 Found 1270 hits with Last Name = 'ye' and Initial = 'xy'
Found 1270 hits with Last Name = 'ye' and Initial = 'xy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50595097

(CHEMBL5170337)Show SMILES COC[C@H]1CCCN1c1cc(Nc2ccccn2)nc(n1)-n1cccn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50507371
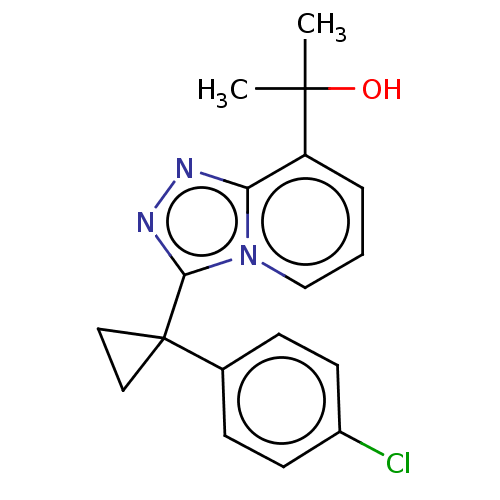
(BMS-823778)Show InChI InChI=1S/C18H18ClN3O/c1-17(2,23)14-4-3-11-22-15(14)20-21-16(22)18(9-10-18)12-5-7-13(19)8-6-12/h3-8,11,23H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... |
ACS Med Chem Lett 9: 1170-1174 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00307
BindingDB Entry DOI: 10.7270/Q20R9SP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens) | BDBM50092173
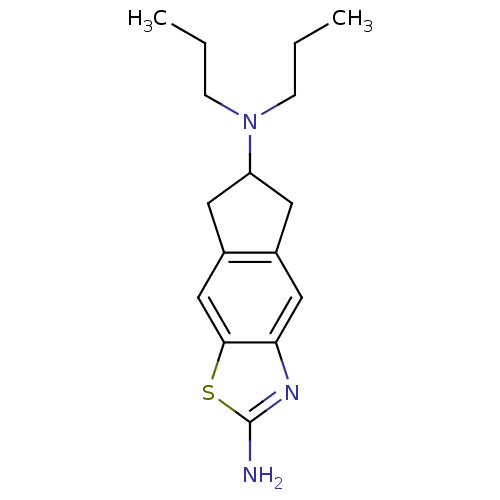
(CHEMBL325710 | N*6*,N*6*-Dipropyl-6,7-dihydro-5H-1...)Show InChI InChI=1S/C16H23N3S/c1-3-5-19(6-4-2)13-7-11-9-14-15(10-12(11)8-13)20-16(17)18-14/h9-10,13H,3-8H2,1-2H3,(H2,17,18) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50123627

((S)-6-Dipropylamino-5,6,7,8-tetrahydro-naphthalene...)Show InChI InChI=1S/C16H25NO2/c1-3-9-17(10-4-2)13-6-7-14-12(11-13)5-8-15(18)16(14)19/h5,8,13,18-19H,3-4,6-7,9-11H2,1-2H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50048466
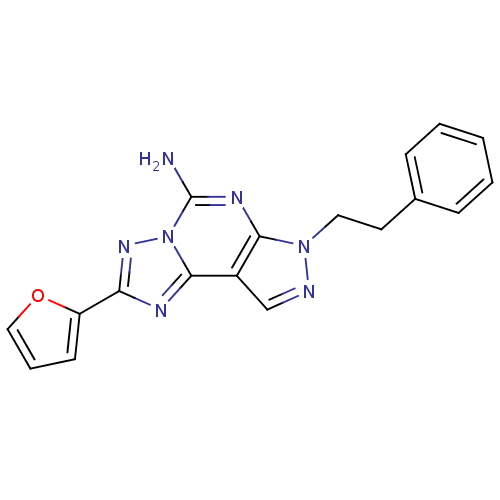
(2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...)Show InChI InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50123627

((S)-6-Dipropylamino-5,6,7,8-tetrahydro-naphthalene...)Show InChI InChI=1S/C16H25NO2/c1-3-9-17(10-4-2)13-6-7-14-12(11-13)5-8-15(18)16(14)19/h5,8,13,18-19H,3-4,6-7,9-11H2,1-2H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239606

(CHEMBL4080667)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)[C@H]3O)c1ccccc1 |r,wD:17.21,TLB:16:15:8.9.10:12,7:8:14.16.15:10.11.12,7:8:12:14.15.17,THB:16:9:12:14.15.17,18:17:8.9.10:12,17:15:8:10.11.12,17:11:8:14.16.15,19:8:14.16.15:10.11.12,19:8:12:14.15.17,(43.24,-21.55,;43.72,-20.08,;43.03,-18.71,;44.4,-18.01,;45.1,-19.38,;44.88,-16.54,;43.63,-15.64,;46.35,-16.07,;47.49,-17.11,;48.99,-16.68,;48.98,-15.1,;50.02,-13.87,;48.67,-14.34,;48.68,-15.83,;50,-16.32,;51.4,-15.97,;50.4,-17.25,;51.42,-14.44,;52.71,-13.59,;47.48,-18.64,;46.13,-19.39,;46.12,-20.93,;47.44,-21.71,;48.79,-20.95,;48.79,-19.41,)| Show InChI InChI=1S/C21H27NO3/c23-18-11-22(12-18)19(24)10-21(15-4-2-1-3-5-15)16-6-13-7-17(21)9-14(8-16)20(13)25/h1-5,13-14,16-18,20,23,25H,6-12H2/t13?,14?,16?,17?,20-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50152240

