

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50077669 ((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077678 ((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077678 ((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124179 (US8754075, 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124167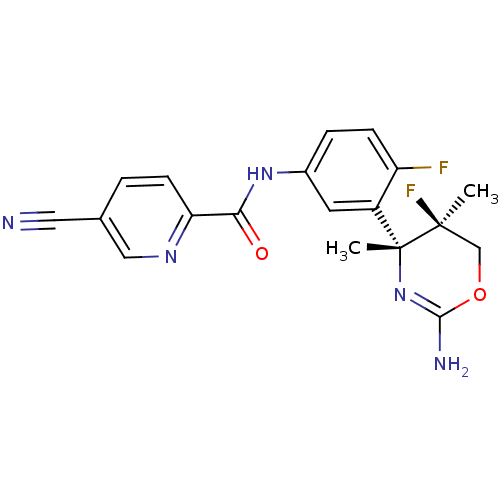 (US8754075, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124132 (US8754075, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077702 (4-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077693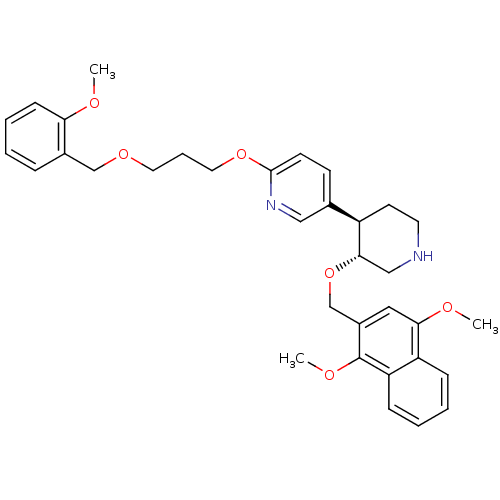 ((3'R,4'R)-3'-(1,4-Dimethoxy-naphthalen-2-ylmethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124133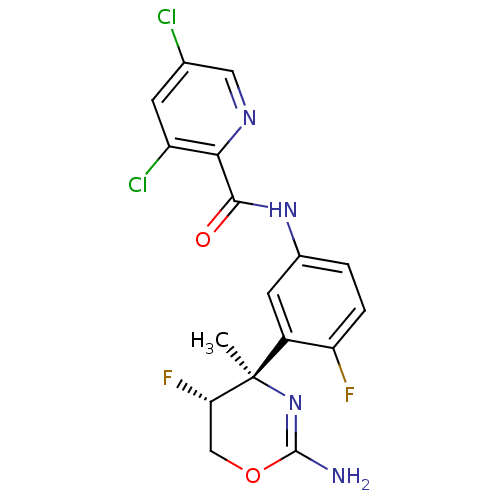 (US8754075, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077699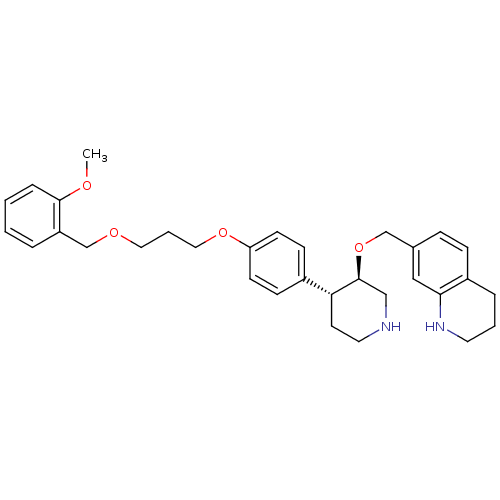 