 Found 60 hits with Last Name = 'yang' and Initial = 'jh'
Found 60 hits with Last Name = 'yang' and Initial = 'jh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB assessed as reduction in H2O2 production from p-tyramine incubated for 15 mins by Amplex red reagent based fluor... |
J Nat Prod 80: 798-804 (2017)
Article DOI: 10.1021/acs.jnatprod.6b00391
BindingDB Entry DOI: 10.7270/Q26T0Q3S |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50260171

(CHEMBL4062286)Show SMILES COc1c(OC\C=C(/C)C[C@H]2OC(=O)C(CCC(N)=O)=C2)ccc2ccc(=O)oc12 |r,c:19| Show InChI InChI=1S/C22H23NO7/c1-13(11-16-12-15(22(26)29-16)4-7-18(23)24)9-10-28-17-6-3-14-5-8-19(25)30-20(14)21(17)27-2/h3,5-6,8-9,12,16H,4,7,10-11H2,1-2H3,(H2,23,24)/b13-9+/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB assessed as reduction in H2O2 production from p-tyramine incubated for 15 mins by Amplex red reagent based fluor... |
J Nat Prod 80: 798-804 (2017)
Article DOI: 10.1021/acs.jnatprod.6b00391
BindingDB Entry DOI: 10.7270/Q26T0Q3S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219240

(4-[4-(benzyloxy)piperidino]butyl-N-(4-chlorophenyl...)Show SMILES Clc1ccc(NC(=O)OCCCCN2CCC(CC2)OCc2ccccc2)cc1 Show InChI InChI=1S/C23H29ClN2O3/c24-20-8-10-21(11-9-20)25-23(27)28-17-5-4-14-26-15-12-22(13-16-26)29-18-19-6-2-1-3-7-19/h1-3,6-11,22H,4-5,12-18H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219232