(CHEMBL184309 | N*5*-{2-[4-(2,4-Difluoro-phenyl)-pi...)Show SMILES CN(CCN1CCN(CC1)c1ccc(F)cc1F)c1nc(N)n2nc(nc2n1)-c1ccco1 Show InChI InChI=1S/C21H23F2N9O/c1-29(6-7-30-8-10-31(11-9-30)16-5-4-14(22)13-15(16)23)20-26-19(24)32-21(27-20)25-18(28-32)17-3-2-12-33-17/h2-5,12-13H,6-11H2,1H3,(H2,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50499055
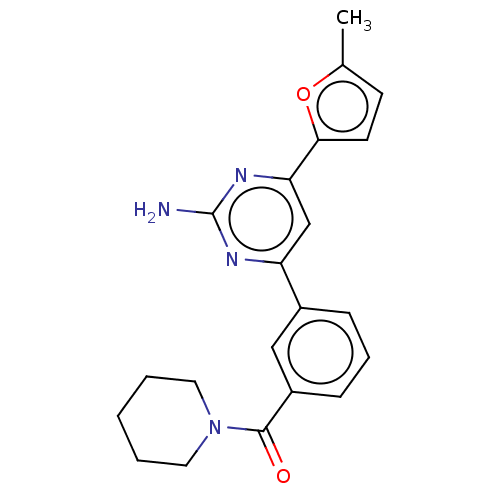
(CHEMBL3735985)Show SMILES Cc1ccc(o1)-c1cc(nc(N)n1)-c1cccc(c1)C(=O)N1CCCCC1 Show InChI InChI=1S/C21H22N4O2/c1-14-8-9-19(27-14)18-13-17(23-21(22)24-18)15-6-5-7-16(12-15)20(26)25-10-3-2-4-11-25/h5-9,12-13H,2-4,10-11H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202086
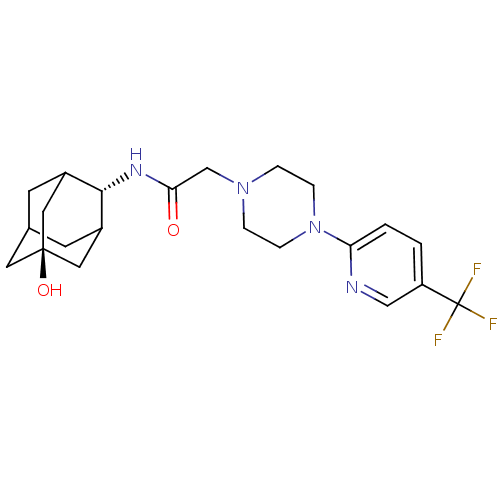
(CHEMBL219142 | N-[(E)-5-hydroxy-2-adamantyl]-2-{4-...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:4:3:30:6.5.7,4:5:2.3.29:30,THB:7:5:2:29.28.30,7:28:2:6.4.5,8:7:2.3.29:30,(1.8,-8.48,;3.15,-9.25,;1.93,-10.52,;3.44,-10.11,;4.84,-10.69,;5.87,-9.43,;4.47,-9.76,;5.9,-7.9,;7.19,-7.07,;8.56,-7.77,;8.64,-9.31,;9.85,-6.93,;11.23,-7.63,;11.25,-9.18,;12.59,-9.92,;13.92,-9.13,;13.88,-7.59,;12.53,-6.84,;15.23,-9.92,;15.2,-11.46,;16.52,-12.26,;17.87,-11.51,;17.89,-9.96,;16.57,-9.18,;19.19,-12.31,;20.51,-13.07,;18.4,-13.63,;19.98,-10.98,;4.51,-7.31,;3.45,-8.53,;3.16,-7.77,)| Show InChI InChI=1S/C22H29F3N4O2/c23-22(24,25)17-1-2-18(26-12-17)29-5-3-28(4-6-29)13-19(30)27-20-15-7-14-8-16(20)11-21(31,9-14)10-15/h1-2,12,14-16,20,31H,3-11,13H2,(H,27,30)/t14?,15?,16?,20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD-1 |
Bioorg Med Chem Lett 21: 6699-704 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.055
BindingDB Entry DOI: 10.7270/Q2Q240NV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50499055

(CHEMBL3735985)Show SMILES Cc1ccc(o1)-c1cc(nc(N)n1)-c1cccc(c1)C(=O)N1CCCCC1 Show InChI InChI=1S/C21H22N4O2/c1-14-8-9-19(27-14)18-13-17(23-21(22)24-18)15-6-5-7-16(12-15)20(26)25-10-3-2-4-11-25/h5-9,12-13H,2-4,10-11H2,1H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50152215

(7N-[1-(2-chloro-4-pyridylmethyl)-(2R)-tetrahydro-1...)Show SMILES Nc1nc(NC[C@H]2CCCN2Cc2ccnc(Cl)c2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C19H20ClN9O/c20-15-9-12(5-6-22-15)11-28-7-1-3-13(28)10-23-18-25-17(21)29-19(26-18)24-16(27-29)14-4-2-8-30-14/h2,4-6,8-9,13H,1,3,7,10-11H2,(H3,21,23,24,25,26,27)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50011294
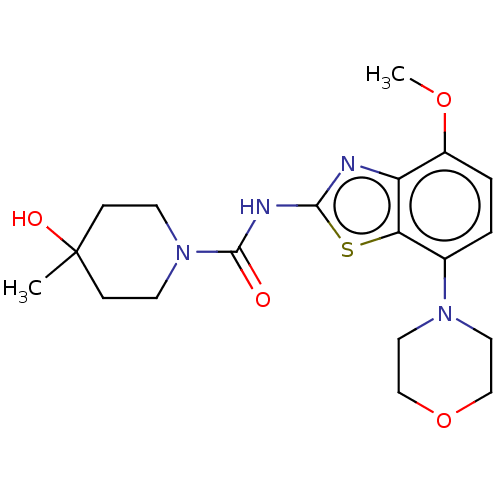
(A2A | Ro-4494351 | Ro-4494351-002 | Ro-4494351000 ...)Show SMILES COc1ccc(N2CCOCC2)c2sc(NC(=O)N3CCC(C)(O)CC3)nc12 Show InChI InChI=1S/C19H26N4O4S/c1-19(25)5-7-23(8-6-19)18(24)21-17-20-15-14(26-2)4-3-13(16(15)28-17)22-9-11-27-12-10-22/h3-4,25H,5-12H2,1-2H3,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50092173

(CHEMBL325710 | N*6*,N*6*-Dipropyl-6,7-dihydro-5H-1...)Show InChI InChI=1S/C16H23N3S/c1-3-5-19(6-4-2)13-7-11-9-14-15(10-12(11)8-13)20-16(17)18-14/h9-10,13H,3-8H2,1-2H3,(H2,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50594647

(CHEMBL5182020)Show SMILES Cc1cccc(Nc2nc(NCCCO)nc3n(ncc23)-c2ccccc2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50156628

((R)-2-furan-2-yl-7-(hexahydropyrrolo[1,2-a]pyrazin...)Show SMILES Nc1nc(cc2nc(nn12)-c1ccco1)N1CCN2CCC[C@@H]2C1 |r| Show InChI InChI=1S/C16H19N7O/c17-16-19-13(22-7-6-21-5-1-3-11(21)10-22)9-14-18-15(20-23(14)16)12-4-2-8-24-12/h2,4,8-9,11H,1,3,5-7,10H2,(H2,17,19)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50151188
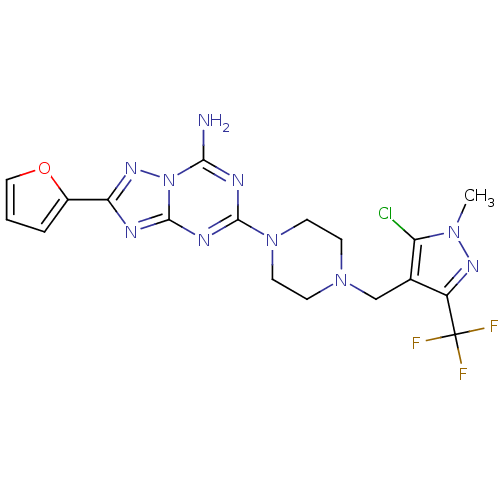
(5-(4-((5-chloro-1-methyl-3-(trifluoromethyl)-1H-py...)Show SMILES Cn1nc(c(CN2CCN(CC2)c2nc(N)n3nc(nc3n2)-c2ccco2)c1Cl)C(F)(F)F Show InChI InChI=1S/C18H18ClF3N10O/c1-29-13(19)10(12(27-29)18(20,21)22)9-30-4-6-31(7-5-30)16-25-15(23)32-17(26-16)24-14(28-32)11-3-2-8-33-11/h2-3,8H,4-7,9H2,1H3,(H2,23,24,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50048466

(2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...)Show InChI InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50152215

(7N-[1-(2-chloro-4-pyridylmethyl)-(2R)-tetrahydro-1...)Show SMILES Nc1nc(NC[C@H]2CCCN2Cc2ccnc(Cl)c2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C19H20ClN9O/c20-15-9-12(5-6-22-15)11-28-7-1-3-13(28)10-23-18-25-17(21)29-19(26-18)24-16(27-29)14-4-2-8-30-14/h2,4-6,8-9,13H,1,3,7,10-11H2,(H3,21,23,24,25,26,27)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50594951
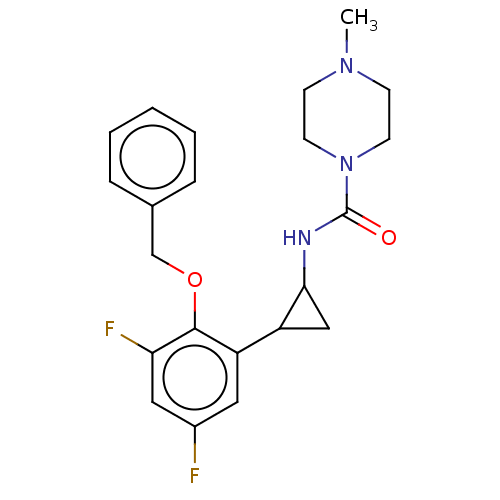
(CHEMBL5174572)Show SMILES CN1CCN(CC1)C(=O)NC1CC1c1cc(F)cc(F)c1OCc1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114143
BindingDB Entry DOI: 10.7270/Q28W3J9V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50507371

(BMS-823778)Show InChI InChI=1S/C18H18ClN3O/c1-17(2,23)14-4-3-11-22-15(14)20-21-16(22)18(9-10-18)12-5-7-13(19)8-6-12/h3-8,11,23H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... |
ACS Med Chem Lett 9: 1170-1174 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00307
BindingDB Entry DOI: 10.7270/Q20R9SP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50594647

(CHEMBL5182020)Show SMILES Cc1cccc(Nc2nc(NCCCO)nc3n(ncc23)-c2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50152240

(CHEMBL184309 | N*5*-{2-[4-(2,4-Difluoro-phenyl)-pi...)Show SMILES CN(CCN1CCN(CC1)c1ccc(F)cc1F)c1nc(N)n2nc(nc2n1)-c1ccco1 Show InChI InChI=1S/C21H23F2N9O/c1-29(6-7-30-8-10-31(11-9-30)16-5-4-14(22)13-15(16)23)20-26-19(24)32-21(27-20)25-18(28-32)17-3-2-12-33-17/h2-5,12-13H,6-11H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50156628