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077692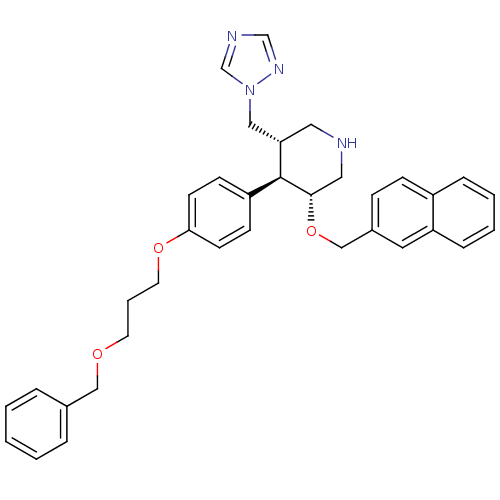 ((3R,4R,5S)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432614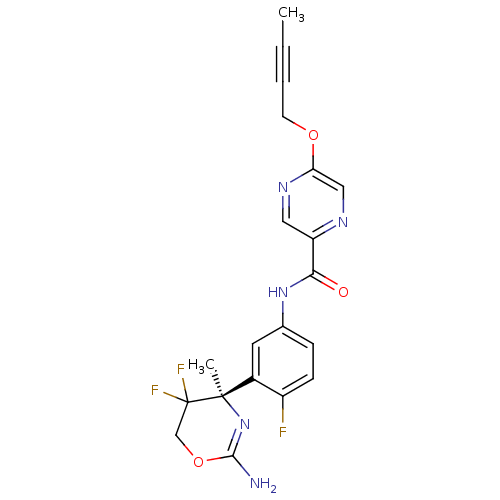 (CHEMBL2347198) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432615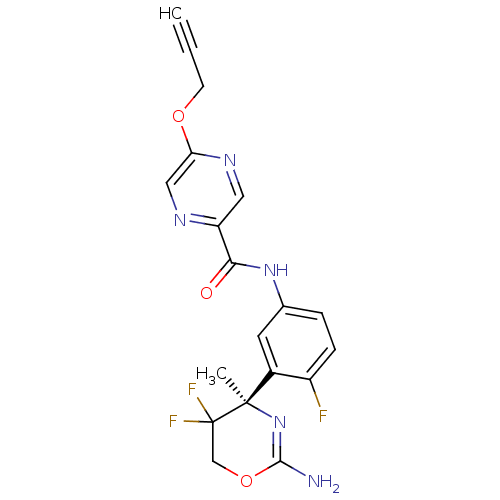 (CHEMBL2347197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432632 (CHEMBL2347211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432608 (CHEMBL2347204 | US8754075, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432608 (CHEMBL2347204 | US8754075, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM124141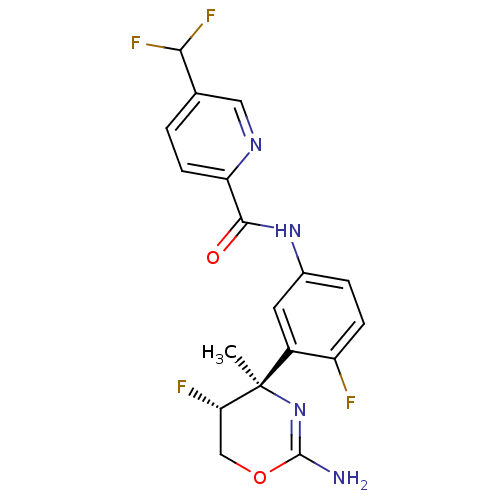 (US8754075, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124134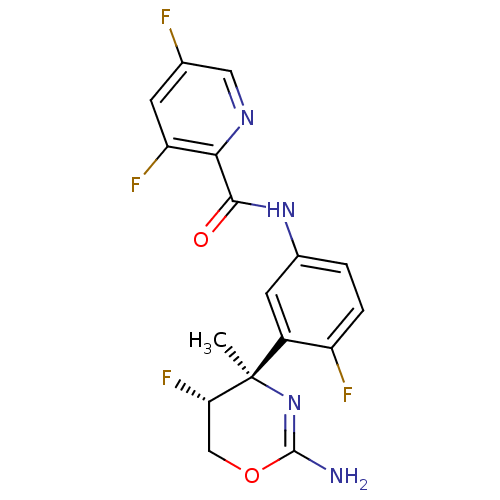 (US8754075, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124135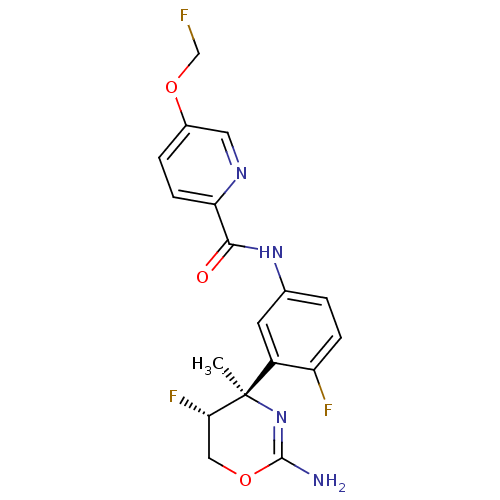 (US8754075, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124174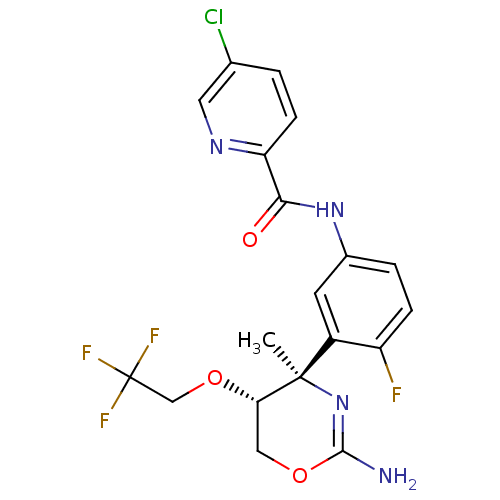 (US8754075, 45) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124176 (US8754075, 47) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM124132 (US8754075, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077688 ((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077703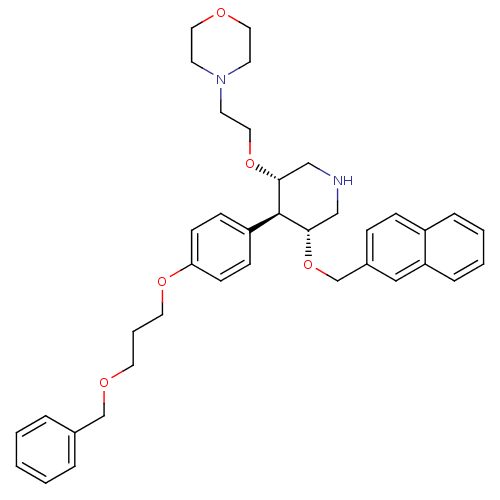 (4-{2-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432601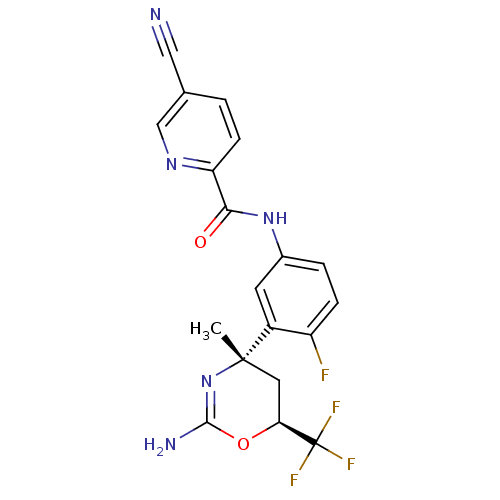 (CHEMBL2347208 | US9296734, 102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124137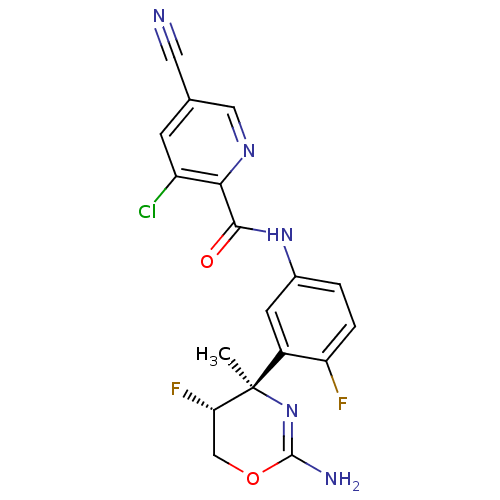 (US8754075, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50478057 (CHEMBL256059) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells assessed as reduction of amyloid beta level by ELISA | Bioorg Med Chem Lett 18: 304-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.074 BindingDB Entry DOI: 10.7270/Q2J105X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124136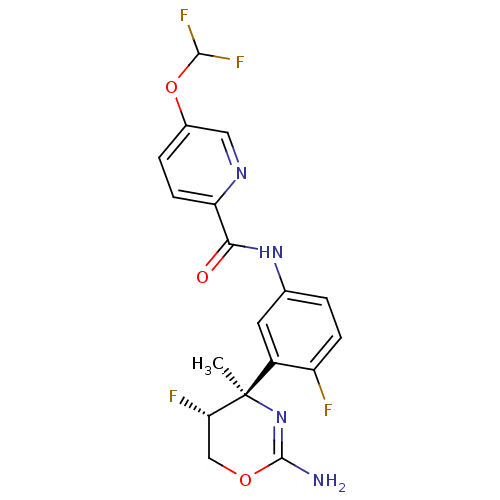 (US8754075, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124175 (US8754075, 46) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124138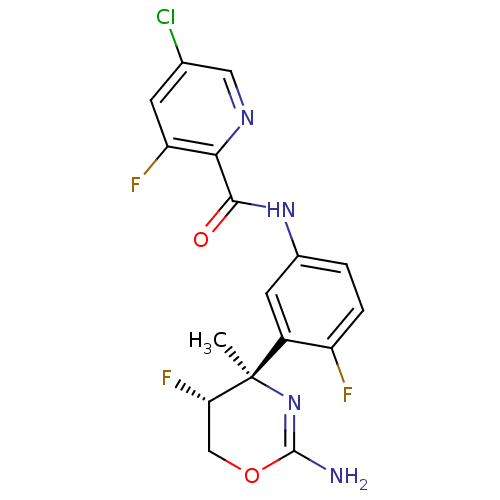 (US8754075, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124139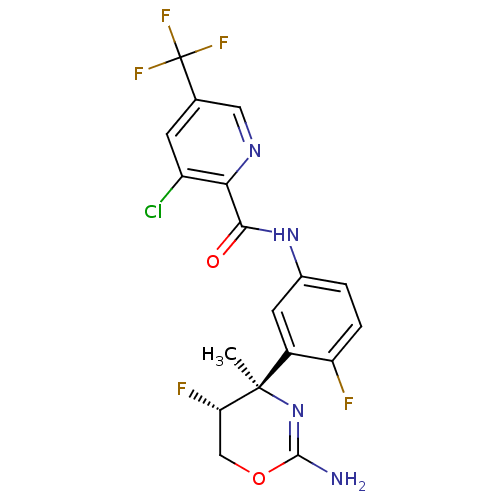 (US8754075, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124168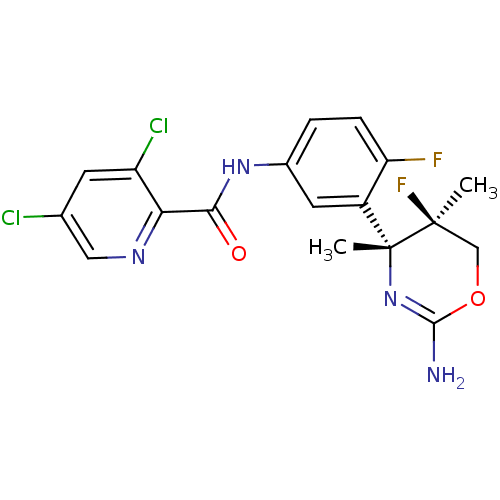 (US8754075, 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124169 (US8754075, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077704 (CHEMBL31880 | Sulfuric acid mono-[(3S,4R,5R)-4-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124141 (US8754075, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124170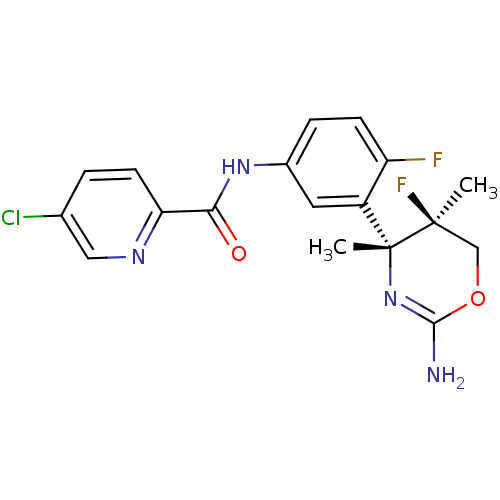 (US8754075, 41) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124177 (US8754075, 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077709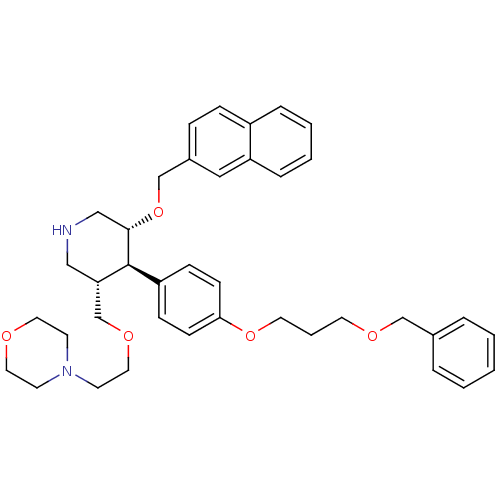 (4-{2-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432631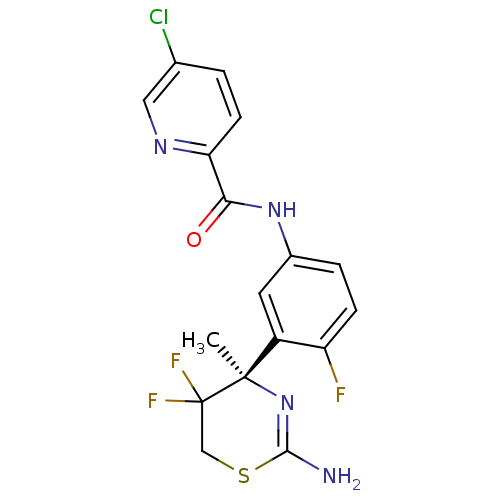 (CHEMBL2347212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50478077 (CHEMBL403332) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells assessed as reduction of amyloid beta level by ELISA | Bioorg Med Chem Lett 18: 304-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.074 BindingDB Entry DOI: 10.7270/Q2J105X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124142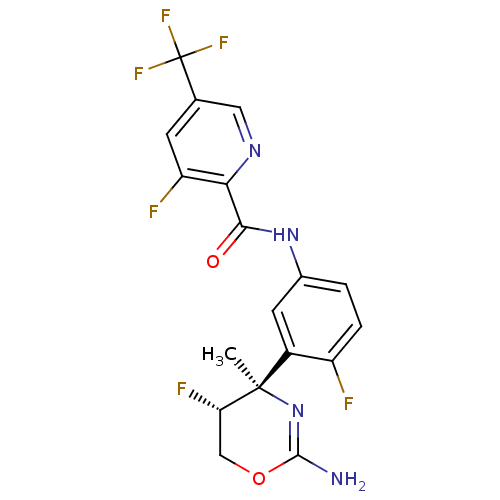 (US8754075, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077695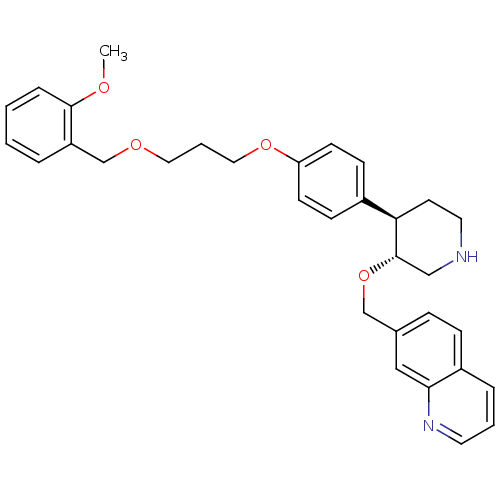 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124144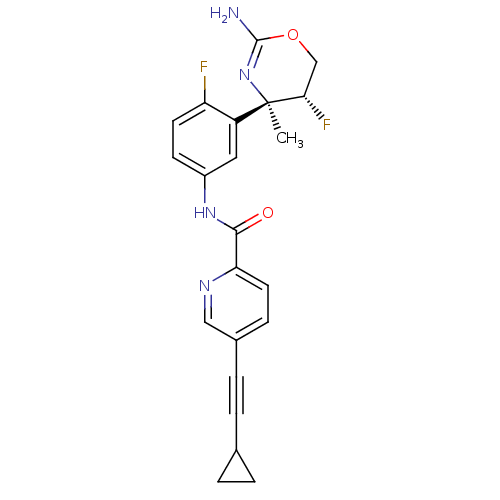 (US8754075, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM124168 (US8754075, 39) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124143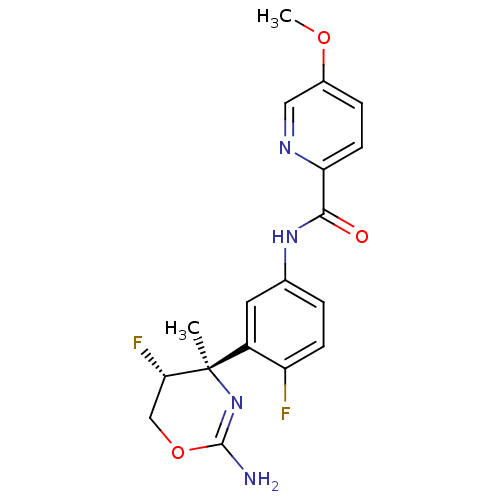 (US8754075, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124145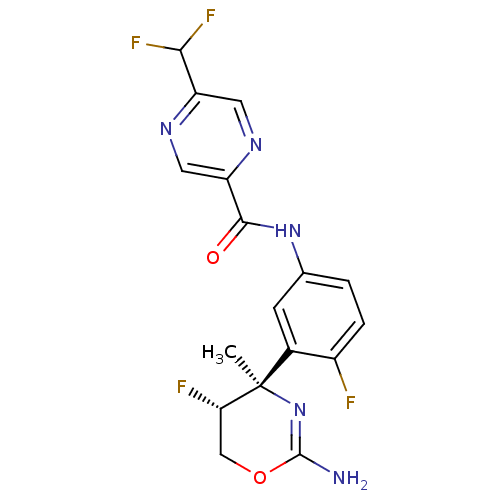 (US8754075, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM123867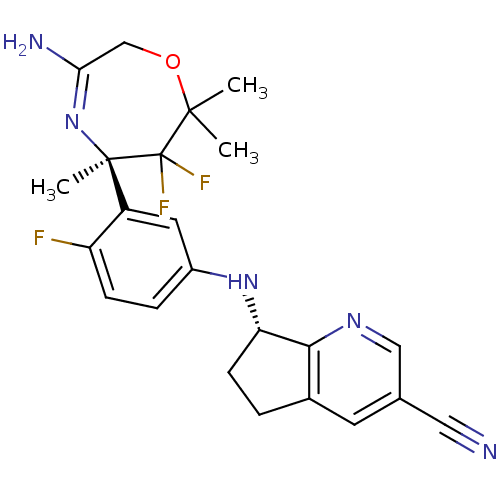 (US8748418, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077689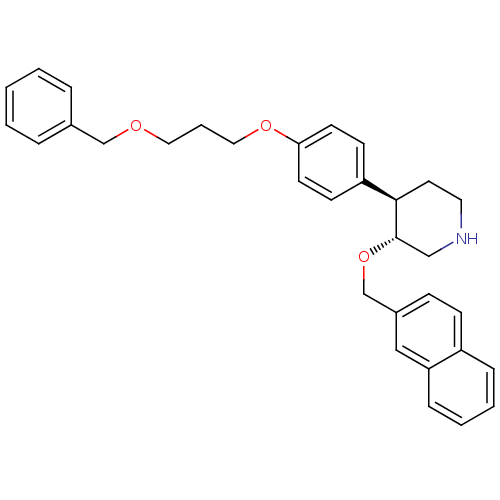 ((3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM124146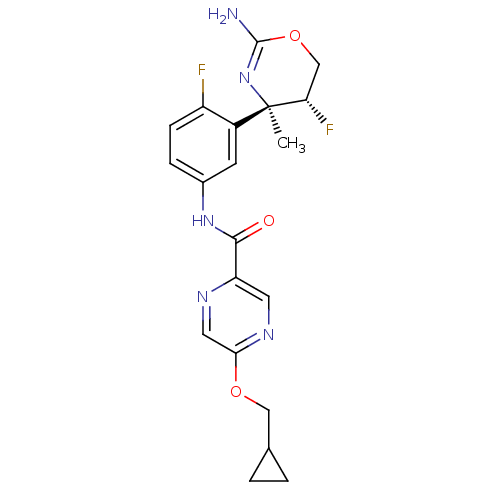 (US8754075, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077694 (7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 429 total ) | Next | Last >> |