(4-[4-(benzhydryloxy)piperidino]butyl-4-chlorobenzo...)Show SMILES Clc1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32ClNO3/c30-26-15-13-25(14-16-26)29(32)33-22-8-7-19-31-20-17-27(18-21-31)34-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219230
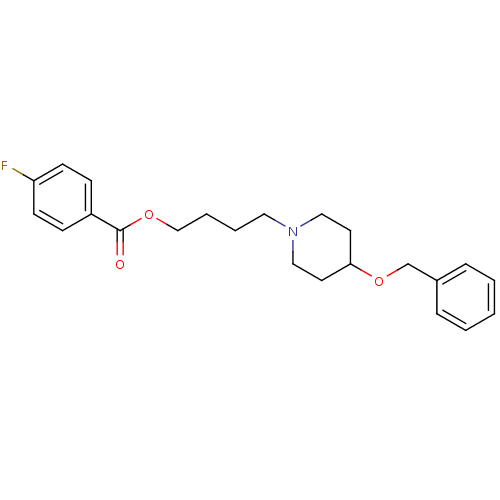
(4-[4-(benzyloxy)piperidino]butyl-4-fluorobenzoate ...)Show InChI InChI=1S/C23H28FNO3/c24-21-10-8-20(9-11-21)23(26)27-17-5-4-14-25-15-12-22(13-16-25)28-18-19-6-2-1-3-7-19/h1-3,6-11,22H,4-5,12-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219235
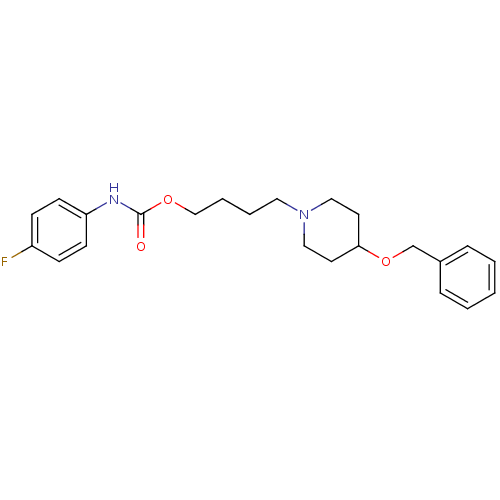
(4-[4-(benzyloxy)piperidino]butyl-N-(4-fluorophenyl...)Show InChI InChI=1S/C23H29FN2O3/c24-20-8-10-21(11-9-20)25-23(27)28-17-5-4-14-26-15-12-22(13-16-26)29-18-19-6-2-1-3-7-19/h1-3,6-11,22H,4-5,12-18H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219245
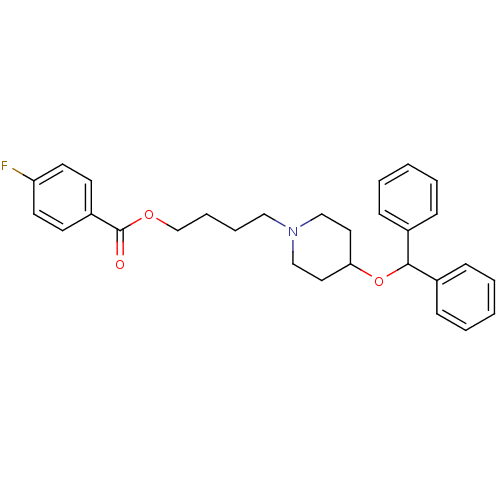
(4-[4-(benzhydryloxy)piperidino]butyl 4-fluorobenzo...)Show SMILES Fc1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32FNO3/c30-26-15-13-25(14-16-26)29(32)33-22-8-7-19-31-20-17-27(18-21-31)34-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) assessed as N-methyldihydronicotinamide oxidation per mg of protein a... |
J Nat Prod 74: 129-36 (2011)
Article DOI: 10.1021/np100373f
BindingDB Entry DOI: 10.7270/Q20865MS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NACHT, LRR and PYD domains-containing protein 3
(Homo sapiens (Human)) | BDBM50553951
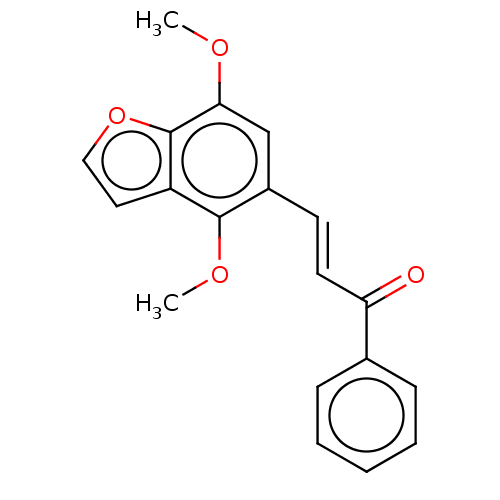
(CHEMBL4782189) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NLRP3 inflammasome activation in LPS-primed human PMA-differentiated THP-1 cells assessed as reduction in nigericin-induced IL-1beta le... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00478
BindingDB Entry DOI: 10.7270/Q2TF01ZH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219238
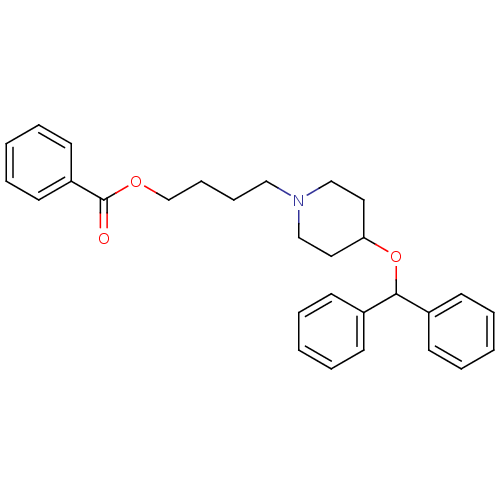
(4-[4-(benzhydryloxy)piperidino]butyl benzoate | CH...)Show SMILES O=C(OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33NO3/c31-29(26-16-8-3-9-17-26)32-23-11-10-20-30-21-18-27(19-22-30)33-28(24-12-4-1-5-13-24)25-14-6-2-7-15-25/h1-9,12-17,27-28H,10-11,18-23H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219233
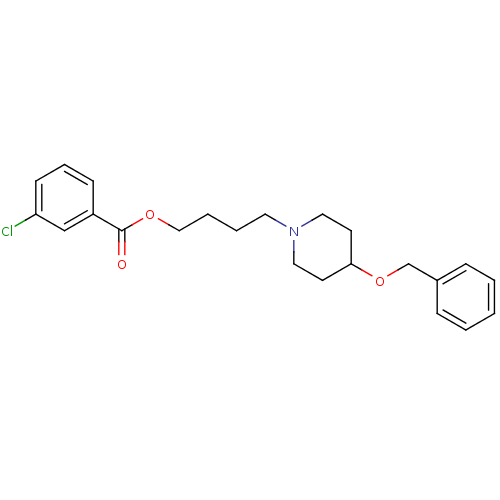
(4-[4-(benzyloxy)piperidino]butyl-3-chlorobenzoate ...)Show InChI InChI=1S/C23H28ClNO3/c24-21-10-6-9-20(17-21)23(26)27-16-5-4-13-25-14-11-22(12-15-25)28-18-19-7-2-1-3-8-19/h1-3,6-10,17,22H,4-5,11-16,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219231

