 Found 50 hits with Last Name = 'xin' and Initial = 'mh'
Found 50 hits with Last Name = 'xin' and Initial = 'mh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR-2 (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR tyrosine kinase (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR tyrosine kinase L858R mutant (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50500089
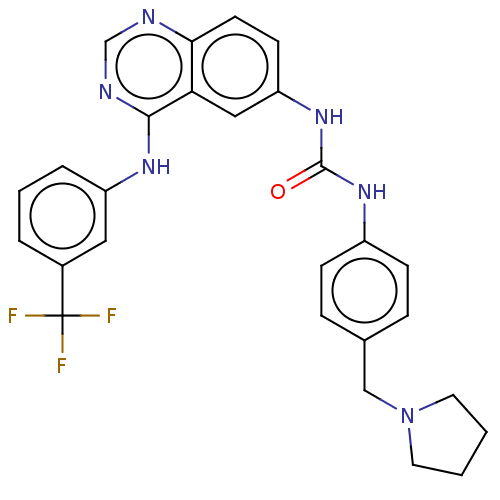
(CHEMBL3740910)Show SMILES FC(F)(F)c1cccc(Nc2ncnc3ccc(NC(=O)Nc4ccc(CN5CCCC5)cc4)cc23)c1 Show InChI InChI=1S/C27H25F3N6O/c28-27(29,30)19-4-3-5-21(14-19)33-25-23-15-22(10-11-24(23)31-17-32-25)35-26(37)34-20-8-6-18(7-9-20)16-36-12-1-2-13-36/h3-11,14-15,17H,1-2,12-13,16H2,(H,31,32,33)(H2,34,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR tyrosine kinase (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50500090
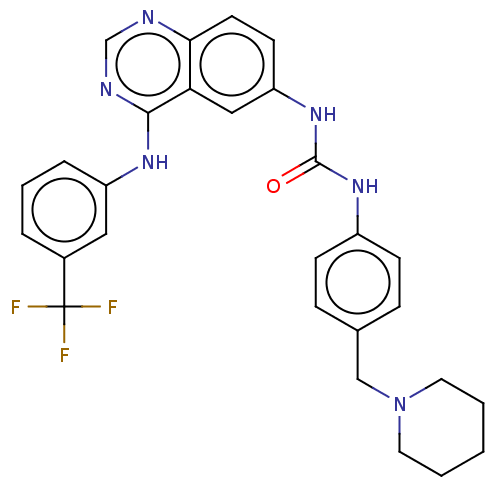
(CHEMBL3741473)Show SMILES FC(F)(F)c1cccc(Nc2ncnc3ccc(NC(=O)Nc4ccc(CN5CCCCC5)cc4)cc23)c1 Show InChI InChI=1S/C28H27F3N6O/c29-28(30,31)20-5-4-6-22(15-20)34-26-24-16-23(11-12-25(24)32-18-33-26)36-27(38)35-21-9-7-19(8-10-21)17-37-13-2-1-3-14-37/h4-12,15-16,18H,1-3,13-14,17H2,(H,32,33,34)(H2,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR tyrosine kinase (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50233667
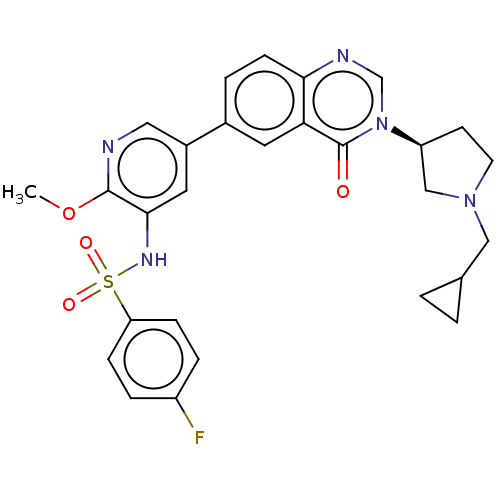
(CHEMBL4088969)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(CC4CC4)C3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H28FN5O4S/c1-38-27-26(32-39(36,37)23-7-5-21(29)6-8-23)13-20(14-30-27)19-4-9-25-24(12-19)28(35)34(17-31-25)22-10-11-33(16-22)15-18-2-3-18/h4-9,12-14,17-18,22,32H,2-3,10-11,15-16H2,1H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha/p85alpha (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50108116
