

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286099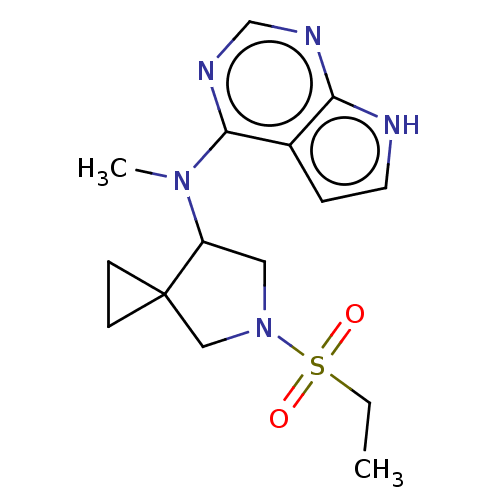 (N-(5-(ethylsulfonyl)-5-azaspiro[2.4]heptan-7-yl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286098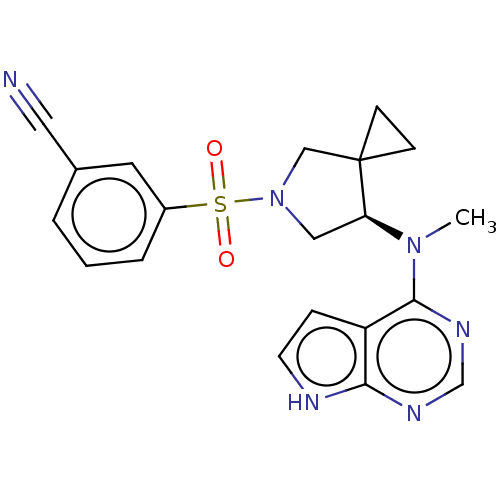 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286095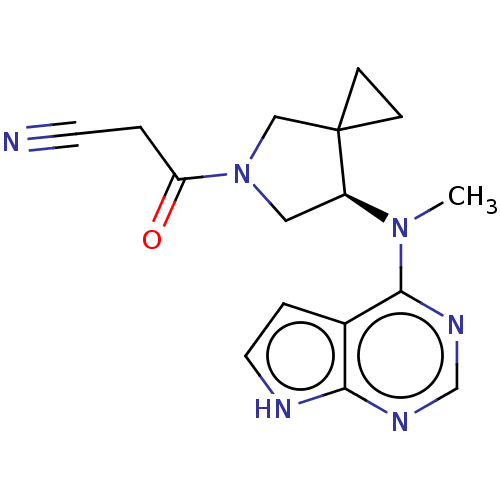 ((R)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286098 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.7 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286096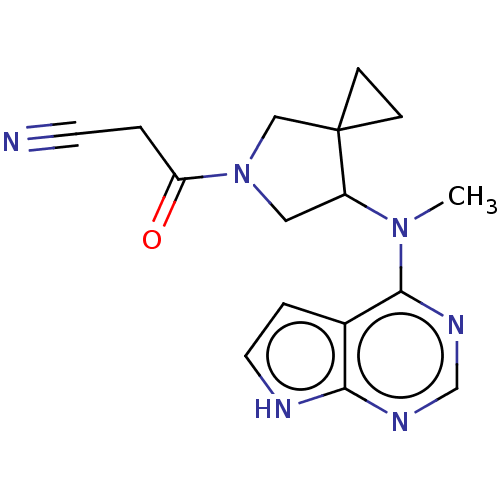 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29.3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286096 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40.4 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286098 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 58.6 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM286098 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286096 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286099 (N-(5-(ethylsulfonyl)-5-azaspiro[2.4]heptan-7-yl)-N...