(S)-2-[(S)-2-((2S,3R)-2-{(S)-2-[(S)-5-Guani...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50240902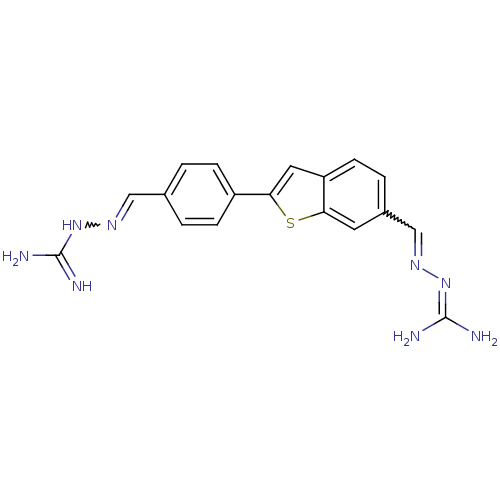 ((2E)-2-{4-[6-((E)-{[(E)-amino(imino)methyl]hydrazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50558084 (CHEMBL4799933) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 20 uM A... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00396 BindingDB Entry DOI: 10.7270/Q2930XVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260293 ((S)-6-amino-2-((2S,3R)-2-((S)-2-((S)-5-guanidino-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50096680 ((E)-2-(2-Chloro-phenyl)-ethenesulfonic acid [4-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant Cdc25B phosphatase enzyme | Bioorg Med Chem Lett 11: 313-7 (2001) BindingDB Entry DOI: 10.7270/Q2DR2TRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50240901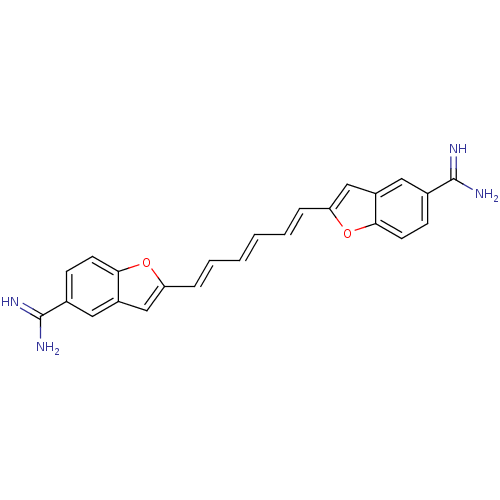 (2-((1E,3E,5E)-6-{5-[(E)-amino(imino)methyl]-1-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242334 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384438 (CHEMBL2035504) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50100895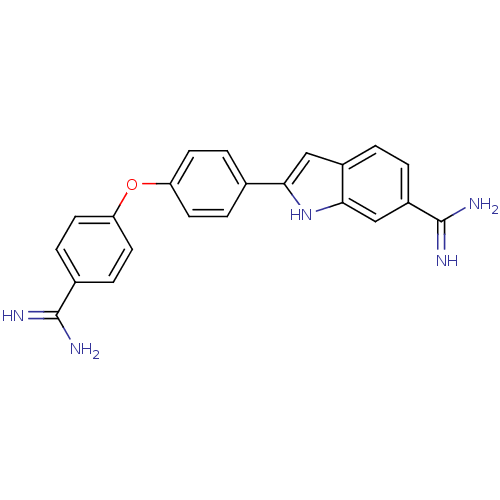 (2-(4-(4-carbamimidoylphenoxy)phenyl)-1H-indole-6-c...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260300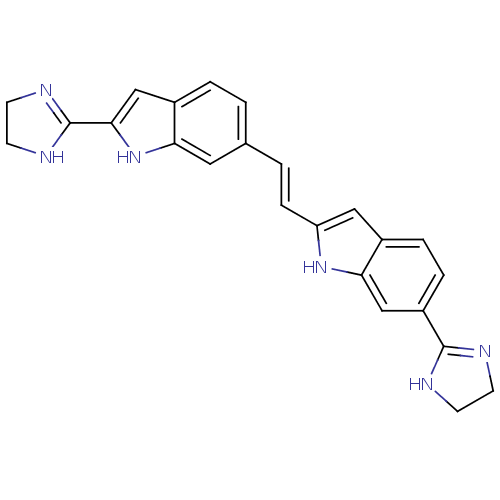 (6-(4,5-dihydro-1H-imidazol-2-yl)-2-(2-(2-(4,5-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260292 ((2S,3R)-2-{(S)-2-[(S)-5-Guanidino-2-((S)-2-mercapt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50096693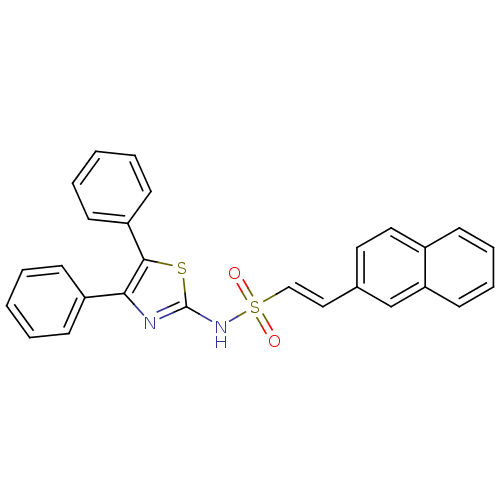 ((E)-2-Naphthalen-2-yl-ethenesulfonic acid (4,5-dip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant Cdc25B phosphatase enzyme | Bioorg Med Chem Lett 11: 313-7 (2001) BindingDB Entry DOI: 10.7270/Q2DR2TRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260291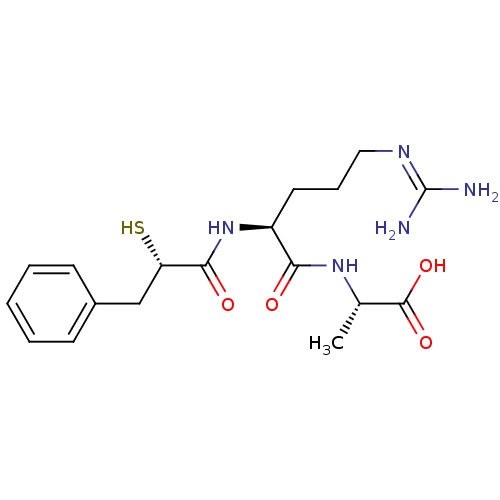 ((S)-2-[(S)-5-Guanidino-2-((S)-2-mercapto-3-phenyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242311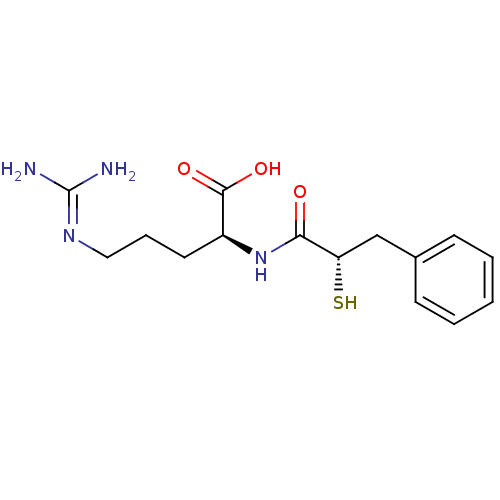 ((S)-5-Guanidino-2-((S)-2-mercapto-3-phenyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242335 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM209932 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptide peptidase-like 2A (Homo sapiens) | BDBM50262993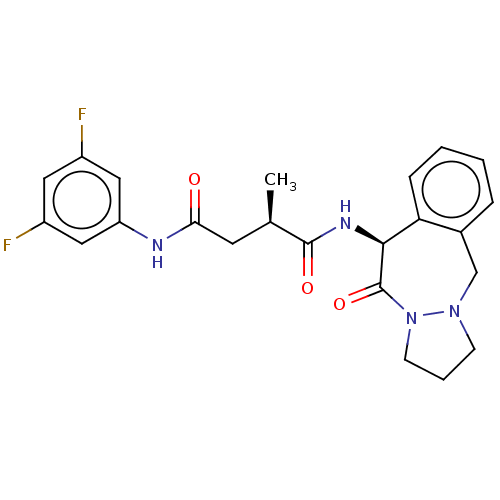 (CHEMBL4059711) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation , 10675 John Jay Hopkins Drive, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Inhibition of human SPPL2a expressed in human U2OS cells using EGFP-labeled TNFalpha (1 to 76 residues) NTF as substrate after 24 hrs by Hoechst stai... | J Med Chem 61: 865-880 (2018) Article DOI: 10.1021/acs.jmedchem.7b01371 BindingDB Entry DOI: 10.7270/Q2JM2D49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptide peptidase-like 2A (Homo sapiens) | BDBM50263008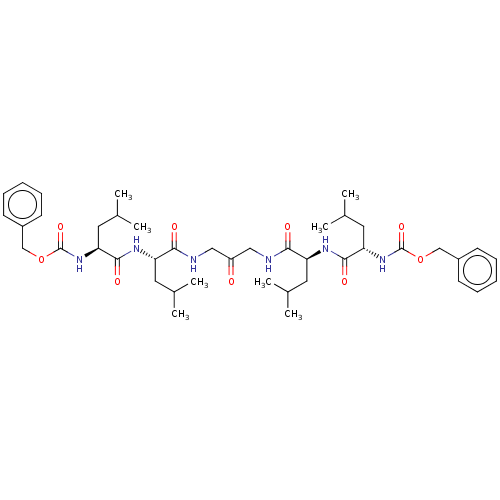 (CHEMBL255473) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation , 10675 John Jay Hopkins Drive, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Inhibition of human SPPL2a expressed in human U2OS cells using EGFP-labeled TNFalpha (1 to 76 residues) NTF as