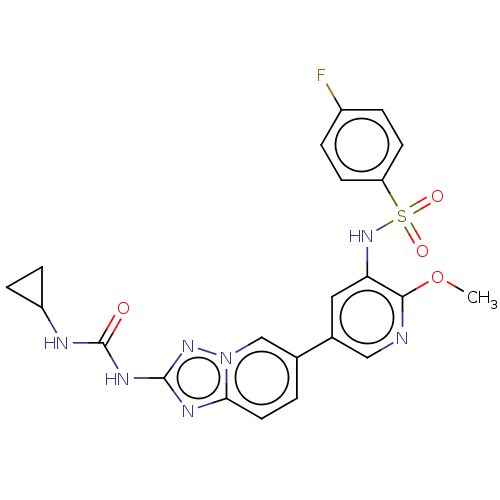
(CHEMBL3601923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2nc(NC(=O)NC3CC3)nn2c1 Show InChI InChI=1S/C22H20FN7O4S/c1-34-20-18(29-35(32,33)17-7-3-15(23)4-8-17)10-14(11-24-20)13-2-9-19-26-21(28-30(19)12-13)27-22(31)25-16-5-6-16/h2-4,7-12,16,29H,5-6H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50233666
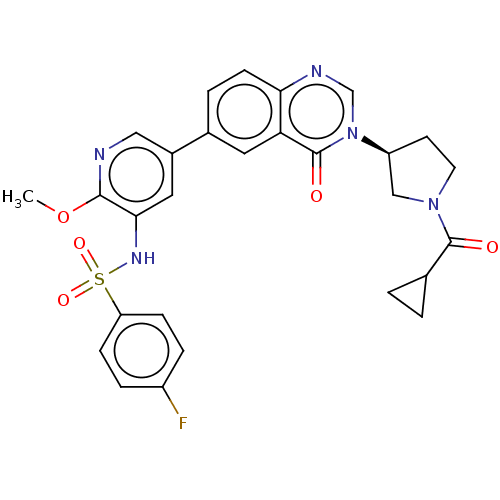
(CHEMBL4097625)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(C3)C(=O)C3CC3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H26FN5O5S/c1-39-26-25(32-40(37,38)22-7-5-20(29)6-8-22)13-19(14-30-26)18-4-9-24-23(12-18)28(36)34(16-31-24)21-10-11-33(15-21)27(35)17-2-3-17/h4-9,12-14,16-17,21,32H,2-3,10-11,15H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha/p85alpha (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50500088
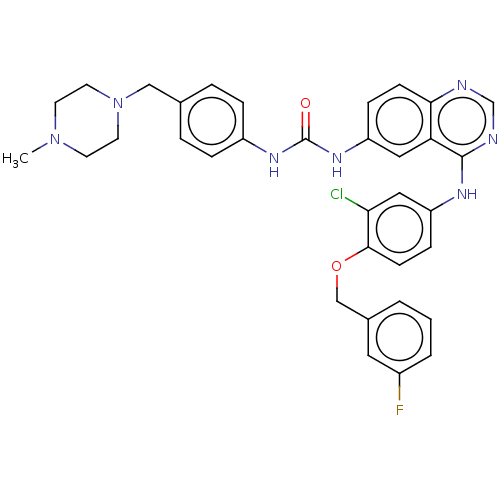
(CHEMBL3740144)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3ccc4ncnc(Nc5ccc(OCc6cccc(F)c6)c(Cl)c5)c4c3)cc2)CC1 Show InChI InChI=1S/C34H33ClFN7O2/c1-42-13-15-43(16-14-42)20-23-5-7-26(8-6-23)40-34(44)41-27-9-11-31-29(18-27)33(38-22-37-31)39-28-10-12-32(30(35)19-28)45-21-24-3-2-4-25(36)17-24/h2-12,17-19,22H,13-16,20-21H2,1H3,(H,37,38,39)(H2,40,41,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR tyrosine kinase (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50500090