((R)-2-furan-2-yl-7-(hexahydropyrrolo[1,2-a]pyrazin...)Show SMILES Nc1nc(cc2nc(nn12)-c1ccco1)N1CCN2CCC[C@@H]2C1 |r| Show InChI InChI=1S/C16H19N7O/c17-16-19-13(22-7-6-21-5-1-3-11(21)10-22)9-14-18-15(20-23(14)16)12-4-2-8-24-12/h2,4,8-9,11H,1,3,5-7,10H2,(H2,17,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50011294

(A2A | Ro-4494351 | Ro-4494351-002 | Ro-4494351000 ...)Show SMILES COc1ccc(N2CCOCC2)c2sc(NC(=O)N3CCC(C)(O)CC3)nc12 Show InChI InChI=1S/C19H26N4O4S/c1-19(25)5-7-23(8-6-19)18(24)21-17-20-15-14(26-2)4-3-13(16(15)28-17)22-9-11-27-12-10-22/h3-4,25H,5-12H2,1-2H3,(H,20,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50133184
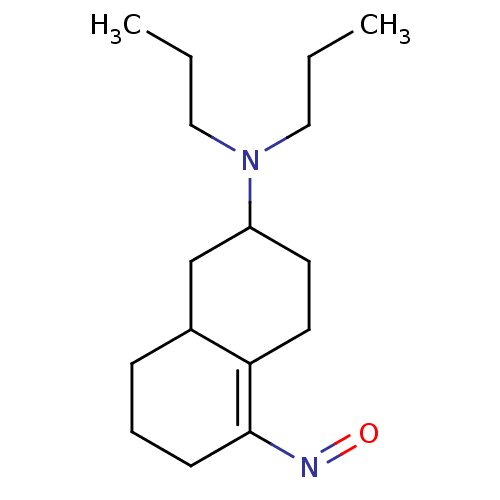
(6-Dipropylamino-3,4,5,6,7,8-hexahydro-2H-naphthale...)Show InChI InChI=1S/C16H28N2O/c1-3-10-18(11-4-2)14-8-9-15-13(12-14)6-5-7-16(15)17-19/h13-14H,3-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 6B
(Homo sapiens (Human)) | BDBM50594969
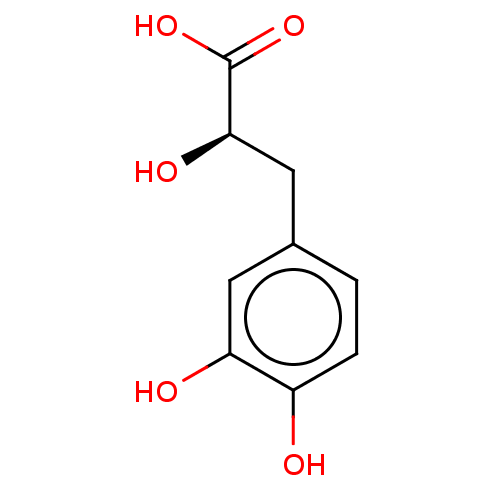
(CHEMBL4218391) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 7.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114143
BindingDB Entry DOI: 10.7270/Q28W3J9V |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 6B
(Homo sapiens (Human)) | BDBM50594970
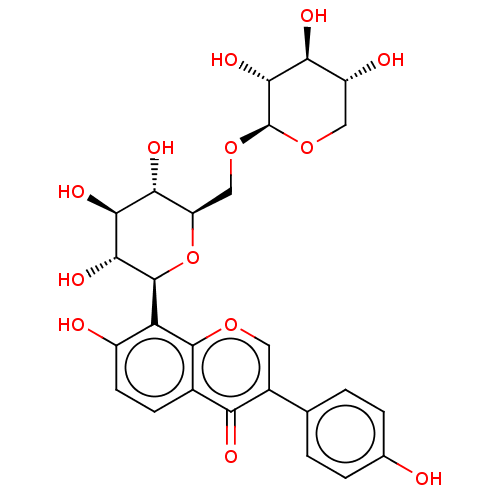
(CHEMBL5196038)Show SMILES O[C@@H]1CO[C@@H](OC[C@H]2O[C@H]([C@H](O)[C@@H](O)[C@@H]2O)c2c(O)ccc3c2occ(-c2ccc(O)cc2)c3=O)[C@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114143
BindingDB Entry DOI: 10.7270/Q28W3J9V |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50151188

(5-(4-((5-chloro-1-methyl-3-(trifluoromethyl)-1H-py...)Show SMILES Cn1nc(c(CN2CCN(CC2)c2nc(N)n3nc(nc3n2)-c2ccco2)c1Cl)C(F)(F)F Show InChI InChI=1S/C18H18ClF3N10O/c1-29-13(19)10(12(27-29)18(20,21)22)9-30-4-6-31(7-5-30)16-25-15(23)32-17(26-16)24-14(28-32)11-3-2-8-33-11/h2-3,8H,4-7,9H2,1H3,(H2,23,24,25,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 2
(Homo sapiens (Human)) | BDBM50594951

(CHEMBL5174572)Show SMILES CN1CCN(CC1)C(=O)NC1CC1c1cc(F)cc(F)c1OCc1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114143
BindingDB Entry DOI: 10.7270/Q28W3J9V |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50594951

(CHEMBL5174572)Show SMILES CN1CCN(CC1)C(=O)NC1CC1c1cc(F)cc(F)c1OCc1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114143
BindingDB Entry DOI: 10.7270/Q28W3J9V |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50594951

