

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141067 (3-[4-(2,4-Difluoro-phenoxy)-phenyl]-pyrazole-1-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141062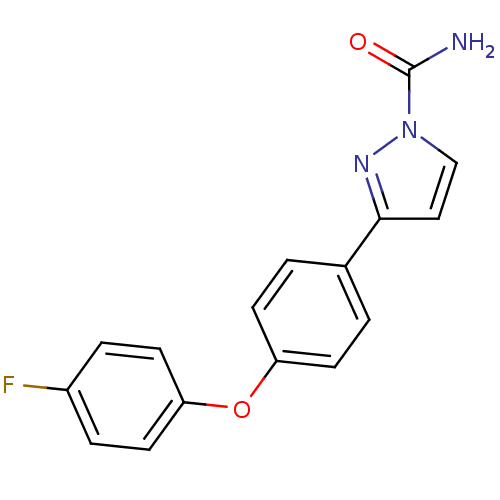 (3-[4-(4-Fluoro-phenoxy)-phenyl]-pyrazole-1-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141070 (3-[4-(4-Nitro-phenoxy)-phenyl]-pyrazole-1-carboxyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141072 (3-(4-Phenoxy-phenyl)-pyrazole-1-carboxylic acid am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141065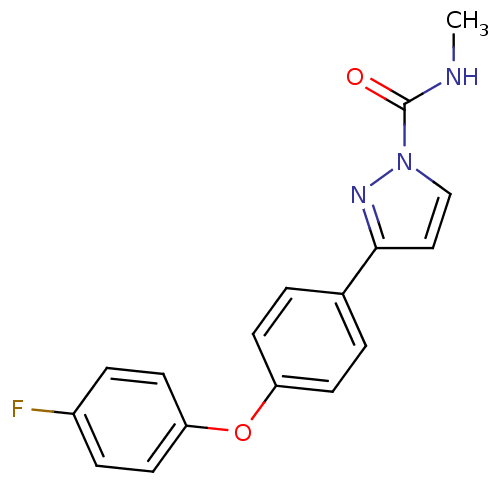 (3-[4-(4-Fluoro-phenoxy)-phenyl]-pyrazole-1-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141073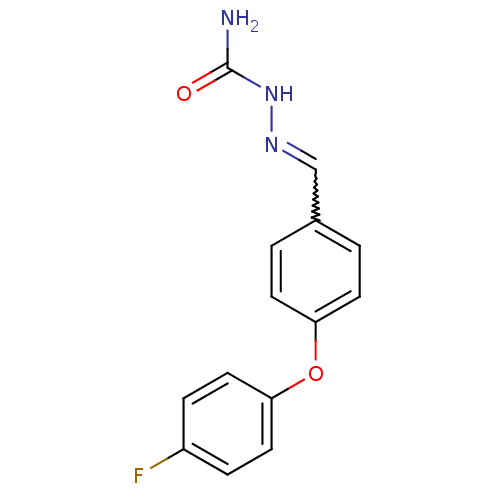 ((E)-2-(4-(4-fluorophenoxy)benzylidene)hydrazinecar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141066 (3-[4-(4-Fluoro-phenoxy)-phenyl]-1H-pyrazole | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141064 (3-(4-Phenoxy-phenyl)-1H-pyrazole | CHEMBL41864) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141071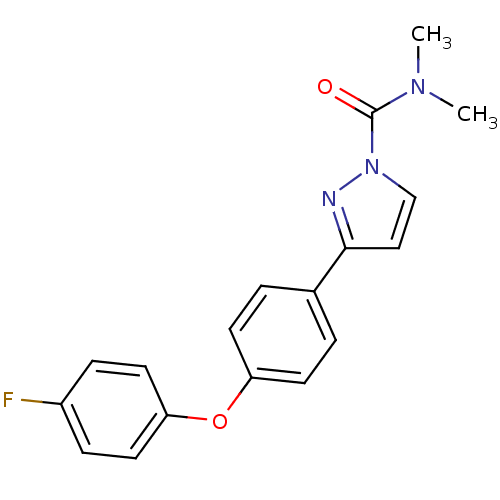 (3-[4-(4-Fluoro-phenoxy)-phenyl]-pyrazole-1-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141069 (3-[4-(4-Fluoro-phenoxy)-phenyl]-1-methyl-1H-pyrazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50031299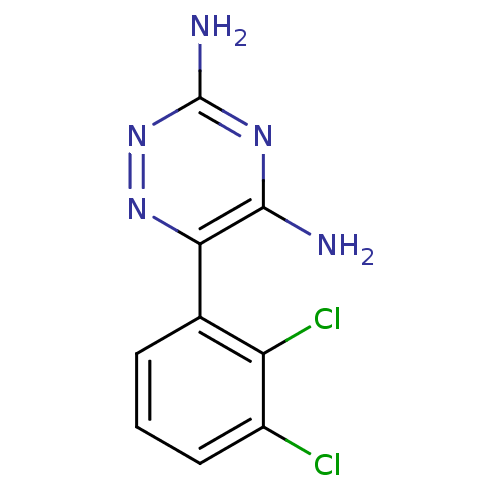 (6-(2,3-Dichloro-phenyl)-[1,2,4]triazine-3,5-diamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50003655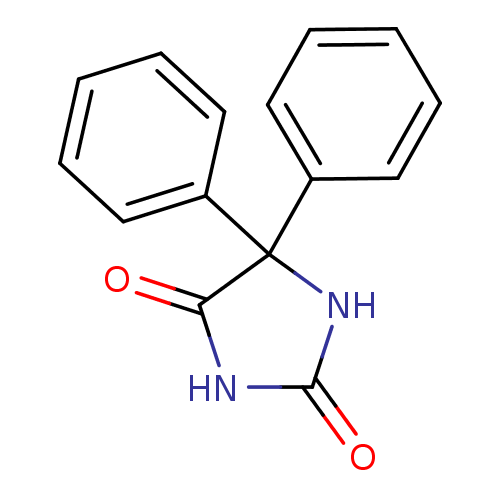 (Phenytoin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50003659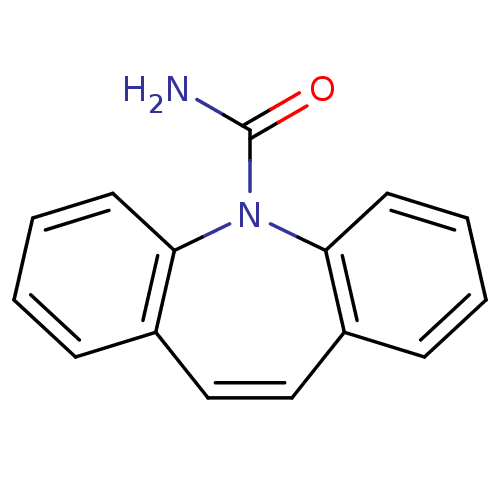 (5H-dibenzo[b,f]azepine-5-carboxamide | CARBAMAZEPI...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50078831 (3-Phenyl-1H-pyrazole | CHEMBL38876) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 4 subunit alpha (Homo sapiens (Human)) | BDBM50141063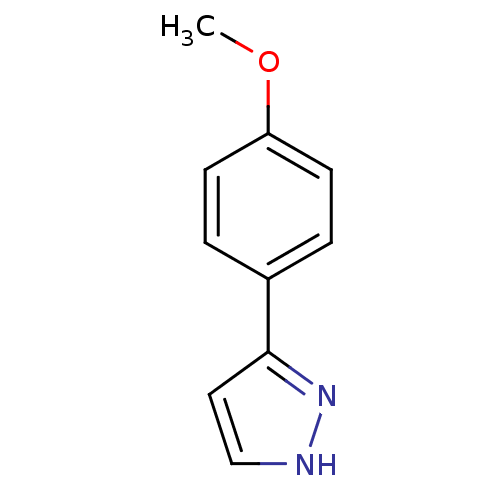 (3-(4-Methoxy-phenyl)-1H-pyrazole | CHEMBL39210) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. Curated by ChEMBL | Assay Description Affinity for inactive human SkM1 sodium channel expressed in HEK293 cells | J Med Chem 47: 1547-52 (2004) Article DOI: 10.1021/jm030498q BindingDB Entry DOI: 10.7270/Q2SX6F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50450704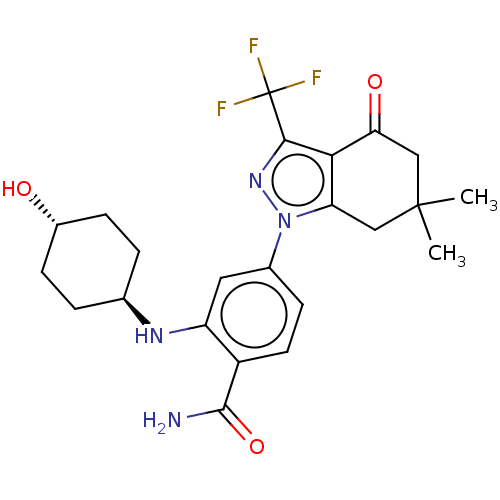 (CHEMBL560895 | SNX-2112) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human A375 cells assessed as pS6 degradation after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50450704 (CHEMBL560895 | SNX-2112) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human A375 cells assessed as Hsp70 induction after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50008057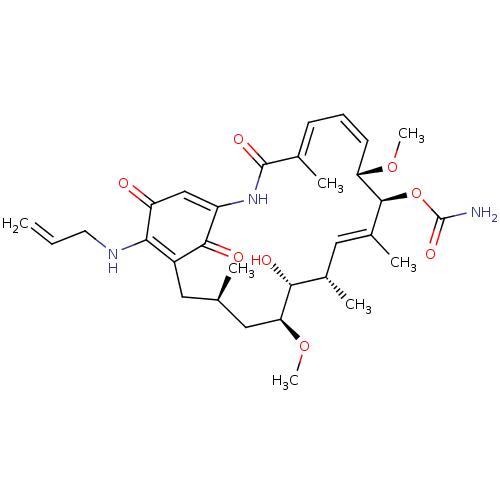 (BMS-722782 | CHEBI:64153 | TANESPIMYCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human AU565 cells assessed as Her2 degradation after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50008057 (BMS-722782 | CHEBI:64153 | TANESPIMYCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human A375 cells assessed as Hsp70 induction after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50056605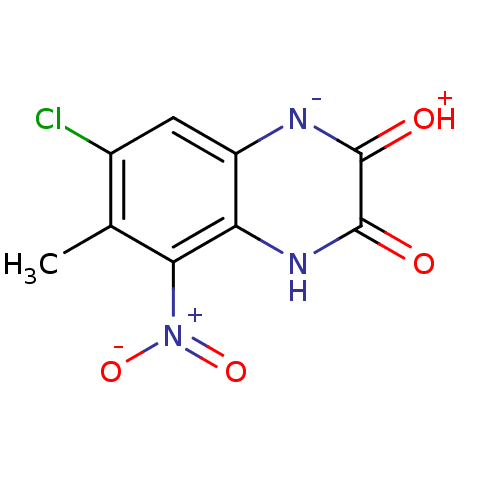 (7-Chloro-6-methyl-5-nitro-1,4-dihydro-quinoxaline-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc. Curated by ChEMBL | Assay Description Potency for the N-methyl-D-aspartate glutamate receptor 1 glycine site by displacement of [3H]5,7-dichlorokynurenic acid (DCKA) binding in rat brain ... | J Med Chem 40: 730-8 (1997) Article DOI: 10.1021/jm960654b BindingDB Entry DOI: 10.7270/Q2NZ86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091637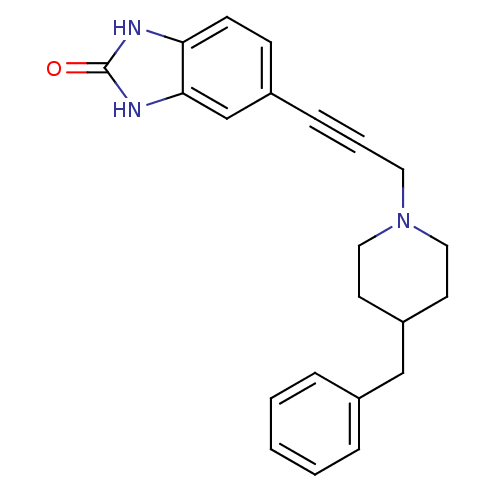 (5-(3-(4-benzylpiperidin-1-yl)prop-1-ynyl)-1H-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50038161 (6,7-Dichloro-3-hydroxy-5-nitro-3,4-dihydro-1H-quin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc. Curated by ChEMBL | Assay Description Potency for the N-methyl-D-aspartate glutamate receptor 1 glycine site by displacement of [3H]5,7-dichlorokynurenic acid (DCKA) binding in rat brain ... | J Med Chem 40: 730-8 (1997) Article DOI: 10.1021/jm960654b BindingDB Entry DOI: 10.7270/Q2NZ86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091635 (5-{3-[4-(3-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50052635 (5,6,7-Trichloro-1H-quinoline-2,3,4-trione 3-oxime ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes | J Med Chem 39: 3248-55 (1996) Article DOI: 10.1021/jm960214k BindingDB