(4-[4-(benzyloxy)piperidino]butyl-3-fluorobenzoate ...)Show InChI InChI=1S/C23H28FNO3/c24-21-10-6-9-20(17-21)23(26)27-16-5-4-13-25-14-11-22(12-15-25)28-18-19-7-2-1-3-8-19/h1-3,6-10,17,22H,4-5,11-16,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219248

(4-[4-(benzyloxy)piperidino]butyl benzoate | CHEMBL...)Show InChI InChI=1S/C23H29NO3/c25-23(21-11-5-2-6-12-21)26-18-8-7-15-24-16-13-22(14-17-24)27-19-20-9-3-1-4-10-20/h1-6,9-12,22H,7-8,13-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219249

(4-[4-(benzhydryloxy)piperidino]butyl-4-(tert-butyl...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C33H41NO3/c1-33(2,3)29-18-16-28(17-19-29)32(35)36-25-11-10-22-34-23-20-30(21-24-34)37-31(26-12-6-4-7-13-26)27-14-8-5-9-15-27/h4-9,12-19,30-31H,10-11,20-25H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219241
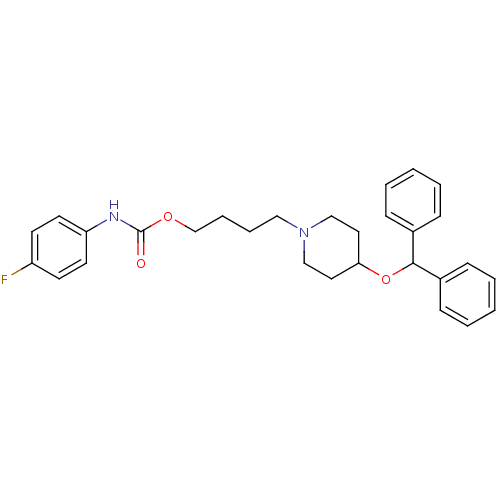
(4-[4-(benzhydryloxy)piperidino]butyl-N-(4-fluoroph...)Show SMILES Fc1ccc(NC(=O)OCCCCN2CCC(CC2)OC(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H33FN2O3/c30-25-13-15-26(16-14-25)31-29(33)34-22-8-7-19-32-20-17-27(18-21-32)35-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2,(H,31,33) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219242

(4-[4-(benzhydryloxy)piperidino]butyl-N-(4-chloroph...)Show SMILES Clc1ccc(NC(=O)OCCCCN2CCC(CC2)OC(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H33ClN2O3/c30-25-13-15-26(16-14-25)31-29(33)34-22-8-7-19-32-20-17-27(18-21-32)35-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2,(H,31,33) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219234

(4-[4-(benzhydryloxy)piperidino]butyl 3-chlorobenzo...)Show SMILES Clc1cccc(c1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32ClNO3/c30-26-15-9-14-25(22-26)29(32)33-21-8-7-18-31-19-16-27(17-20-31)34-28(23-10-3-1-4-11-23)24-12-5-2-6-13-24/h1-6,9-15,22,27-28H,7-8,16-21H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219244
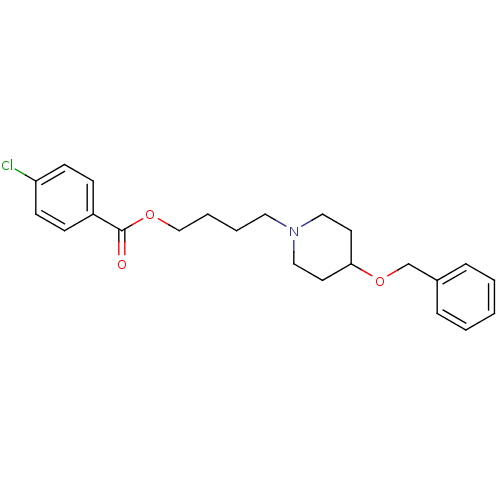
(4-[4-(benzyloxy)piperidino]butyl-4-chlorobenzoate ...)Show InChI InChI=1S/C23H28ClNO3/c24-21-10-8-20(9-11-21)23(26)27-17-5-4-14-25-15-12-22(13-16-25)28-18-19-6-2-1-3-7-19/h1-3,6-11,22H,4-5,12-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50339150