(CHEMBL3741473)Show SMILES FC(F)(F)c1cccc(Nc2ncnc3ccc(NC(=O)Nc4ccc(CN5CCCCC5)cc4)cc23)c1 Show InChI InChI=1S/C28H27F3N6O/c29-28(30,31)20-5-4-6-22(15-20)34-26-24-16-23(11-12-25(24)32-18-33-26)36-27(38)35-21-9-7-19(8-10-21)17-37-13-2-1-3-14-37/h4-12,15-16,18H,1-3,13-14,17H2,(H,32,33,34)(H2,35,36,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR tyrosine kinase L858R mutant (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50500089

(CHEMBL3740910)Show SMILES FC(F)(F)c1cccc(Nc2ncnc3ccc(NC(=O)Nc4ccc(CN5CCCC5)cc4)cc23)c1 Show InChI InChI=1S/C27H25F3N6O/c28-27(29,30)19-4-3-5-21(14-19)33-25-23-15-22(10-11-24(23)31-17-32-25)35-26(37)34-20-8-6-18(7-9-20)16-36-12-1-2-13-36/h3-11,14-15,17H,1-2,12-13,16H2,(H,31,32,33)(H2,34,35,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR tyrosine kinase L858R mutant (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50108117
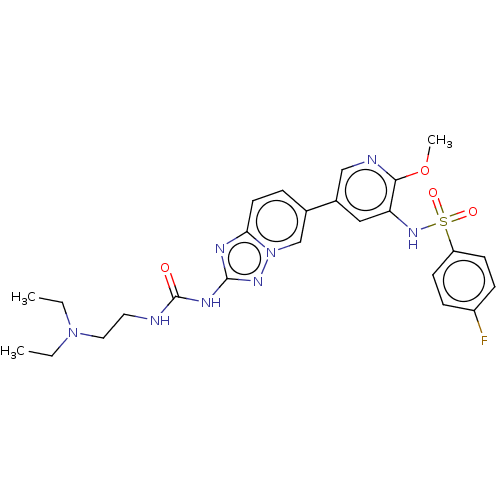
(CHEMBL3601925)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cn2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H29FN8O4S/c1-4-33(5-2)13-12-27-25(35)30-24-29-22-11-6-17(16-34(22)31-24)18-14-21(23(38-3)28-15-18)32-39(36,37)20-9-7-19(26)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,27,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50117874
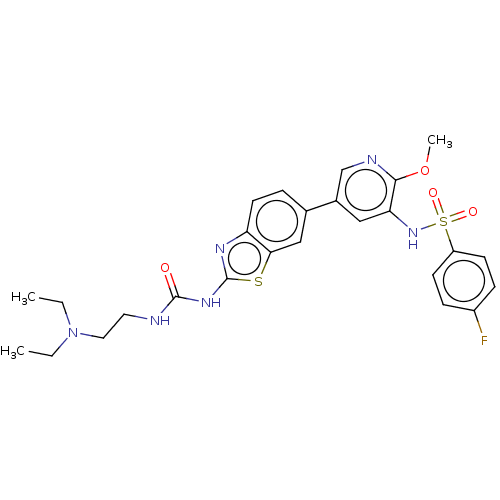
(CHEMBL3612310)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cc2s1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C26H29FN6O4S2/c1-4-33(5-2)13-12-28-25(34)31-26-30-21-11-6-17(15-23(21)38-26)18-14-22(24(37-3)29-16-18)32-39(35,36)20-9-7-19(27)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,28,30,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of p110alpha/p85alpha (unknown origin) incubated for 40 mins by luciferase based ATP depletion assay |
Bioorg Med Chem 23: 6477-85 (2015)
Article DOI: 10.1016/j.bmc.2015.08.013
BindingDB Entry DOI: 10.7270/Q24J0GX8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50108116