(CHEMBL5174572)Show SMILES CN1CCN(CC1)C(=O)NC1CC1c1cc(F)cc(F)c1OCc1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114143
BindingDB Entry DOI: 10.7270/Q28W3J9V |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50594947
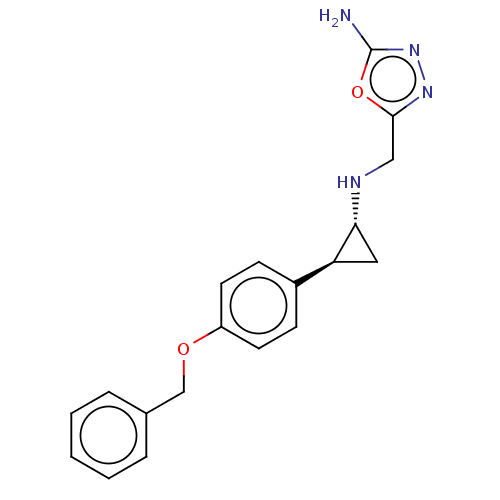
(ORY-2001 | VAFIDEMSTAT | Vafidemstat)Show SMILES Nc1nnc(CN[C@@H]2C[C@H]2c2ccc(OCc3ccccc3)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114143
BindingDB Entry DOI: 10.7270/Q28W3J9V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239610
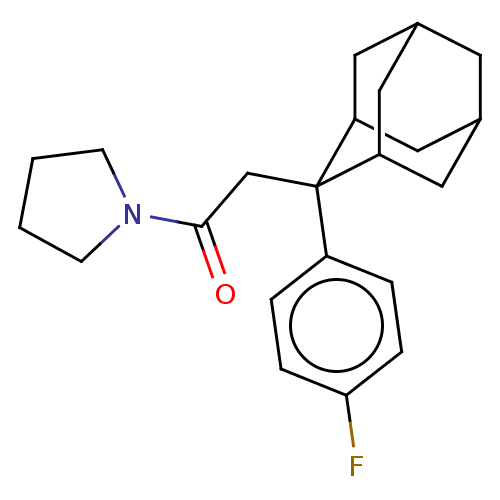
(CHEMBL4073961)Show SMILES Fc1ccc(cc1)C1(CC(=O)N2CCCC2)C2CC3CC(C2)CC1C3 |TLB:24:23:21:17.18.19,8:7:17.24.18:22.20.21,4:7:21:17.18.19,THB:24:18:7.23.22:21,19:18:7:22.20.21,19:20:7:17.24.18,8:7:21:17.18.19,4:7:17.24.18:22.20.21,(23.94,-24.95,;23.96,-23.41,;22.63,-22.62,;22.65,-21.09,;23.99,-20.34,;25.31,-21.11,;25.3,-22.64,;24.01,-18.8,;22.86,-17.77,;21.4,-18.25,;20.15,-17.34,;20.92,-19.71,;19.45,-20.18,;19.45,-21.72,;20.91,-22.2,;21.82,-20.95,;25.21,-17.53,;26.53,-18.01,;27.92,-17.67,;27.94,-16.14,;26.54,-15.56,;25.2,-16.04,;25.5,-16.8,;25.5,-18.39,;26.91,-18.95,)| Show InChI InChI=1S/C22H28FNO/c23-20-5-3-17(4-6-20)22(14-21(25)24-7-1-2-8-24)18-10-15-9-16(12-18)13-19(22)11-15/h3-6,15-16,18-19H,1-2,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50507376
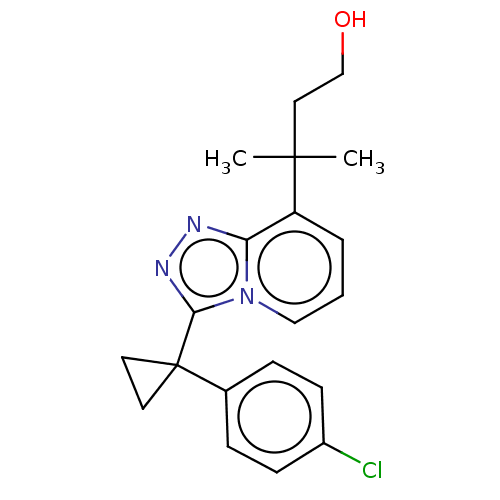
(CHEMBL4453347)Show SMILES CC(C)(CCO)c1cccn2c(nnc12)C1(CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H22ClN3O/c1-19(2,11-13-25)16-4-3-12-24-17(16)22-23-18(24)20(9-10-20)14-5-7-15(21)8-6-14/h3-8,12,25H,9-11,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... |
ACS Med Chem Lett 9: 1170-1174 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00307
BindingDB Entry DOI: 10.7270/Q20R9SP3 |
More data for this
Ligand-Target Pair | |
Stanniocalcin-1
(Mus musculus) | BDBM201239
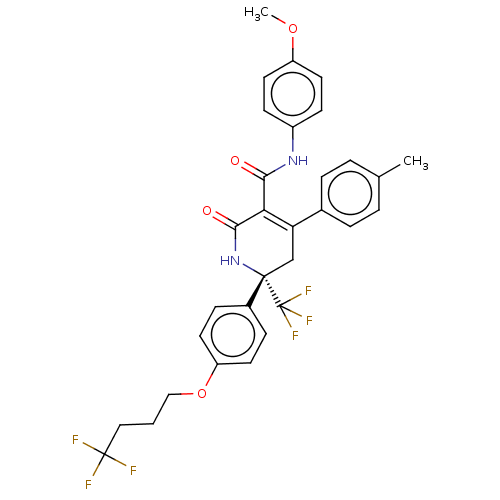
(US9187424, 6-2)Show SMILES COc1ccc(NC(=O)C2=C(C[C@](NC2=O)(c2ccc(OCCCC(F)(F)F)cc2)C(F)(F)F)c2ccc(C)cc2)cc1 |r,t:9| Show InChI InChI=1S/C31H28F6N2O4/c1-19-4-6-20(7-5-19)25-18-29(31(35,36)37,21-8-12-24(13-9-21)43-17-3-16-30(32,33)34)39-28(41)26(25)27(40)38-22-10-14-23(42-2)15-11-22/h4-15H,3,16-18H2,1-2H3,(H,38,40)(H,39,41)/t29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse STC1 in cell based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01356
BindingDB Entry DOI: 10.7270/Q2RF5ZVM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239632
(CHEMBL4071232)Show SMILES OCC1CN(C1)C(=O)CC1(C2CC3CC(C2)CC1C3)c1ccc(F)cc1 |TLB:18:17:15:11.12.13,8:9:11.18.12:16.14.15,19:9:15:11.12.13,THB:18:12:9.17.16:15,13:12:9:16.14.15,13:14:9:11.18.12,8:9:15:11.12.13,19:9:11.18.12:16.14.15,(48.51,-12.14,;50.02,-11.82,;50.49,-10.36,;49.8,-8.98,;51.18,-8.28,;51.87,-9.66,;51.66,-6.82,;50.41,-5.91,;53.13,-6.34,;54.27,-7.37,;55.47,-6.1,;56.79,-6.59,;58.18,-6.24,;58.2,-4.71,;56.8,-4.13,;55.46,-4.61,;55.76,-5.37,;55.77,-6.96,;57.18,-7.52,;54.26,-8.91,;52.91,-9.66,;52.9,-11.19,;54.22,-11.98,;54.21,-13.52,;55.57,-11.21,;55.58,-9.68,)| Show InChI InChI=1S/C22H28FNO2/c23-20-3-1-17(2-4-20)22(10-21(26)24-11-16(12-24)13-25)18-6-14-5-15(8-18)9-19(22)7-14/h1-4,14-16,18-19,25H,5-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Stanniocalcin-1
(Homo sapiens) | BDBM201239