(5,7-dihydroxy-2-(4-hydroxyphenyl)-3-((2R,3S,4S,5S,...)Show SMILES C[C@H]1O[C@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)cc2)[C@@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O10/c1-8-15(25)17(27)18(28)21(29-8)31-20-16(26)14-12(24)6-11(23)7-13(14)30-19(20)9-2-4-10(22)5-3-9/h2-8,15,17-18,21-23,25-28H,1H3/t8-,15-,17+,18+,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) assessed as N-methyldihydronicotinamide oxidation per mg of protein a... |
J Nat Prod 74: 129-36 (2011)
Article DOI: 10.1021/np100373f
BindingDB Entry DOI: 10.7270/Q20865MS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219239

(4-[4-(benzhydryloxy)piperidino]butyl 4-isopropylbe...)Show SMILES CC(C)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H39NO3/c1-25(2)26-15-17-29(18-16-26)32(34)35-24-10-9-21-33-22-19-30(20-23-33)36-31(27-11-5-3-6-12-27)28-13-7-4-8-14-28/h3-8,11-18,25,30-31H,9-10,19-24H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219236
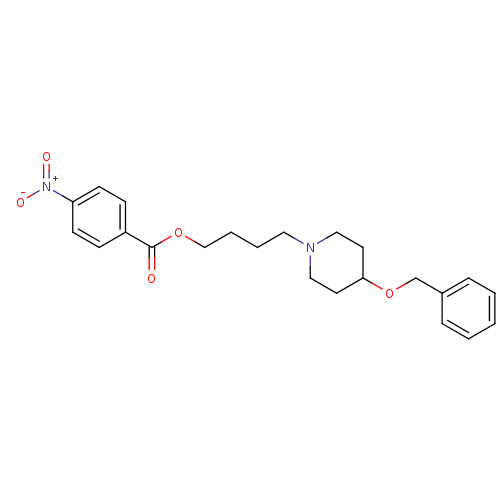
(4-[4-(benzyloxy)piperidino]butyl-4-nitrobenzoate |...)Show SMILES [O-][N+](=O)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OCc1ccccc1 Show InChI InChI=1S/C23H28N2O5/c26-23(20-8-10-21(11-9-20)25(27)28)29-17-5-4-14-24-15-12-22(13-16-24)30-18-19-6-2-1-3-7-19/h1-3,6-11,22H,4-5,12-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM29136
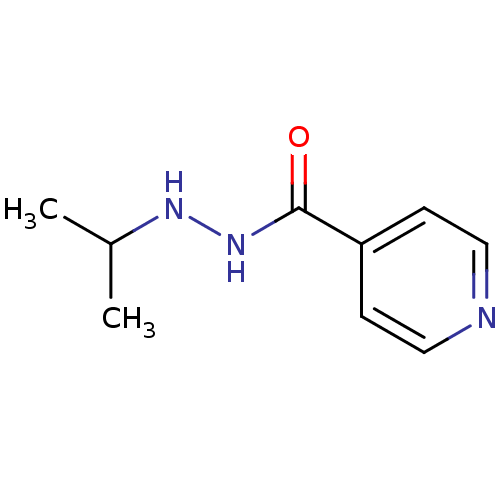
(CHEMBL92401 | Euphozid | Iprazid | Iproniazid)Show InChI InChI=1S/C9H13N3O/c1-7(2)11-12-9(13)8-3-5-10-6-4-8/h3-7,11H,1-2H3,(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA assessed as reduction in H2O2 production from p-tyramine incubated for 15 mins by Amplex red reagent based fluor... |
J Nat Prod 80: 798-804 (2017)
Article DOI: 10.1021/acs.jnatprod.6b00391
BindingDB Entry DOI: 10.7270/Q26T0Q3S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219237
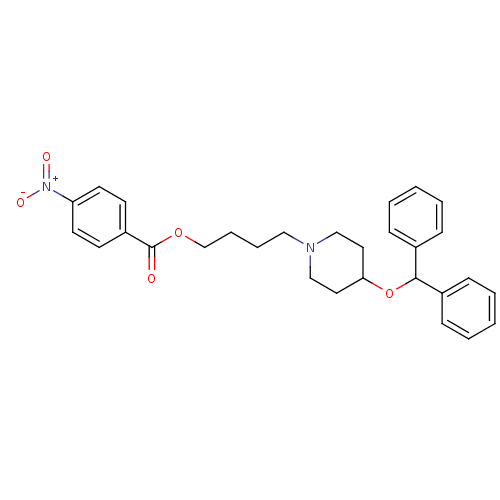
(4-[4-(benzhydryloxy)piperidino]butyl-4-nitrobenzoa...)Show SMILES [O-][N+](=O)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32N2O5/c32-29(25-13-15-26(16-14-25)31(33)34)35-22-8-7-19-30-20-17-27(18-21-30)36-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM29136

(CHEMBL92401 | Euphozid | Iprazid | Iproniazid)Show InChI InChI=1S/C9H13N3O/c1-7(2)11-12-9(13)8-3-5-10-6-4-8/h3-7,11H,1-2H3,(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB assessed as reduction in H2O2 production from p-tyramine incubated for 15 mins by Amplex red reagent based fluor... |
J Nat Prod 80: 798-804 (2017)
Article DOI: 10.1021/acs.jnatprod.6b00391
BindingDB Entry DOI: 10.7270/Q26T0Q3S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219246
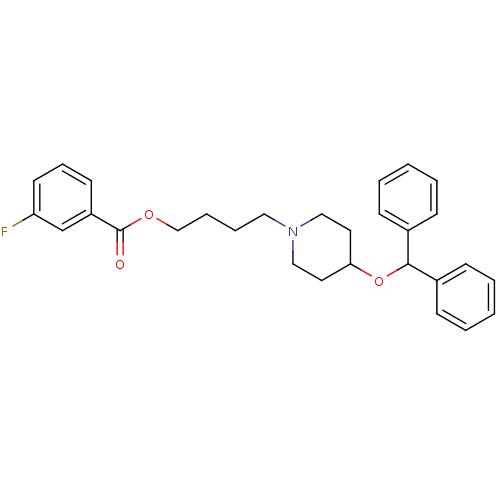
(4-[4-(benzhydryloxy)piperidino]butyl 3-fluorobenzo...)Show SMILES Fc1cccc(c1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32FNO3/c30-26-15-9-14-25(22-26)29(32)33-21-8-7-18-31-19-16-27(17-20-31)34-28(23-10-3-1-4-11-23)24-12-5-2-6-13-24/h1-6,9-15,22,27-28H,7-8,16-21H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219229

(4-[4-(benzyloxy)piperidino]butyl-4-isopropylbenzoa...)Show SMILES CC(C)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OCc1ccccc1 Show InChI InChI=1S/C26H35NO3/c1-21(2)23-10-12-24(13-11-23)26(28)29-19-7-6-16-27-17-14-25(15-18-27)30-20-22-8-4-3-5-9-22/h3-5,8-13,21,25H,6-7,14-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219247
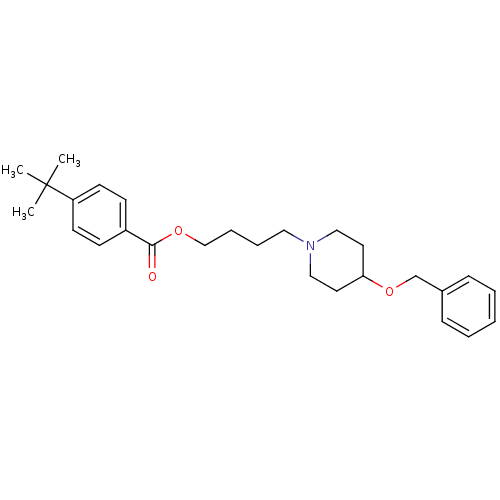
(4-[4-(benzyloxy)piperidino]butyl-4-(tert-butyl)-be...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OCc1ccccc1 Show InChI InChI=1S/C27H37NO3/c1-27(2,3)24-13-11-23(12-14-24)26(29)30-20-8-7-17-28-18-15-25(16-19-28)31-21-22-9-5-4-6-10-22/h4-6,9-14,25H,7-8,15-21H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219242