substrate after 24 hrs by Hoechst stai... | J Med Chem 61: 865-880 (2018) Article DOI: 10.1021/acs.jmedchem.7b01371 BindingDB Entry DOI: 10.7270/Q2JM2D49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50514008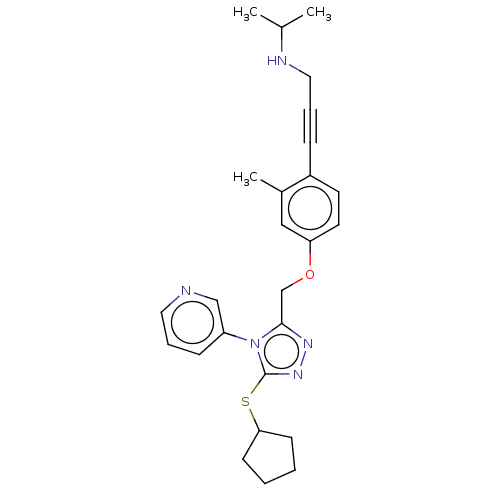 (CHEMBL4450581 | US10894782, No 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50514008 (CHEMBL4450581 | US10894782, No 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of p97 (unknown origin) assessed as reduction in ATPase activity by biomol green reagent based assay | J Med Chem 63: 1892-1907 (2020) Article DOI: 10.1021/acs.jmedchem.9b01318 BindingDB Entry DOI: 10.7270/Q2N58QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50514004 (CHEMBL4476693) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of p97 (unknown origin) assessed as reduction in ATPase activity by biomol green reagent based assay | J Med Chem 63: 1892-1907 (2020) Article DOI: 10.1021/acs.jmedchem.9b01318 BindingDB Entry DOI: 10.7270/Q2N58QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478271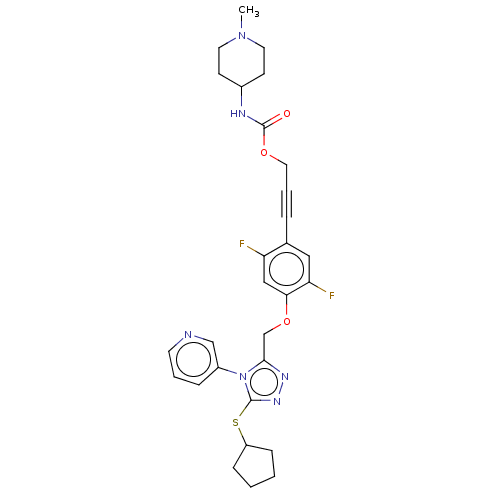 (US10894782, No 124) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptide peptidase-like 2A (Homo sapiens) | BDBM50241259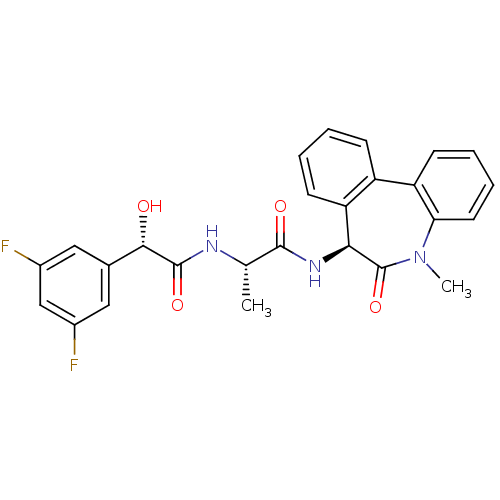 ((2S)-2-((S)-2-(3,5-difluorophenyl)-2-hydroxyacetam...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation , 10675 John Jay Hopkins Drive, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Inhibition of human SPPL2a expressed in human U2OS cells using EGFP-labeled TNFalpha (1 to 76 residues) NTF as substrate after 24 hrs by Hoechst stai... | J Med