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286095 ((R)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286095 ((R)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286099 (N-(5-(ethylsulfonyl)-5-azaspiro[2.4]heptan-7-yl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM286096 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 694 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286097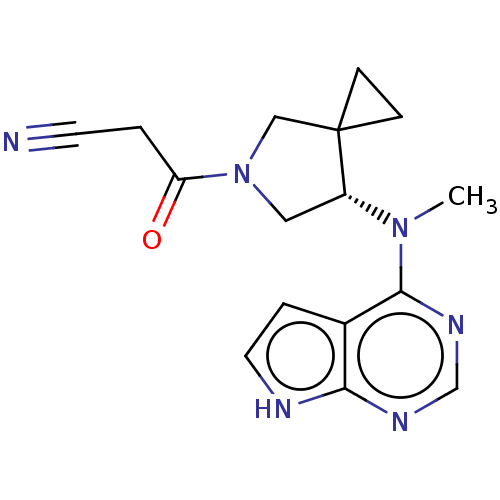 ((S)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 787 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286097 ((S)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM286097 ((S)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286097 ((S)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM286099 (N-(5-(ethylsulfonyl)-5-azaspiro[2.4]heptan-7-yl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM286095 ((R)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397111 (N-(1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-(2-thiaz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397112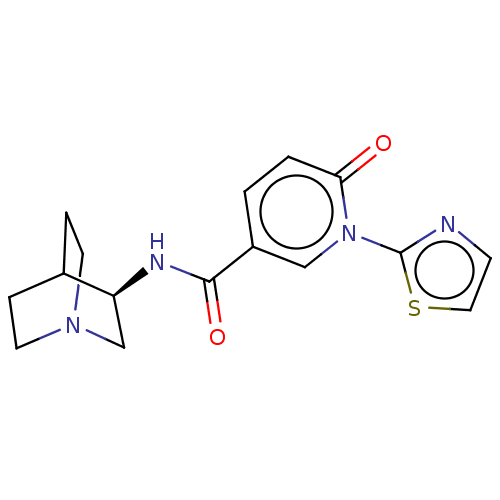 (N-[(3R)-1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397113 (N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397114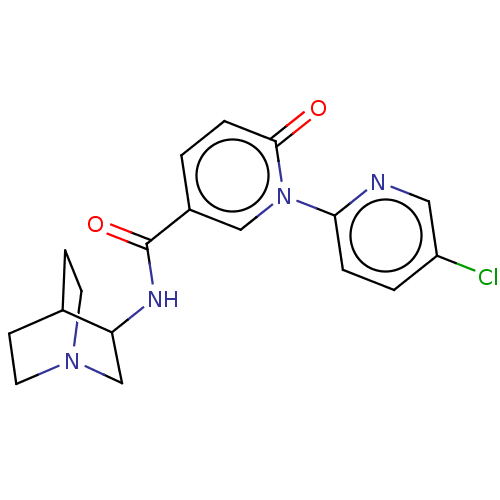 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(5-chloro-2-py...