(4-[4-(benzhydryloxy)piperidino]butyl-N-(4-chloroph...)Show SMILES Clc1ccc(NC(=O)OCCCCN2CCC(CC2)OC(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H33ClN2O3/c30-25-13-15-26(16-14-25)31-29(33)34-22-8-7-19-32-20-17-27(18-21-32)35-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50219229

(4-[4-(benzyloxy)piperidino]butyl-4-isopropylbenzoa...)Show SMILES CC(C)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OCc1ccccc1 Show InChI InChI=1S/C26H35NO3/c1-21(2)23-10-12-24(13-11-23)26(28)29-19-7-6-16-27-17-14-25(15-18-27)30-20-22-8-4-3-5-9-22/h3-5,8-13,21,25H,6-7,14-20H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219247

(4-[4-(benzyloxy)piperidino]butyl-4-(tert-butyl)-be...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OCc1ccccc1 Show InChI InChI=1S/C27H37NO3/c1-27(2,3)24-13-11-23(12-14-24)26(29)30-20-8-7-17-28-18-15-25(16-19-28)31-21-22-9-5-4-6-10-22/h4-6,9-14,25H,7-8,15-21H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219241

(4-[4-(benzhydryloxy)piperidino]butyl-N-(4-fluoroph...)Show SMILES Fc1ccc(NC(=O)OCCCCN2CCC(CC2)OC(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H33FN2O3/c30-25-13-15-26(16-14-25)31-29(33)34-22-8-7-19-32-20-17-27(18-21-32)35-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219245

(4-[4-(benzhydryloxy)piperidino]butyl 4-fluorobenzo...)Show SMILES Fc1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32FNO3/c30-26-15-13-25(14-16-26)29(32)33-22-8-7-19-31-20-17-27(18-21-31)34-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219239

(4-[4-(benzhydryloxy)piperidino]butyl 4-isopropylbe...)Show SMILES CC(C)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H39NO3/c1-25(2)26-15-17-29(18-16-26)32(34)35-24-10-9-21-33-22-19-30(20-23-33)36-31(27-11-5-3-6-12-27)28-13-7-4-8-14-28/h3-8,11-18,25,30-31H,9-10,19-24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
NACHT, LRR and PYD domains-containing protein 3
(Homo sapiens (Human)) | BDBM50140172

(CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...)Show SMILES COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NLRP3 inflammasome activation in LPS-primed human PMA-differentiated THP-1 cells assessed as reduction in nigericin-induced IL-1beta le... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00478
BindingDB Entry DOI: 10.7270/Q2TF01ZH |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50339149

(CHEMBL1689259 | beta-(3,4-dihydroxyphenyl)ethyl-3-...)Show SMILES OC[C@H]1O[C@@H](OCCc2ccc(O)c(O)c2)[C@H](O[C@@H]2OC[C@](O)(CO)[C@H]2O)[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@@H]1O |r| Show InChI InChI=1S/C28H34O15/c29-11-20-22(36)23(42-21(35)6-3-14-1-4-16(31)18(33)9-14)24(43-27-25(37)28(38,12-30)13-40-27)26(41-20)39-8-7-15-2-5-17(32)19(34)10-15/h1-6,9-10,20,22-27,29-34,36-38H,7-8,11-13H2/b6-3+/t20-,22-,23+,24-,25+,26-,27+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University
Curated by ChEMBL
| Assay Description
Inhibition of aromatase preincubated with 2.6 mM NADP+ for 10 mins before substrate addition measured after 30 mins by fluorescence assay |
J Nat Prod 74: 129-36 (2011)
Article DOI: 10.1021/np100373f
BindingDB Entry DOI: 10.7270/Q20865MS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219237

(4-[4-(benzhydryloxy)piperidino]butyl-4-nitrobenzoa...)Show SMILES [O-][N+](=O)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32N2O5/c32-29(25-13-15-26(16-14-25)31(33)34)35-22-8-7-19-30-20-17-27(18-21-30)36-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50339147

(CHEMBL1689257 | beta-(4-hydroxyphenyl)ethyl-4-O-E-...)Show SMILES OC[C@H]1O[C@@H](OCCc2ccc(O)cc2)[C@H](O[C@@H]2OC[C@](O)(CO)[C@H]2O)[C@@H](O)[C@@H]1OC(=O)\C=C\c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C28H34O14/c29-12-20-23(41-21(34)8-4-16-3-7-18(32)19(33)11-16)22(35)24(42-27-25(36)28(37,13-30)14-39-27)26(40-20)38-10-9-15-1-5-17(31)6-2-15/h1-8,11,20,22-27,29-33,35-37H,9-10,12-14H2/b8-4+/t20-,22+,23-,24-,25+,26-,27+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University
Curated by ChEMBL
| Assay Description
Inhibition of aromatase preincubated with 2.6 mM NADP+ for 10 mins before substrate addition measured after 30 mins by fluorescence assay |
J Nat Prod 74: 129-36 (2011)
Article DOI: 10.1021/np100373f
BindingDB Entry DOI: 10.7270/Q20865MS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219238

(4-[4-(benzhydryloxy)piperidino]butyl benzoate | CH...)Show SMILES O=C(OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33NO3/c31-29(26-16-8-3-9-17-26)32-23-11-10-20-30-21-18-27(19-22-30)33-28(24-12-4-1-5-13-24)25-14-6-2-7-15-25/h1-9,12-17,27-28H,10-11,18-23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219246

(4-[4-(benzhydryloxy)piperidino]butyl 3-fluorobenzo...)Show SMILES Fc1cccc(c1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32FNO3/c30-26-15-9-14-25(22-26)29(32)33-21-8-7-18-31-19-16-27(17-20-31)34-28(23-10-3-1-4-11-23)24-12-5-2-6-13-24/h1-6,9-15,22,27-28H,7-8,16-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50339148
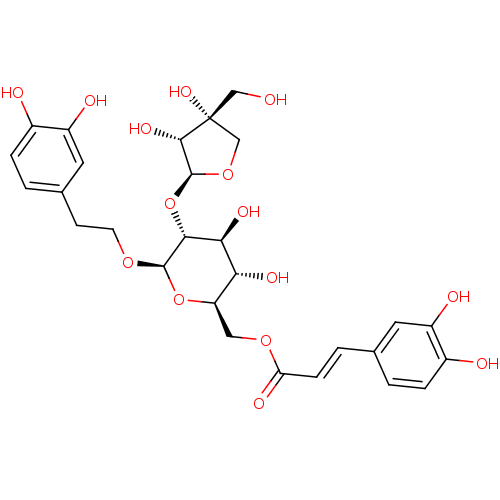
(CHEMBL1689258 | beta-(3,4-dihydroxyphenyl)ethyl-6-...)Show SMILES OC[C@@]1(O)CO[C@@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](COC(=O)\C=C\c3ccc(O)c(O)c3)O[C@H]2OCCc2ccc(O)c(O)c2)[C@@H]1O |r| Show InChI InChI=1S/C28H34O15/c29-12-28(38)13-41-27(25(28)37)43-24-23(36)22(35)20(11-40-21(34)6-3-14-1-4-16(30)18(32)9-14)42-26(24)39-8-7-15-2-5-17(31)19(33)10-15/h1-6,9-10,20,22-27,29-33,35-38H,7-8,11-13H2/b6-3+/t20-,22-,23+,24-,25+,26-,27+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University
Curated by ChEMBL
| Assay Description
Inhibition of aromatase preincubated with 2.6 mM NADP+ for 10 mins before substrate addition measured after 30 mins by fluorescence assay |
J Nat Prod 74: 129-36 (2011)
Article DOI: 10.1021/np100373f
BindingDB Entry DOI: 10.7270/Q20865MS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50269516
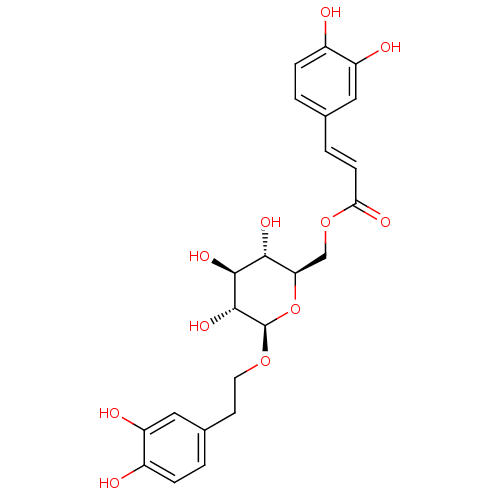
(CHEMBL518414 | Calceolarioside B)Show SMILES O[C@@H]1[C@@H](COC(=O)\C=C\c2ccc(O)c(O)c2)O[C@@H](OCCc2ccc(O)c(O)c2)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H26O11/c24-14-4-1-12(9-16(14)26)3-6-19(28)33-11-18-20(29)21(30)22(31)23(34-18)32-8-7-13-2-5-15(25)17(27)10-13/h1-6,9-10,18,20-27,29-31H,7-8,11H2/b6-3+/t18-,20-,21+,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University
Curated by ChEMBL
| Assay Description
Inhibition of aromatase preincubated with 2.6 mM NADP+ for 10 mins before substrate addition measured after 30 mins by fluorescence assay |
J Nat Prod 74: 129-36 (2011)
Article DOI: 10.1021/np100373f
BindingDB Entry DOI: 10.7270/Q20865MS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219232