Chem 61: 865-880 (2018) Article DOI: 10.1021/acs.jmedchem.7b01371 BindingDB Entry DOI: 10.7270/Q2JM2D49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50424917 (CHEMBL2311578) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 100 uM ATP as subst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00396 BindingDB Entry DOI: 10.7270/Q2930XVT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478177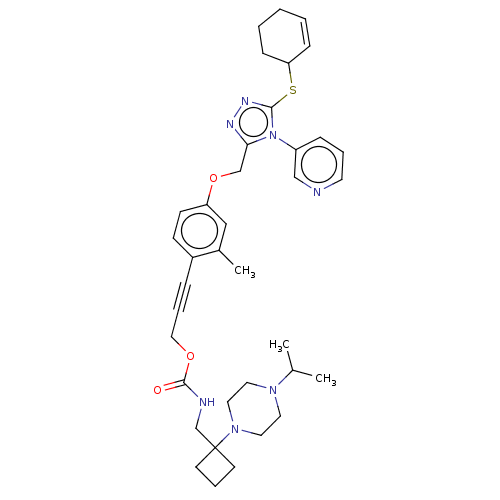 (US10894782, No 30) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478266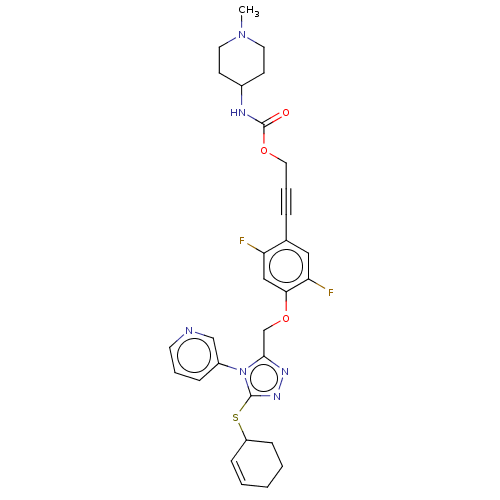 (US10894782, No 119) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478267 (US10894782, No 120) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478224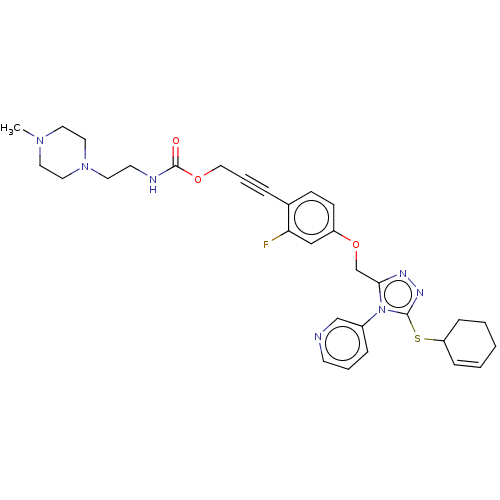 (US10894782, No 107 | US10894782, No 77) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50468106 (CHEMBL4280801 | US11247985, Table 3.49) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... | Citation and Details BindingDB Entry DOI: 10.7270/Q25Q509B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM536747 (US11247985, Table 3.51) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... | Citation and Details BindingDB Entry DOI: 10.7270/Q25Q509B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478222 (US10894782, No 108 | US10894782, No 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478272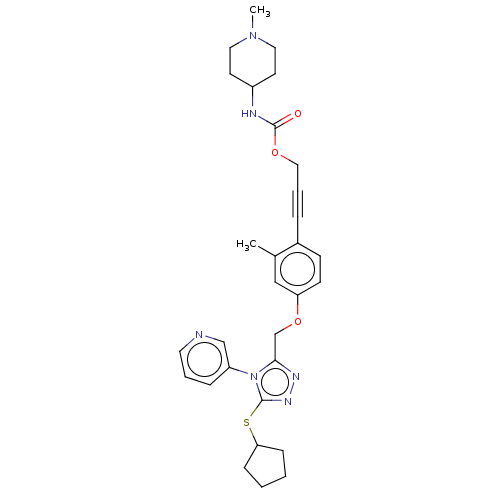 (US10894782, No 125) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478273 (US10894782, No 126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1378 total ) | Next | Last >> |