(CHEMBL3601923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2nc(NC(=O)NC3CC3)nn2c1 Show InChI InChI=1S/C22H20FN7O4S/c1-34-20-18(29-35(32,33)17-7-3-15(23)4-8-17)10-14(11-24-20)13-2-9-19-26-21(28-30(19)12-13)27-22(31)25-16-5-6-16/h2-4,7-12,16,29H,5-6H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50108117

(CHEMBL3601925)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cn2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H29FN8O4S/c1-4-33(5-2)13-12-27-25(35)30-24-29-22-11-6-17(16-34(22)31-24)18-14-21(23(38-3)28-15-18)32-39(36,37)20-9-7-19(26)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,27,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50108116

(CHEMBL3601923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2nc(NC(=O)NC3CC3)nn2c1 Show InChI InChI=1S/C22H20FN7O4S/c1-34-20-18(29-35(32,33)17-7-3-15(23)4-8-17)10-14(11-24-20)13-2-9-19-26-21(28-30(19)12-13)27-22(31)25-16-5-6-16/h2-4,7-12,16,29H,5-6H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50233667

(CHEMBL4088969)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(CC4CC4)C3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H28FN5O4S/c1-38-27-26(32-39(36,37)23-7-5-21(29)6-8-23)13-20(14-30-27)19-4-9-25-24(12-19)28(35)34(17-31-25)22-10-11-33(16-22)15-18-2-3-18/h4-9,12-14,17-18,22,32H,2-3,10-11,15-16H2,1H3/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110delta/p85alpha (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50108117

(CHEMBL3601925)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cn2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H29FN8O4S/c1-4-33(5-2)13-12-27-25(35)30-24-29-22-11-6-17(16-34(22)31-24)18-14-21(23(38-3)28-15-18)32-39(36,37)20-9-7-19(26)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,27,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50233667

(CHEMBL4088969)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(CC4CC4)C3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H28FN5O4S/c1-38-27-26(32-39(36,37)23-7-5-21(29)6-8-23)13-20(14-30-27)19-4-9-25-24(12-19)28(35)34(17-31-25)22-10-11-33(16-22)15-18-2-3-18/h4-9,12-14,17-18,22,32H,2-3,10-11,15-16H2,1H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110beta/p85alpha (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380363
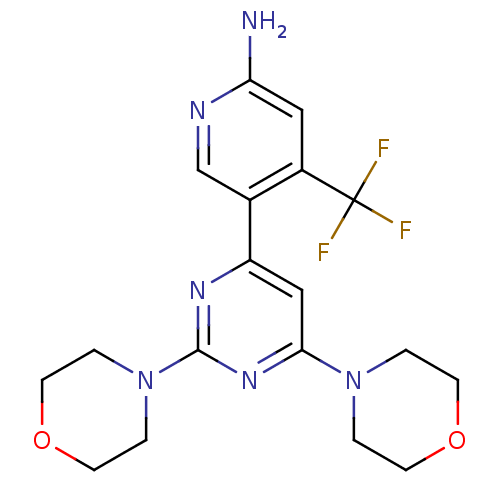
(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 25: 1730-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.067
BindingDB Entry DOI: 10.7270/Q20003RQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Eur J Med Chem 96: 382-95 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.037
BindingDB Entry DOI: 10.7270/Q2XS5X3B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50108117

(CHEMBL3601925)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cn2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H29FN8O4S/c1-4-33(5-2)13-12-27-25(35)30-24-29-22-11-6-17(16-34(22)31-24)18-14-21(23(38-3)28-15-18)32-39(36,37)20-9-7-19(26)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,27,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 (unknown origin) incubated for 40 mins by luciferase based ATP depletion assay |
Bioorg Med Chem 23: 6477-85 (2015)
Article DOI: 10.1016/j.bmc.2015.08.013
BindingDB Entry DOI: 10.7270/Q24J0GX8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50108116