(4-[4-(benzhydryloxy)piperidino]butyl-4-chlorobenzo...)Show SMILES Clc1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32ClNO3/c30-26-15-13-25(14-16-26)29(32)33-22-8-7-19-31-20-17-27(18-21-31)34-28(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,9-16,27-28H,7-8,17-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50342164
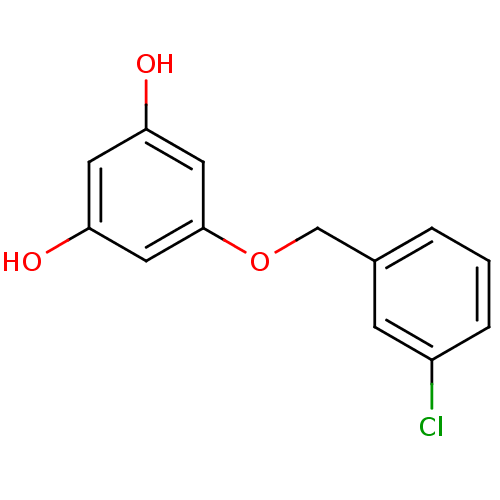
(5-(3-Chlorobenzyloxy)benzene-1,3-diol | CHEMBL1766...)Show InChI InChI=1S/C13H11ClO3/c14-10-3-1-2-9(4-10)8-17-13-6-11(15)5-12(16)7-13/h1-7,15-16H,8H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase activity in alpha-MSH-stimulated mouse B16 cells using L-tyrosine as substrate |
Bioorg Med Chem 19: 2168-75 (2011)
Article DOI: 10.1016/j.bmc.2011.02.044
BindingDB Entry DOI: 10.7270/Q2K937TS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219234

(4-[4-(benzhydryloxy)piperidino]butyl 3-chlorobenzo...)Show SMILES Clc1cccc(c1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32ClNO3/c30-26-15-9-14-25(22-26)29(32)33-21-8-7-18-31-19-16-27(17-20-31)34-28(23-10-3-1-4-11-23)24-12-5-2-6-13-24/h1-6,9-15,22,27-28H,7-8,16-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50342165

(5-(3,4-Dichlorobenzyloxy)benzene-1,3-diol | CHEMBL...)Show InChI InChI=1S/C13H10Cl2O3/c14-12-2-1-8(3-13(12)15)7-18-11-5-9(16)4-10(17)6-11/h1-6,16-17H,7H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase activity in alpha-MSH-stimulated mouse B16 cells using L-tyrosine as substrate |
Bioorg Med Chem 19: 2168-75 (2011)
Article DOI: 10.1016/j.bmc.2011.02.044
BindingDB Entry DOI: 10.7270/Q2K937TS |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-1881 from Androgen receptor of LNCaP cells |
J Nat Prod 80: 798-804 (2017)
Article DOI: 10.1021/acs.jnatprod.6b00391
BindingDB Entry DOI: 10.7270/Q26T0Q3S |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50219249

(4-[4-(benzhydryloxy)piperidino]butyl-4-(tert-butyl...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)OCCCCN1CCC(CC1)OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C33H41NO3/c1-33(2,3)29-18-16-28(17-19-29)32(35)36-25-11-10-22-34-23-20-30(21-24-34)37-31(26-12-6-4-7-13-26)27-14-8-5-9-15-27/h4-9,12-19,30-31H,10-11,20-25H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 15: 6596-607 (2007)
Article DOI: 10.1016/j.bmc.2007.07.003
BindingDB Entry DOI: 10.7270/Q2HM585X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data