(US9187424, 6-2)Show SMILES COc1ccc(NC(=O)C2=C(C[C@](NC2=O)(c2ccc(OCCCC(F)(F)F)cc2)C(F)(F)F)c2ccc(C)cc2)cc1 |r,t:9| Show InChI InChI=1S/C31H28F6N2O4/c1-19-4-6-20(7-5-19)25-18-29(31(35,36)37,21-8-12-24(13-9-21)43-17-3-16-30(32,33)34)39-28(41)26(25)27(40)38-22-10-14-23(42-2)15-11-22/h4-15H,3,16-18H2,1-2H3,(H,38,40)(H,39,41)/t29-/m0/s1 | MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human STC1 in cell based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01356
BindingDB Entry DOI: 10.7270/Q2RF5ZVM |
More data for this
Ligand-Target Pair | |
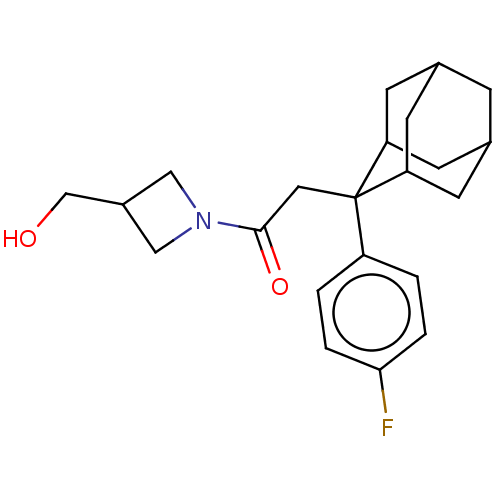
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239628

(CHEMBL4102283)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)C3O)c1ccc(cc1)-c1ccc(F)cc1 |TLB:12:11:8.13.14:16,7:8:16:10.11.17,19:8:10.12.11:14.15.16,THB:18:17:8.13.14:16,12:13:16:10.11.17,17:11:8:14.15.16,17:15:8:10.12.11,7:8:10.12.11:14.15.16,19:8:16:10.11.17,(5.77,-1.2,;6.92,-2.24,;8.46,-2.16,;8.53,-3.7,;7,-3.78,;9.68,-4.73,;9.36,-6.24,;11.15,-4.26,;12.29,-5.3,;13.49,-4.02,;14.82,-4.5,;16.22,-4.16,;15.2,-5.44,;13.79,-4.87,;13.78,-3.28,;14.83,-2.05,;13.48,-2.53,;16.23,-2.63,;17.52,-1.78,;12.27,-6.84,;10.94,-7.58,;10.92,-9.12,;12.24,-9.91,;13.59,-9.14,;13.6,-7.61,;12.23,-11.45,;10.89,-12.21,;10.87,-13.75,;12.2,-14.54,;12.19,-16.08,;13.55,-13.77,;13.56,-12.23,)| Show InChI InChI=1S/C27H30FNO3/c28-23-7-3-17(4-8-23)16-1-5-20(6-2-16)27(13-25(31)29-14-24(30)15-29)21-9-18-10-22(27)12-19(11-21)26(18)32/h1-8,18-19,21-22,24,26,30,32H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239619
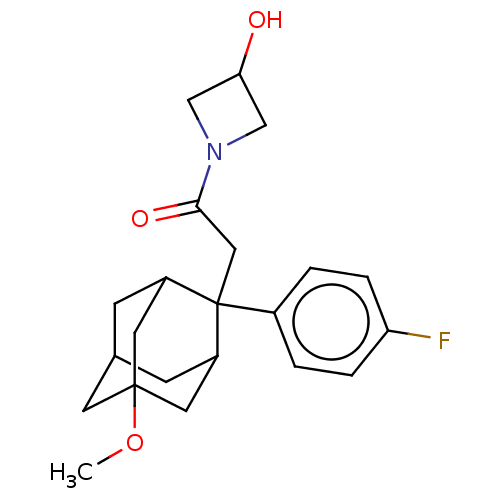
(CHEMBL4087497)Show SMILES COC12CC3CC(C1)C(CC(=O)N1CC(O)C1)(C(C3)C2)c1ccc(F)cc1 |TLB:18:17:7:5.4.3,20:8:5.18.4:19.2.7,20:8:7:5.4.3,THB:18:4:8.17.19:7,3:4:8:19.2.7,3:2:8:5.18.4,1:2:8:5.18.4,9:8:5.18.4:19.2.7,9:8:7:5.4.3,(28.8,-17.02,;27.27,-17.21,;26.66,-18.63,;28.07,-19.21,;28.05,-20.74,;26.65,-21.08,;25.33,-20.59,;25.32,-19.11,;24.13,-21.87,;22.99,-20.84,;21.52,-21.31,;20.27,-20.4,;21.04,-22.78,;19.66,-23.47,;20.36,-24.85,;19.88,-26.32,;21.74,-24.15,;25.63,-21.45,;27.04,-22.02,;25.62,-19.86,;24.12,-23.41,;22.77,-24.16,;22.76,-25.7,;24.08,-26.48,;24.07,-28.02,;25.43,-25.72,;25.44,-24.18,)| Show InChI InChI=1S/C22H28FNO3/c1-27-21-8-14-6-16(9-21)22(17(7-14)10-21,15-2-4-18(23)5-3-15)11-20(26)24-12-19(25)13-24/h2-5,14,16-17,19,25H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239609

(CHEMBL4093016)Show SMILES Fc1ccc(cc1)C1(CC(=O)N2CCC2)C2CC3CC(C2)CC1C3 |TLB:23:22:20:16.17.18,8:7:16.23.17:21.19.20,4:7:20:16.17.18,THB:23:17:7.22.21:20,18:17:7:21.19.20,18:19:7:16.23.17,8:7:20:16.17.18,4:7:16.23.17:21.19.20,(23.9,-12.85,;23.91,-11.3,;22.58,-10.52,;22.6,-8.98,;23.94,-8.23,;25.26,-9,;25.25,-10.54,;23.96,-6.69,;22.81,-5.65,;21.34,-6.13,;20.1,-5.22,;20.86,-7.6,;19.48,-8.3,;20.18,-9.68,;21.56,-8.98,;25.16,-5.41,;26.48,-5.9,;27.88,-5.55,;27.9,-4.02,;26.49,-3.44,;25.15,-3.92,;25.45,-4.68,;25.46,-6.27,;26.87,-6.84,)| Show InChI InChI=1S/C21H26FNO/c22-19-4-2-16(3-5-19)21(13-20(24)23-6-1-7-23)17-9-14-8-15(11-17)12-18(21)10-14/h2-5,14-15,17-18H,1,6-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239616

(CHEMBL4078671)Show SMILES COC1CN(C1)C(=O)CC1(C2CC3CC(C2)CC1C3)c1ccc(F)cc1 |TLB:18:17:15:11.12.13,8:9:11.18.12:16.14.15,19:9:15:11.12.13,THB:18:12:9.17.16:15,13:12:9:16.14.15,13:14:9:11.18.12,8:9:15:11.12.13,19:9:11.18.12:16.14.15,(63.87,-11.5,;65.38,-11.19,;65.86,-9.72,;65.16,-8.35,;66.55,-7.65,;67.23,-9.02,;67.02,-6.18,;65.77,-5.27,;68.49,-5.7,;69.64,-6.74,;70.83,-5.46,;72.15,-5.95,;73.55,-5.61,;73.57,-4.08,;72.16,-3.5,;70.82,-3.98,;71.13,-4.74,;71.13,-6.32,;72.54,-6.88,;69.62,-8.28,;68.28,-9.02,;68.26,-10.56,;69.59,-11.34,;69.57,-12.89,;70.92,-10.58,;70.93,-9.05,)| Show InChI InChI=1S/C22H28FNO2/c1-26-20-12-24(13-20)21(25)11-22(16-2-4-19(23)5-3-16)17-7-14-6-15(9-17)10-18(22)8-14/h2-5,14-15,17-18,20H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239603

(CHEMBL4101370)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)C3O)c1ccc(cc1)-c1ccncc1 |TLB:12:11:8.13.14:16,7:8:16:10.11.17,19:8:10.12.11:14.15.16,THB:18:17:8.13.14:16,12:13:16:10.11.17,17:11:8:14.15.16,17:15:8:10.12.11,7:8:10.12.11:14.15.16,19:8:16:10.11.17,(5.99,-16.91,;7.14,-17.94,;8.68,-17.86,;8.76,-19.41,;7.22,-19.48,;9.9,-20.44,;9.58,-21.95,;11.37,-19.97,;12.51,-21,;13.72,-19.72,;15.04,-20.22,;16.44,-19.87,;15.43,-21.14,;14.01,-20.58,;14.01,-18.99,;15.05,-17.75,;13.71,-18.23,;16.46,-18.34,;17.75,-17.49,;12.5,-22.54,;11.16,-23.3,;11.14,-24.83,;12.46,-25.61,;13.81,-24.85,;13.82,-23.32,;12.45,-27.16,;11.11,-27.92,;11.09,-29.46,;12.42,-30.24,;13.77,-29.48,;13.78,-27.94,)| Show InChI InChI=1S/C26H30N2O3/c29-23-14-28(15-23)24(30)13-26(21-9-18-10-22(26)12-19(11-21)25(18)31)20-3-1-16(2-4-20)17-5-7-27-8-6-17/h1-8,18-19,21-23,25,29,31H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]PIA from adenosine A1 receptor of rat brain membranes |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239614

(CHEMBL4067777)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC(C2)CC1C3)c1ccc(F)cc1 |TLB:17:16:14:10.11.12,7:8:10.17.11:15.13.14,18:8:14:10.11.12,THB:17:11:8.16.15:14,12:11:8:15.13.14,12:13:8:10.17.11,7:8:14:10.11.12,18:8:10.17.11:15.13.14,(49.46,-25.67,;49.94,-24.2,;49.24,-22.82,;50.62,-22.13,;51.32,-23.5,;51.1,-20.66,;49.85,-19.75,;52.57,-20.18,;53.71,-21.22,;54.91,-19.94,;56.23,-20.43,;57.63,-20.08,;57.65,-18.55,;56.24,-17.97,;54.9,-18.45,;55.2,-19.21,;55.21,-20.8,;56.62,-21.36,;53.69,-22.76,;52.35,-23.51,;52.33,-25.04,;53.66,-25.83,;53.65,-27.37,;55.01,-25.06,;55.01,-23.53,)| Show InChI InChI=1S/C21H26FNO2/c22-18-3-1-15(2-4-18)21(10-20(25)23-11-19(24)12-23)16-6-13-5-14(8-16)9-17(21)7-13/h1-4,13-14,16-17,19,24H,5-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50568531
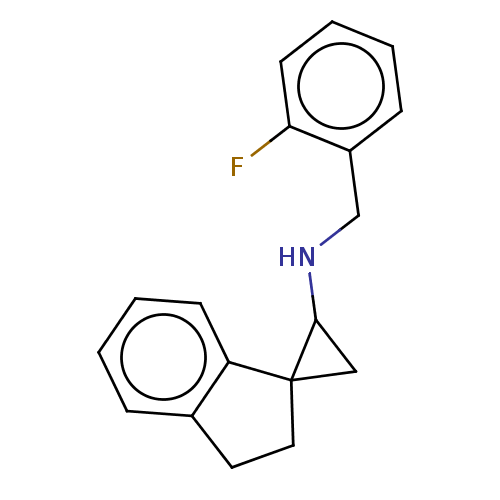
(CHEMBL4855475) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114143
BindingDB Entry DOI: 10.7270/Q28W3J9V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50306431

(2-[(3R)-1'-[(adamantan-2-yl)carbamoyl]-7-bromo-2,3...)Show SMILES OC(=O)C[C@H]1CC2(CCN(CC2)C(=O)NC2C3CC4CC(C3)CC2C4)c2c1cccc2Br |r,TLB:21:20:24:17.16.15,21:16:19.20.22:24,THB:15:16:19:22.23.24,15:23:19:17.21.16,14:15:19.20.22:24| Show InChI InChI=1S/C26H33BrN2O3/c27-21-3-1-2-20-19(13-22(30)31)14-26(23(20)21)4-6-29(7-5-26)25(32)28-24-17-9-15-8-16(11-17)12-18(24)10-15/h1-3,15-19,24H,4-14H2,(H,28,32)(H,30,31)/t15?,16?,17?,18?,19-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD-1 |
Bioorg Med Chem Lett 21: 6699-704 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.055
BindingDB Entry DOI: 10.7270/Q2Q240NV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50597805
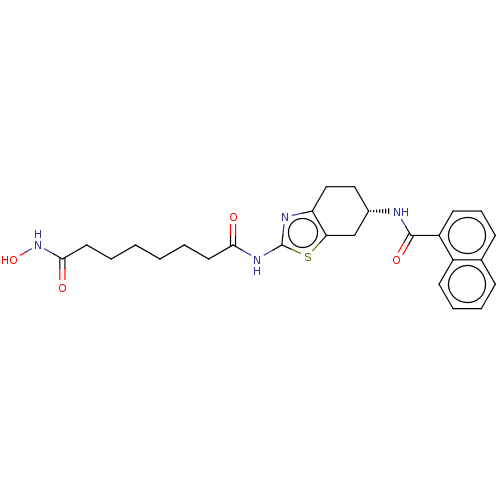
(CHEMBL5199007)Show SMILES ONC(=O)CCCCCCC(=O)Nc1nc2CC[C@@H](Cc2s1)NC(=O)c1cccc2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113946
BindingDB Entry DOI: 10.7270/Q2348QFG |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50579517
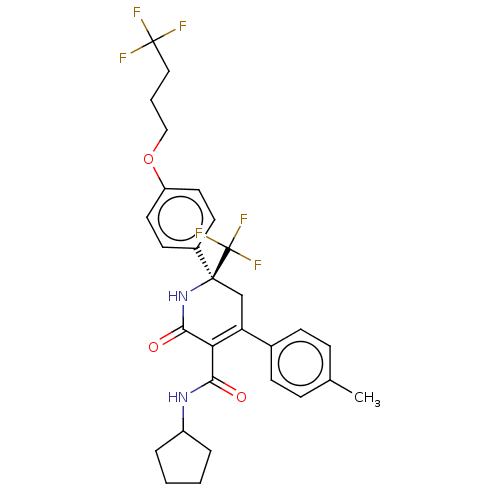
(CHEMBL4847167)Show SMILES Cc1ccc(cc1)C1=C(C(=O)NC2CCCC2)C(=O)N[C@@](C1)(c1ccc(OCCCC(F)(F)F)cc1)C(F)(F)F |r,c:8| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MGAT2 expressed in Sf9 cell membrane using 2-monooleglycerol and [H3]-oleoyl-CoA as substrates incubated for 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01356
BindingDB Entry DOI: 10.7270/Q2RF5ZVM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data