(CHEMBL3601923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2nc(NC(=O)NC3CC3)nn2c1 Show InChI InChI=1S/C22H20FN7O4S/c1-34-20-18(29-35(32,33)17-7-3-15(23)4-8-17)10-14(11-24-20)13-2-9-19-26-21(28-30(19)12-13)27-22(31)25-16-5-6-16/h2-4,7-12,16,29H,5-6H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of p110alpha/p85alpha (unknown origin) incubated for 40 mins by luciferase based ATP depletion assay |
Bioorg Med Chem 23: 6477-85 (2015)
Article DOI: 10.1016/j.bmc.2015.08.013
BindingDB Entry DOI: 10.7270/Q24J0GX8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50117874

(CHEMBL3612310)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cc2s1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C26H29FN6O4S2/c1-4-33(5-2)13-12-28-25(34)31-26-30-21-11-6-17(15-23(21)38-26)18-14-22(24(37-3)29-16-18)32-39(35,36)20-9-7-19(27)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,28,30,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 (unknown origin) incubated for 40 mins by luciferase based ATP depletion assay |
Bioorg Med Chem 23: 6477-85 (2015)
Article DOI: 10.1016/j.bmc.2015.08.013
BindingDB Entry DOI: 10.7270/Q24J0GX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha/p85alpha (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50117874

(CHEMBL3612310)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cc2s1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C26H29FN6O4S2/c1-4-33(5-2)13-12-28-25(34)31-26-30-21-11-6-17(15-23(21)38-26)18-14-22(24(37-3)29-16-18)32-39(35,36)20-9-7-19(27)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,28,30,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of p110beta/p85alpha (unknown origin) incubated for 40 mins by luciferase based ATP depletion assay |
Bioorg Med Chem 23: 6477-85 (2015)
Article DOI: 10.1016/j.bmc.2015.08.013
BindingDB Entry DOI: 10.7270/Q24J0GX8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50233666

(CHEMBL4097625)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(C3)C(=O)C3CC3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H26FN5O5S/c1-39-26-25(32-40(37,38)22-7-5-20(29)6-8-22)13-19(14-30-26)18-4-9-24-23(12-18)28(36)34(16-31-24)21-10-11-33(15-21)27(35)17-2-3-17/h4-9,12-14,16-17,21,32H,2-3,10-11,15H2,1H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110delta/p85alpha (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50233667

(CHEMBL4088969)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(CC4CC4)C3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H28FN5O4S/c1-38-27-26(32-39(36,37)23-7-5-21(29)6-8-23)13-20(14-30-27)19-4-9-25-24(12-19)28(35)34(17-31-25)22-10-11-33(16-22)15-18-2-3-18/h4-9,12-14,17-18,22,32H,2-3,10-11,15-16H2,1H3/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM50233666

(CHEMBL4097625)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(C3)C(=O)C3CC3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H26FN5O5S/c1-39-26-25(32-40(37,38)22-7-5-20(29)6-8-22)13-19(14-30-26)18-4-9-24-23(12-18)28(36)34(16-31-24)21-10-11-33(15-21)27(35)17-2-3-17/h4-9,12-14,16-17,21,32H,2-3,10-11,15H2,1H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma/PIK3R5 (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50108117

(CHEMBL3601925)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cn2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H29FN8O4S/c1-4-33(5-2)13-12-27-25(35)30-24-29-22-11-6-17(16-34(22)31-24)18-14-21(23(38-3)28-15-18)32-39(36,37)20-9-7-19(26)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,27,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of p110delta/p85alpha (unknown origin) incubated for 40 mins by luciferase based ATP depletion assay |
Bioorg Med Chem 23: 6477-85 (2015)
Article DOI: 10.1016/j.bmc.2015.08.013
BindingDB Entry DOI: 10.7270/Q24J0GX8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50117874