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397115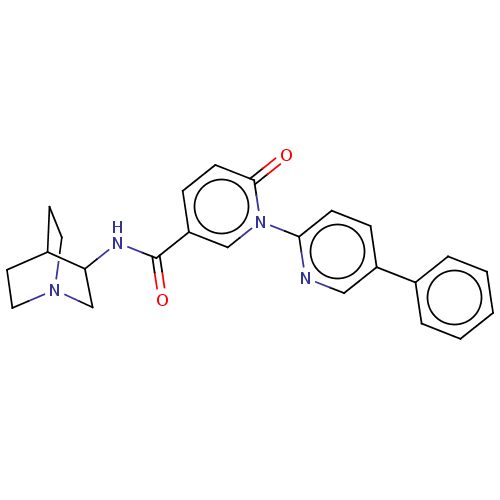 (N-(1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-(5-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397116 (N-(1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-[3-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397117 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(5-methyl-2-th...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397118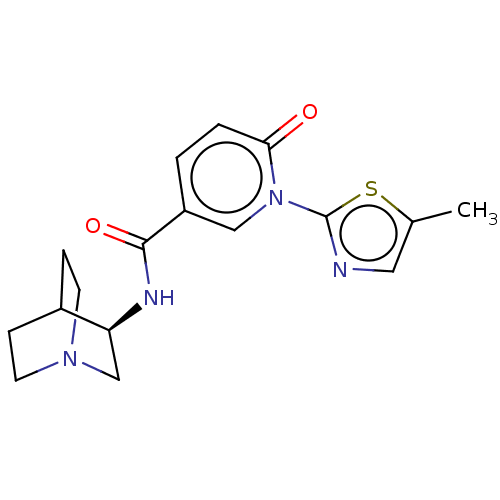 (N-[(3R)-1-azabicyclo[2.2.2]octan-3-yl]-1-(5-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397119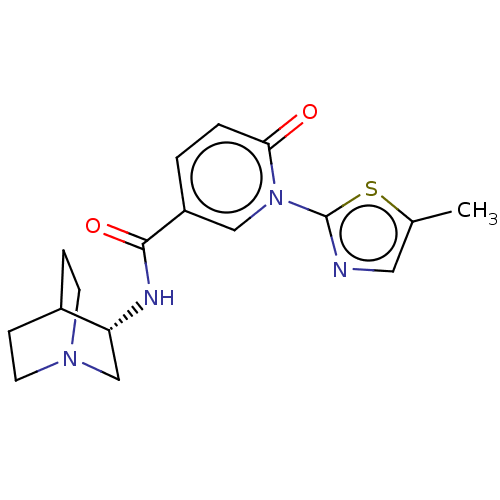 (N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-1-(5-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397120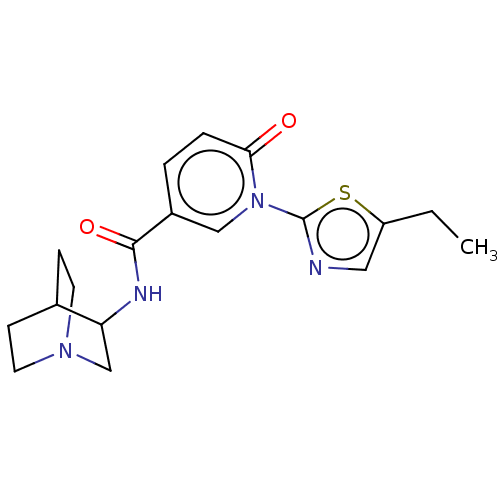 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(5-ethyl-2-thi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397121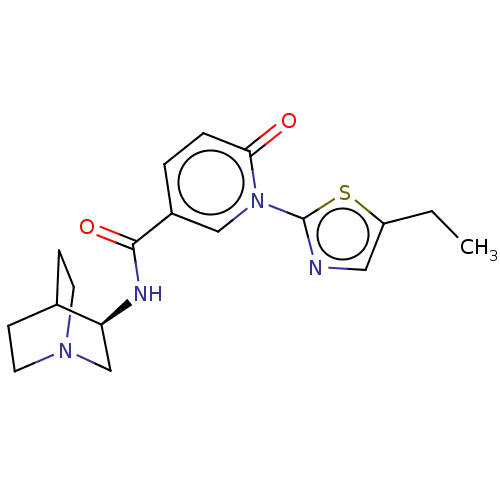 (N-[(3R)-1-azabicyclo[2.2.2]octan-3-yl]-1-(5-ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397122 (N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-1-(5-ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397123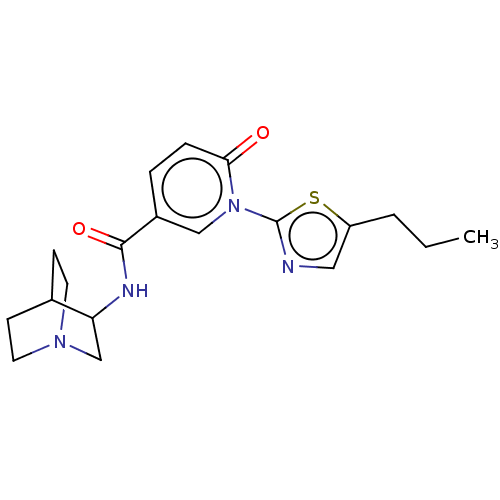 (N-(1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-(5-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397124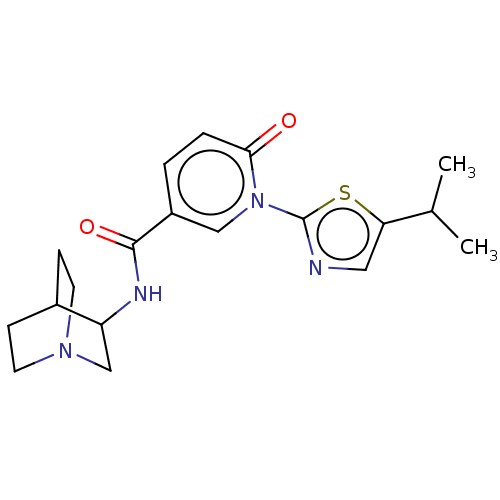 (N-(1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-(5-propa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397125 (N-[(3R)-1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-(5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397126 (N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-(5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397127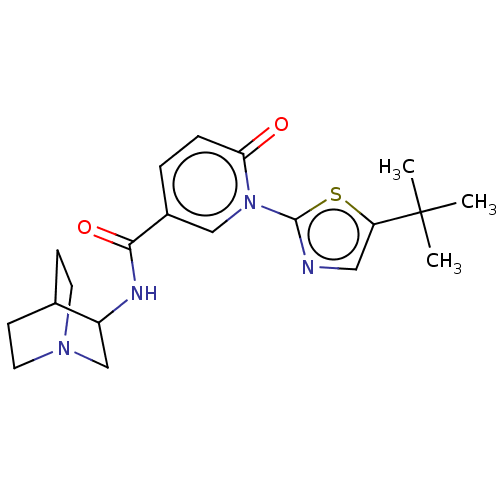 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(5-tert-butyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397128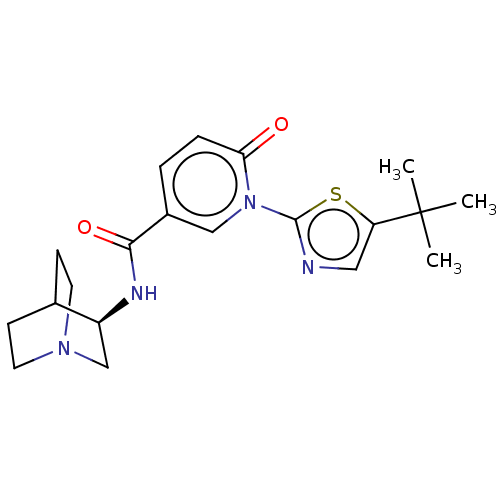 (N-[(3R)-1-azabicyclo[2.2.2]octan-3-yl]-1-[5-tert-b...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397129 (N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-1-[5-tert-b...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397130 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(5-cyclopentyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397131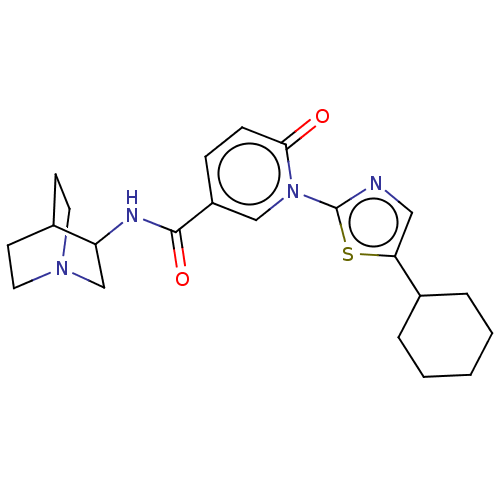 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(5-cyclohexyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397132 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(5-phenyl-2-th...