Entry DOI: 10.7270/Q2QC02KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50480378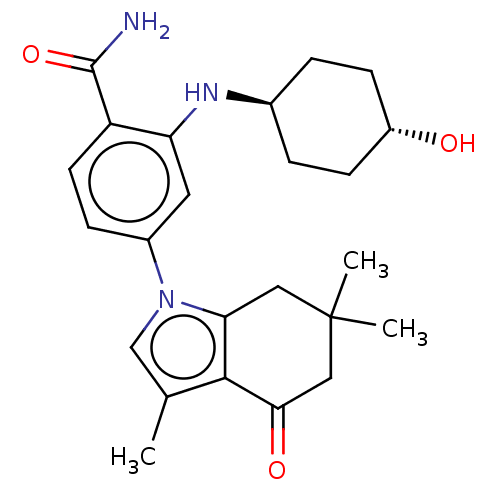 (CHEMBL563608) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human A375 cells assessed as pS6 degradation after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50008057 (BMS-722782 | CHEBI:64153 | TANESPIMYCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human AU565 cells assessed as pERK degradation after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50066544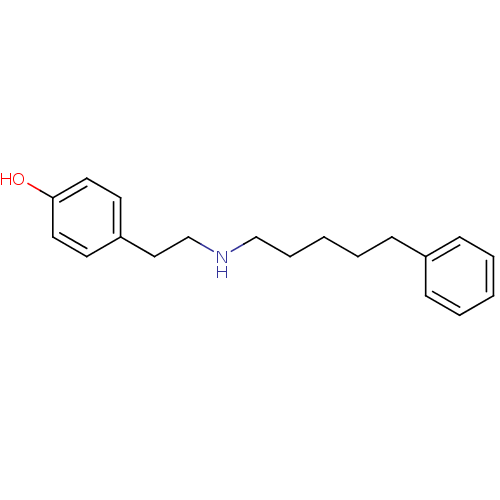 (4-[2-(5-Phenyl-pentylamino)-ethyl]-phenol | CHEMBL...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor | J Med Chem 41: 3499-506 (1998) Article DOI: 10.1021/jm980235+ BindingDB Entry DOI: 10.7270/Q29Z95MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091636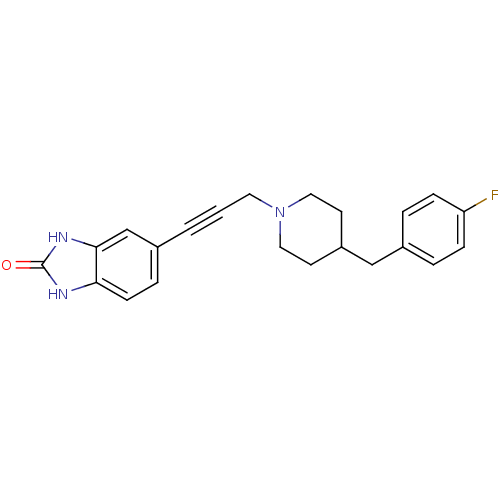 (5-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50056596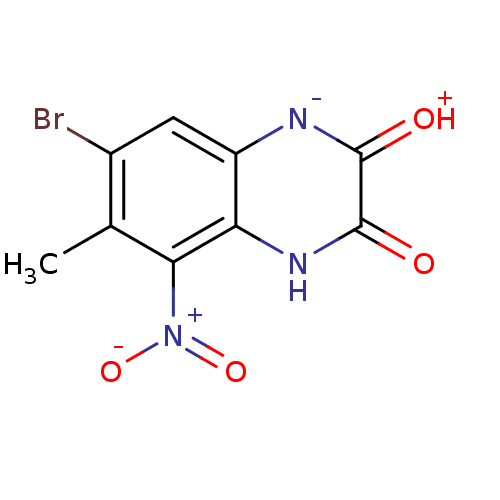 (7-Bromo-6-methyl-5-nitro-1,4-dihydro-quinoxaline-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc. Curated by ChEMBL | Assay Description Potency for the N-methyl-D-aspartate glutamate receptor 1 glycine site by displacement of [3H]5,7-dichlorokynurenic acid (DCKA) binding in rat brain ... | J Med Chem 40: 730-8 (1997) Article DOI: 10.1021/jm960654b BindingDB Entry DOI: 10.7270/Q2NZ86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50080029 (4-((1R,2S)-3-(4-benzylpiperidin-1-yl)-1-hydroxy-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Antagonistic activity against N-methyl-D-aspartate glutamate receptor 1/2B. | J Med Chem 42: 3412-20 (1999) Article DOI: 10.1021/jm990199u BindingDB Entry DOI: 10.7270/Q2RR1XFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50080029 (4-((1R,2S)-3-(4-benzylpiperidin-1-yl)-1-hydroxy-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Antagonist activity against rat 1A/2B subtype of N-methyl-D-aspartate(NMDA) receptor in xenopus oocytes. | Bioorg Med Chem Lett 9: 1619-24 (1999) BindingDB Entry DOI: 10.7270/Q29022ZJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50369783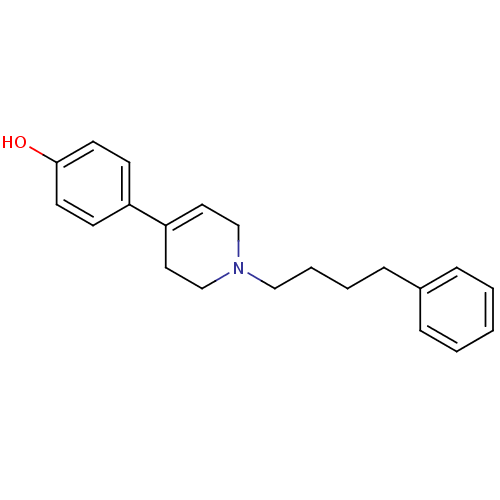 (CHEMBL1203902) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus oocytes expressing rat N-Methyl-D-aspartate (NR1A/2B) Receptor subtype. | J Med Chem 43: 984-94 (2000) BindingDB Entry DOI: 10.7270/Q20G3KVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50480379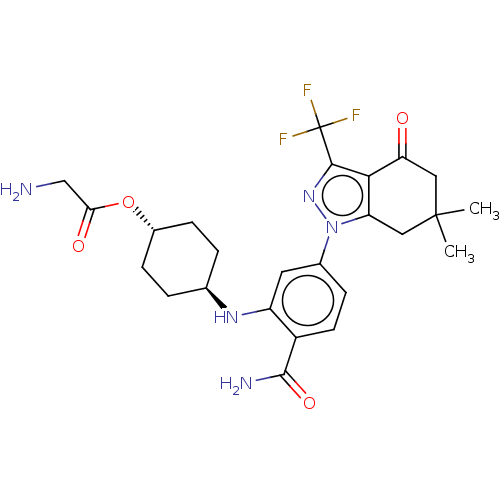 (CHEMBL553939 | SNX-5422) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human AU565 cells assessed as pERK degradation after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50085922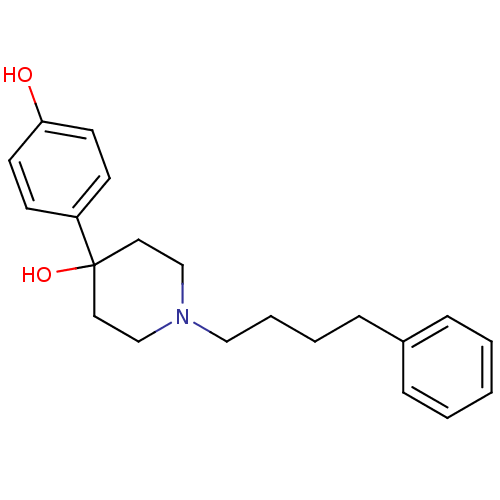 (4-(4-Hydroxy-phenyl)-1-(4-phenyl-butyl)-piperidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus oocytes expressing rat N-Methyl-D-aspartate (NR1A/2B) Receptor subtype. | J Med Chem 43: 984-94 (2000) BindingDB Entry DOI: 10.7270/Q20G3KVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50052629 (6,7-Dichloro-1H-quinoline-2,3,4-trione 3-oxime | C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- DCKA binding to NMDA receptor of rat brain membranes | J Med Chem 39: 3248-55 (1996) Article DOI: 10.1021/jm960214k BindingDB Entry DOI: 10.7270/Q2QC02KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50480379 (CHEMBL553939 | SNX-5422) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human A375 cells assessed as Hsp70 induction after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50480378 (CHEMBL563608) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human A375 cells assessed as Hsp70 induction after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50066538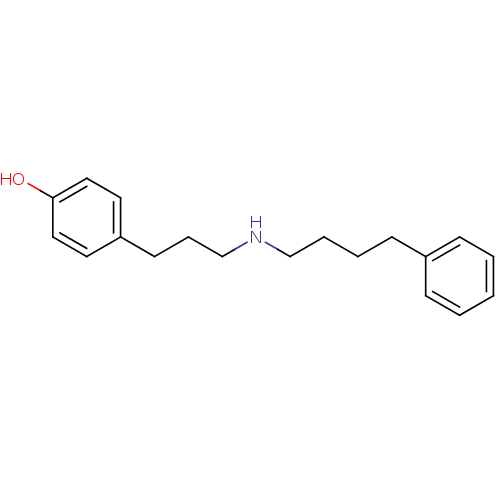 (4-[3-(4-Phenyl-butylamino)-propyl]-phenol | CHEMBL...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Functional antagonism by electrical assays in Xenopus oocytes expressing the 1A/2B NMDA receptor | J Med Chem 41: 3499-506 (1998) Article DOI: 10.1021/jm980235+ BindingDB Entry DOI: 10.7270/Q29Z95MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365264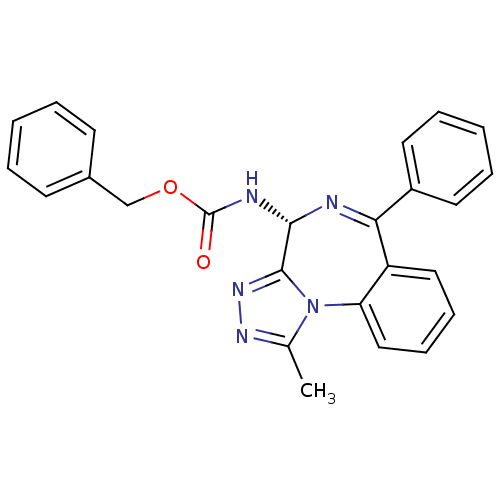 (CHEMBL1738926) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of tetra-acetylated H4 peptide from human Brd4 bromodomain BD12 after 1 hr by FRET analysis | J Med Chem 54: 3827-38 (2011) Article DOI: 10.1021/jm200108t BindingDB Entry DOI: 10.7270/Q2GB2541 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091647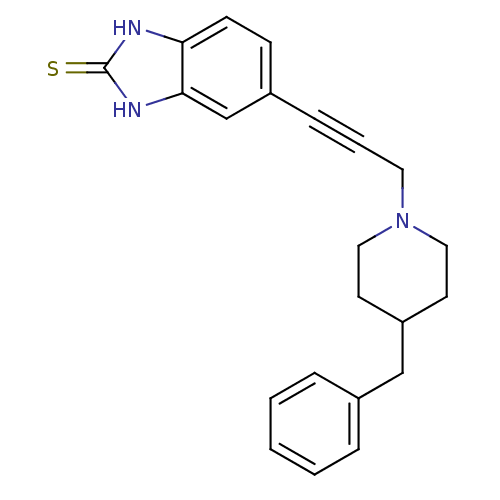 (5-[3-(4-Benzyl-piperidin-1-yl)-prop-1-ynyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50480378 (CHEMBL563608) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Serenex Inc Curated by ChEMBL | Assay Description Inhibition of Hsp90 in human AU565 cells assessed as pERK degradation after 24 hrs by high content screening | J Med Chem 52: 4288-305 (2009) Article DOI: 10.1021/jm900230j BindingDB Entry DOI: 10.7270/Q2MK6GQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50085933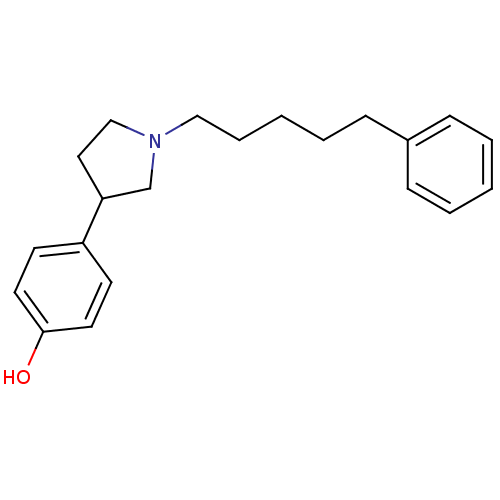 (4-[1-(5-Phenyl-pentyl)-pyrrolidin-3-yl]-phenol | C...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus oocytes expressing rat N-Methyl-D-aspartate (NR1A/2B) Receptor subtype. | J Med Chem 43: 984-94 (2000) BindingDB Entry DOI: 10.7270/Q20G3KVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091632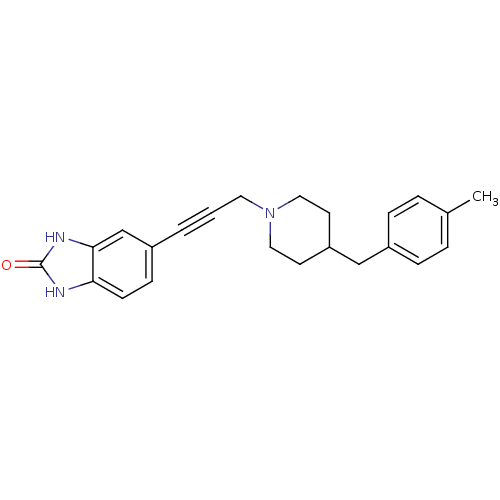 (5-{3-[4-(4-Methyl-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50369786 (CHEMBL1201889) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus oocytes expressing rat N-Methyl-D-aspartate (NR1A/2B) Receptor subtype. | J Med Chem 43: 984-94 (2000) BindingDB Entry DOI: 10.7270/Q20G3KVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50085927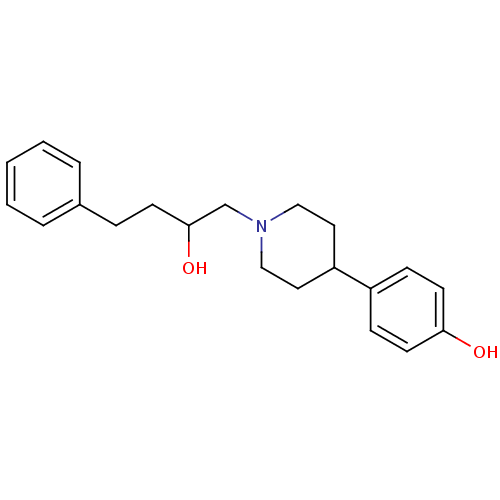 (4-[1-(2-Hydroxy-4-phenyl-butyl)-piperidin-4-yl]-ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus oocytes expressing rat N-Methyl-D-aspartate (NR1A/2B) Receptor subtype. | J Med Chem 43: 984-94 (2000) BindingDB Entry DOI: 10.7270/Q20G3KVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091626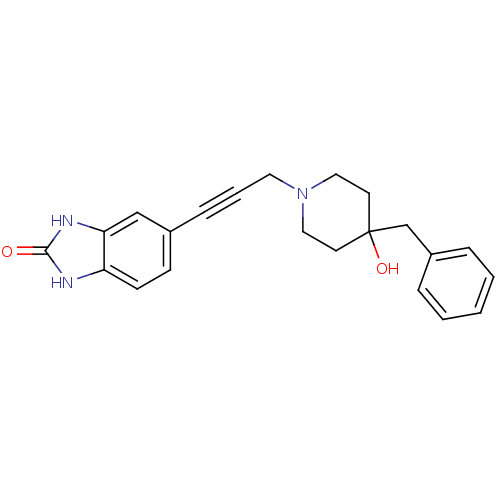 (5-[3-(4-Benzyl-4-hydroxy-piperidin-1-yl)-prop-1-yn...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091643 (5-{3-[4-(4-Chloro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50066538 (4-[3-(4-Phenyl-butylamino)-propyl]-phenol | CHEMBL...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Antagonist activity against rat 1A/2B subtype of N-methyl-D-aspartate(NMDA) receptor in xenopus oocytes. | Bioorg Med Chem Lett 9: 1619-24 (1999) BindingDB Entry DOI: 10.7270/Q29022ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50056618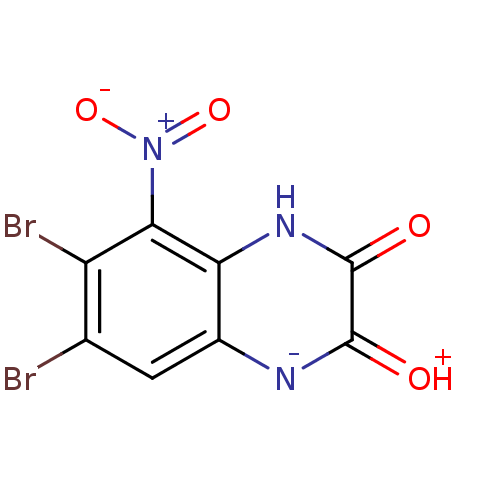 (6,7-Dibromo-5-nitro-1,4-dihydro-quinoxaline-2,3-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys Inc. Curated by ChEMBL | Assay Description Potency for the N-methyl-D-aspartate glutamate receptor 1 glycine site by displacement of [3H]5,7-dichlorokynurenic acid (DCKA) binding in rat brain ... | J Med Chem 40: 730-8 (1997) Article DOI: 10.1021/jm960654b BindingDB Entry DOI: 10.7270/Q2NZ86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50369791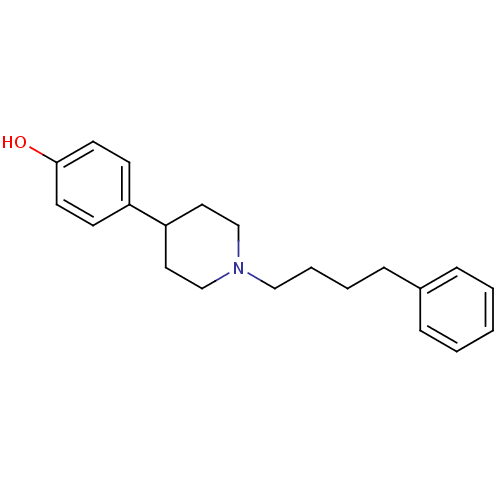 (CHEMBL1202296) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus oocytes expressing rat N-Methyl-D-aspartate (NR1A/2B) Receptor subtype. | J Med Chem 43: 984-94 (2000) BindingDB Entry DOI: 10.7270/Q20G3KVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 701 total ) | Next | Last >> |