(CHEMBL3612310)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cc2s1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C26H29FN6O4S2/c1-4-33(5-2)13-12-28-25(34)31-26-30-21-11-6-17(15-23(21)38-26)18-14-22(24(37-3)29-16-18)32-39(35,36)20-9-7-19(27)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,28,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of p110delta/p85alpha (unknown origin) incubated for 40 mins by luciferase based ATP depletion assay |
Bioorg Med Chem 23: 6477-85 (2015)
Article DOI: 10.1016/j.bmc.2015.08.013
BindingDB Entry DOI: 10.7270/Q24J0GX8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma/PIK3R5 (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM50233667

(CHEMBL4088969)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(CC4CC4)C3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H28FN5O4S/c1-38-27-26(32-39(36,37)23-7-5-21(29)6-8-23)13-20(14-30-27)19-4-9-25-24(12-19)28(35)34(17-31-25)22-10-11-33(16-22)15-18-2-3-18/h4-9,12-14,17-18,22,32H,2-3,10-11,15-16H2,1H3/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110gamma/PIK3R5 (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110delta/p85alpha (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50233666

(CHEMBL4097625)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(C3)C(=O)C3CC3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H26FN5O5S/c1-39-26-25(32-40(37,38)22-7-5-20(29)6-8-22)13-19(14-30-26)18-4-9-24-23(12-18)28(36)34(16-31-24)21-10-11-33(15-21)27(35)17-2-3-17/h4-9,12-14,16-17,21,32H,2-3,10-11,15H2,1H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50233666

(CHEMBL4097625)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2ncn([C@H]3CCN(C3)C(=O)C3CC3)c(=O)c2c1 |r| Show InChI InChI=1S/C28H26FN5O5S/c1-39-26-25(32-40(37,38)22-7-5-20(29)6-8-22)13-19(14-30-26)18-4-9-24-23(12-18)28(36)34(16-31-24)21-10-11-33(15-21)27(35)17-2-3-17/h4-9,12-14,16-17,21,32H,2-3,10-11,15H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110beta/p85alpha (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50108116

(CHEMBL3601923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2nc(NC(=O)NC3CC3)nn2c1 Show InChI InChI=1S/C22H20FN7O4S/c1-34-20-18(29-35(32,33)17-7-3-15(23)4-8-17)10-14(11-24-20)13-2-9-19-26-21(28-30(19)12-13)27-22(31)25-16-5-6-16/h2-4,7-12,16,29H,5-6H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 399 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 421 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of P110beta/p85alpha (unknown origin) using L-alpha-phosphatidylinositol as substrate after 40 mins by ATP depletion assay |
Bioorg Med Chem 23: 7765-76 (2015)
Article DOI: 10.1016/j.bmc.2015.11.027
BindingDB Entry DOI: 10.7270/Q2222X19 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 478 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of p110beta/p85alpha (unknown origin) incubated for 40 mins by luciferase based ATP depletion assay |
Bioorg Med Chem 23: 6477-85 (2015)
Article DOI: 10.1016/j.bmc.2015.08.013
BindingDB Entry DOI: 10.7270/Q24J0GX8 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50500089

(CHEMBL3740910)Show SMILES FC(F)(F)c1cccc(Nc2ncnc3ccc(NC(=O)Nc4ccc(CN5CCCC5)cc4)cc23)c1 Show InChI InChI=1S/C27H25F3N6O/c28-27(29,30)19-4-3-5-21(14-19)33-25-23-15-22(10-11-24(23)31-17-32-25)35-26(37)34-20-8-6-18(7-9-20)16-36-12-1-2-13-36/h3-11,14-15,17H,1-2,12-13,16H2,(H,31,32,33)(H2,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 565 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR-2 (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50500088

(CHEMBL3740144)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3ccc4ncnc(Nc5ccc(OCc6cccc(F)c6)c(Cl)c5)c4c3)cc2)CC1 Show InChI InChI=1S/C34H33ClFN7O2/c1-42-13-15-43(16-14-42)20-23-5-7-26(8-6-23)40-34(44)41-27-9-11-31-29(18-27)33(38-22-37-31)39-28-10-12-32(30(35)19-28)45-21-24-3-2-4-25(36)17-24/h2-12,17-19,22H,13-16,20-21H2,1H3,(H,37,38,39)(H2,40,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR-2 (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data