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397133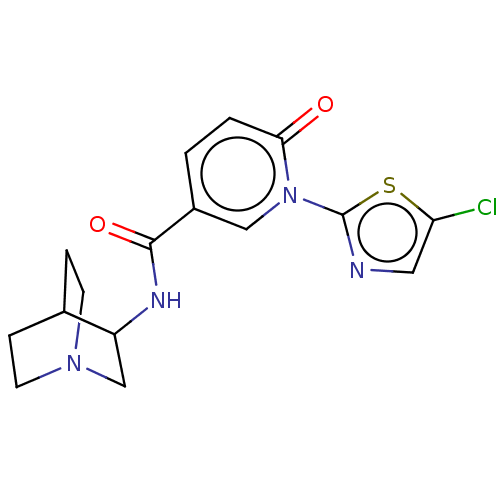 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(5-chloro-2-th...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397134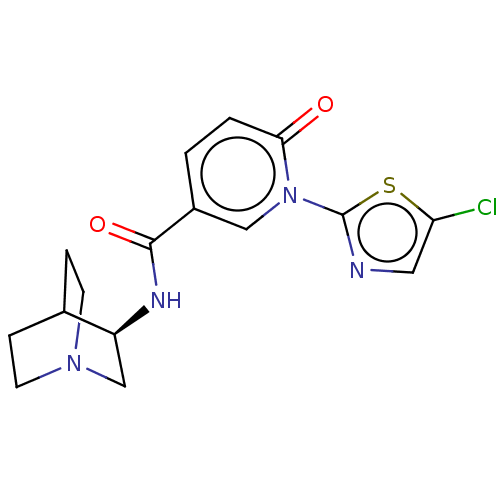 (N-[(3R)-1-azabicyclo[2.2.2]octan-3-yl]-1-(5-chloro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397135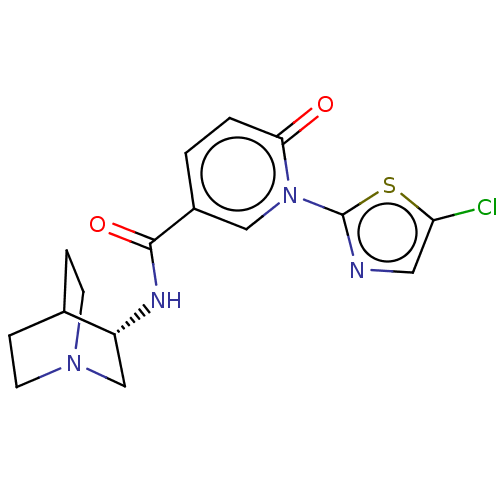 (N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-1-(5-chloro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397136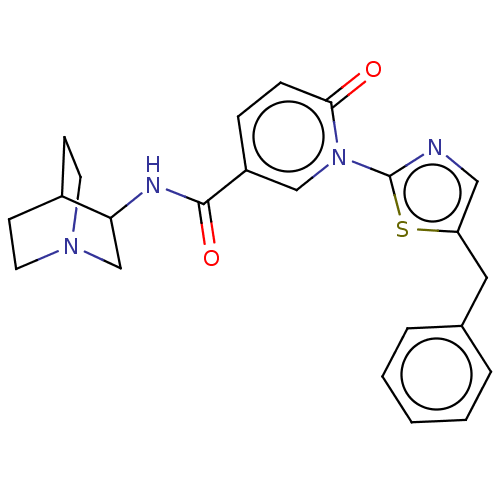 (N-(1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-[5-(phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397137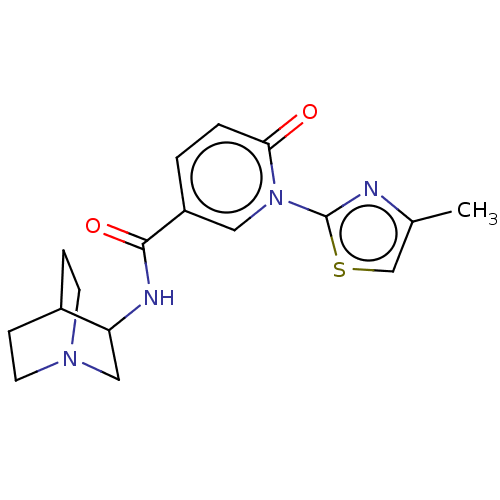 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(4-methyl-2-th...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397138 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-(1,3-benzothia...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397139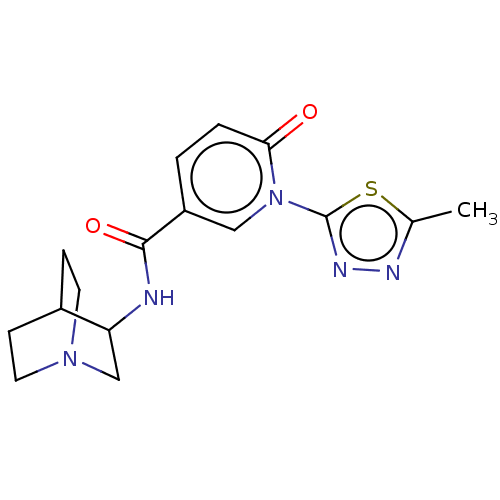 (N-(1-azabicyclo[2.2.2]octan-3-yl)-1-[5-methyl-1,3,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM397140 ((1-azabicyclo[2.2.2]octan-3-yl)-1-[5-methyl-1,3-th...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |