

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Homo sapiens (Human)) | BDBM50137733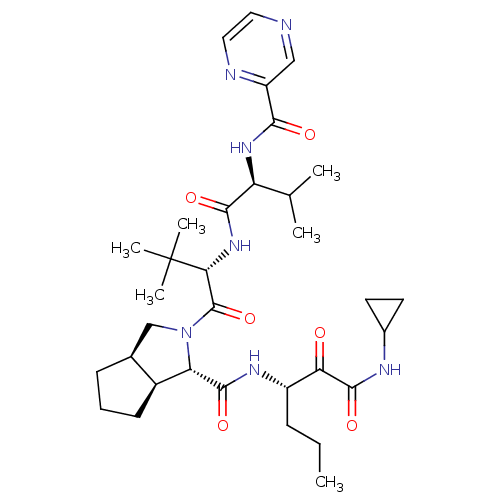 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin B | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50137736 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin B | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin B | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50137730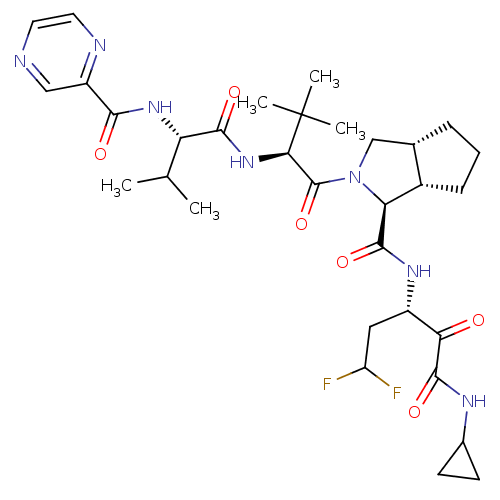 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin B | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150593 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin L | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137724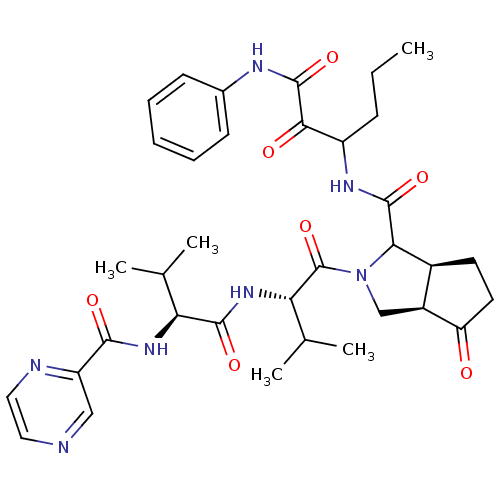 ((3aR,5S)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyraz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50152750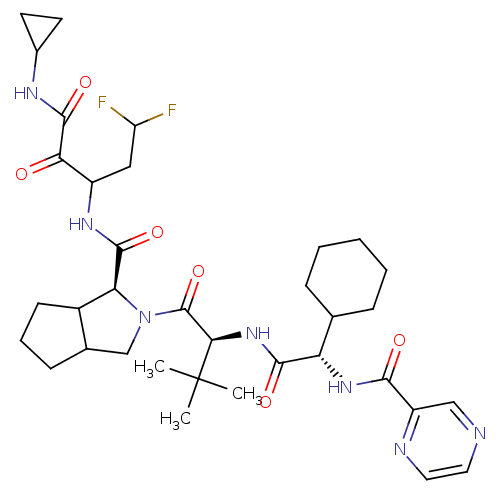 (2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carbonyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HCV NS3 protease in the pNA based inhibition assay | Bioorg Med Chem Lett 14: 5007-11 (2004) Article DOI: 10.1016/j.bmcl.2004.07.007 BindingDB Entry DOI: 10.7270/Q29P314N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150591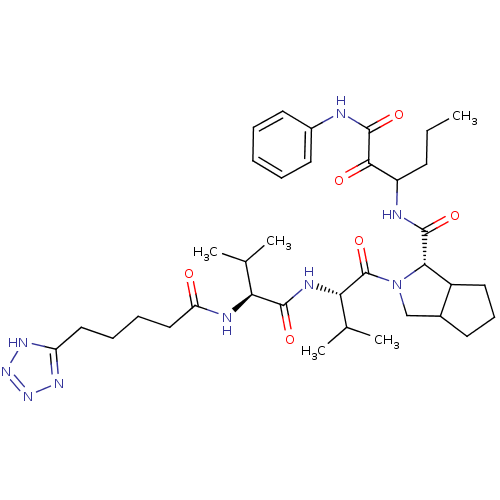 ((S)-2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetraz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50137732 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin L | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150599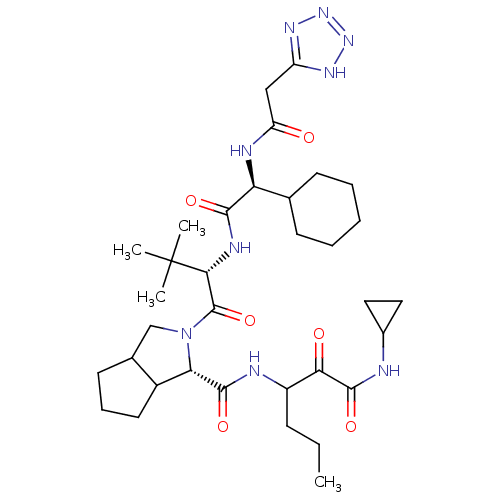 (2-{(S)-2-[(S)-2-Cyclohexyl-2-(2-2H-tetrazol-5-yl-a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50137730 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin L | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150595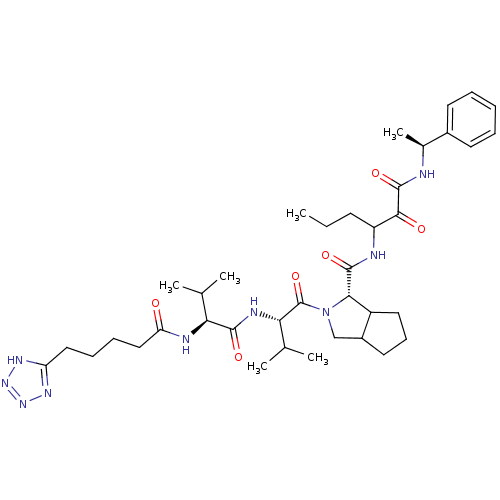 ((S)-2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetraz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50137736 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin L | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50137732 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin B | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50137732 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Chymotrypsin | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150606 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137721 (2-(3-{[(1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50152754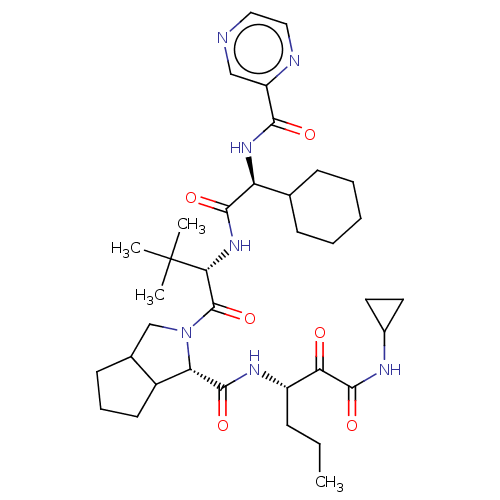 (2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carbonyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HCV NS3 protease in the pNA based inhibition assay | Bioorg Med Chem Lett 14: 5007-11 (2004) Article DOI: 10.1016/j.bmcl.2004.07.007 BindingDB Entry DOI: 10.7270/Q29P314N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150602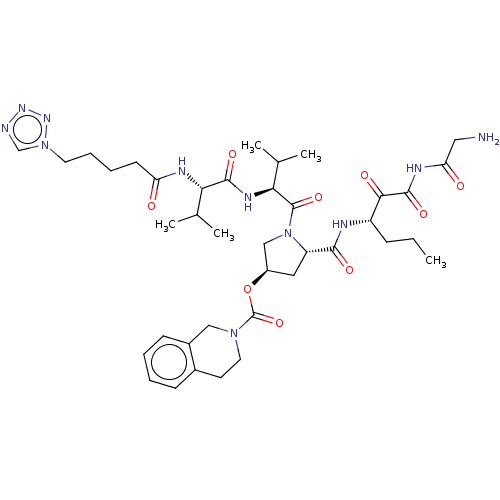 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50137733 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HCV NS3 protease in the pNA based inhibition assay | Bioorg Med Chem Lett 14: 5007-11 (2004) Article DOI: 10.1016/j.bmcl.2004.07.007 BindingDB Entry DOI: 10.7270/Q29P314N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50152753 ((S)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[(pyrazi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HCV NS3 protease in the pNA based inhibition assay | Bioorg Med Chem Lett 14: 5007-11 (2004) Article DOI: 10.1016/j.bmcl.2004.07.007 BindingDB Entry DOI: 10.7270/Q29P314N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50152748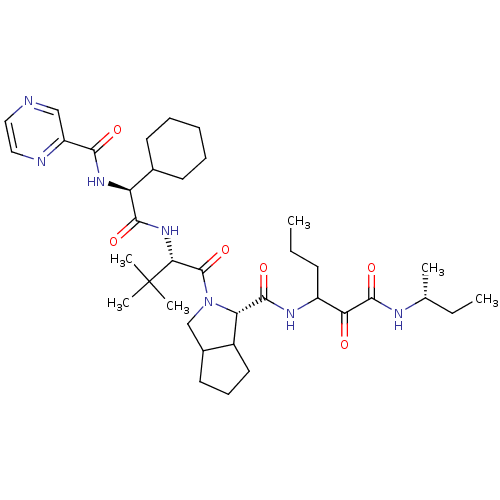 ((S)-2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HCV NS3 protease in the pNA based inhibition assay | Bioorg Med Chem Lett 14: 5007-11 (2004) Article DOI: 10.1016/j.bmcl.2004.07.007 BindingDB Entry DOI: 10.7270/Q29P314N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150604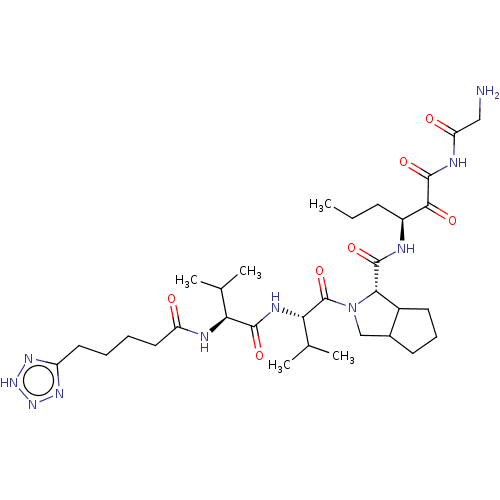 (2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetrazol-5...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137713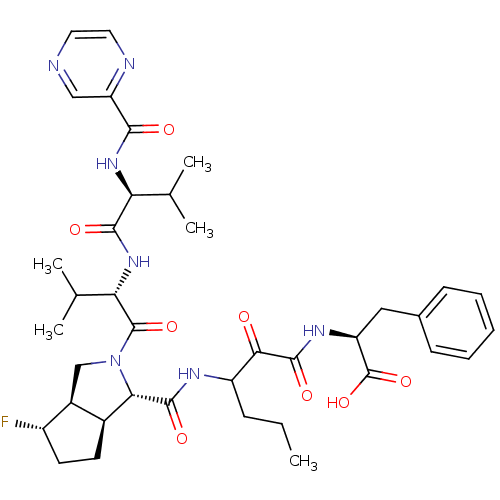 ((S)-2-((S)-3-{[(1S,5S,6R)-4-Fluoro-2-((S)-3-methyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137733 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity of the compound towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137718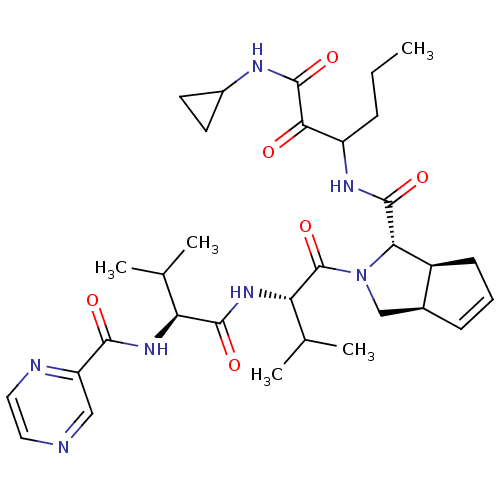 ((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50152751 (2-((S)-2-{(S)-2-Cyclohexyl-2-[((R)-pyrazine-2-carb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HCV NS3 protease in the pNA based inhibition assay | Bioorg Med Chem Lett 14: 5007-11 (2004) Article DOI: 10.1016/j.bmcl.2004.07.007 BindingDB Entry DOI: 10.7270/Q29P314N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150605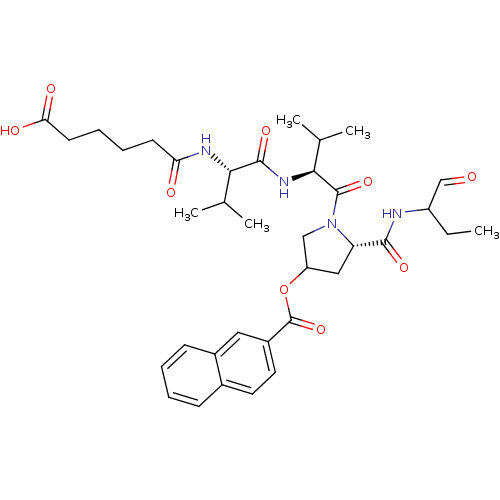 (CHEMBL360805 | Naphthalene-2-carboxylic acid (S)-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150598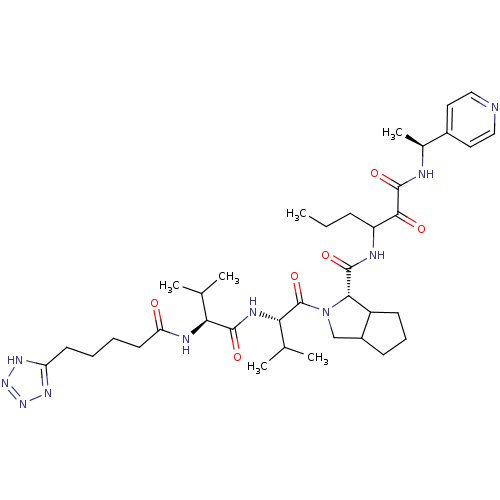 ((S)-2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetraz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137738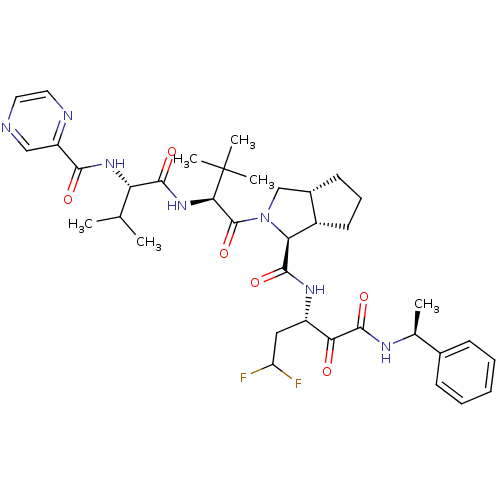 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity of the compound towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity of the compound towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150601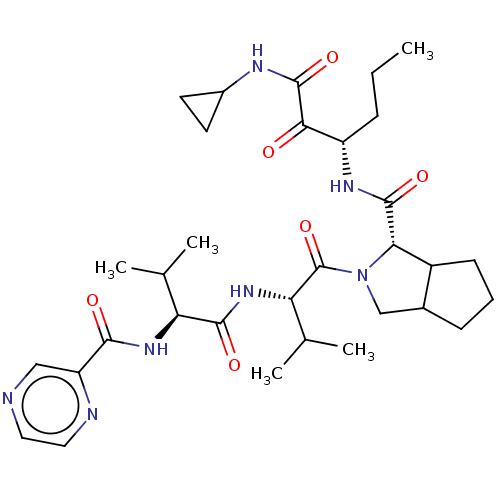 (2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyrazine-2-car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50152755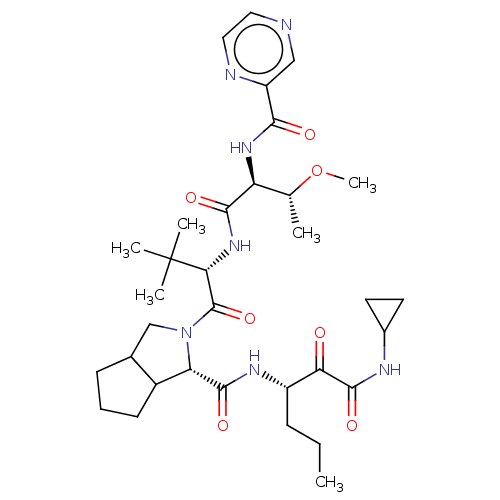 (2-((S)-2-{(S)-3-Methoxy-2-[(pyrazine-2-carbonyl)-a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HCV NS3 protease in the pNA based inhibition assay | Bioorg Med Chem Lett 14: 5007-11 (2004) Article DOI: 10.1016/j.bmcl.2004.07.007 BindingDB Entry DOI: 10.7270/Q29P314N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50137733 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin L | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50152752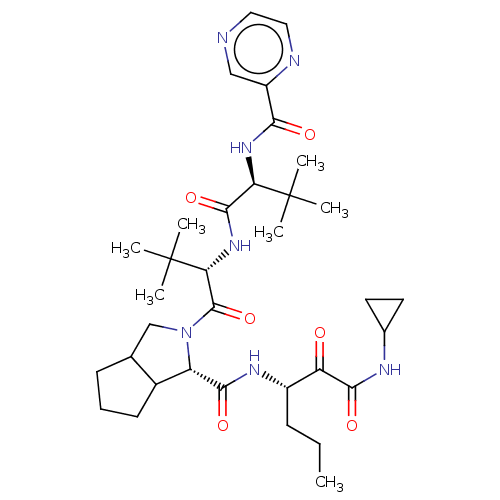 ((S)-2-((S)-2-{(S)-3,3-Dimethyl-2-[(pyrazine-2-carb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HCV NS3 protease in the pNA based inhibition assay | Bioorg Med Chem Lett 14: 5007-11 (2004) Article DOI: 10.1016/j.bmcl.2004.07.007 BindingDB Entry DOI: 10.7270/Q29P314N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150594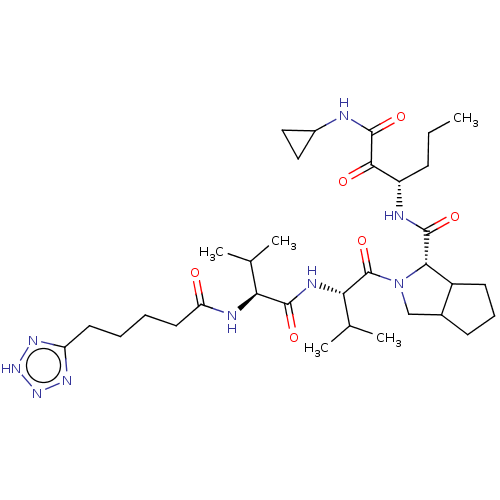 ((S)-2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetraz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137722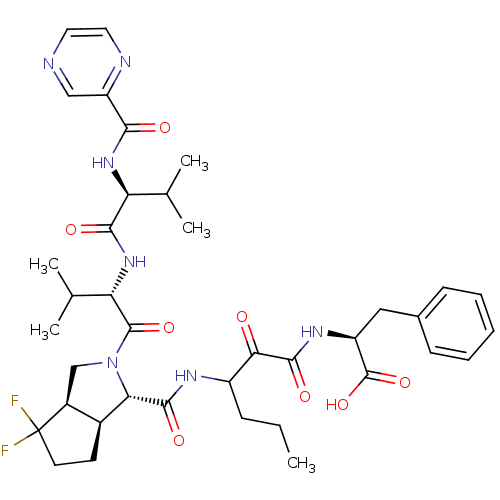 ((S)-2-(3-{[(1S,5S,6R)-4,4-Difluoro-2-((S)-3-methyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137732 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity of the compound towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150607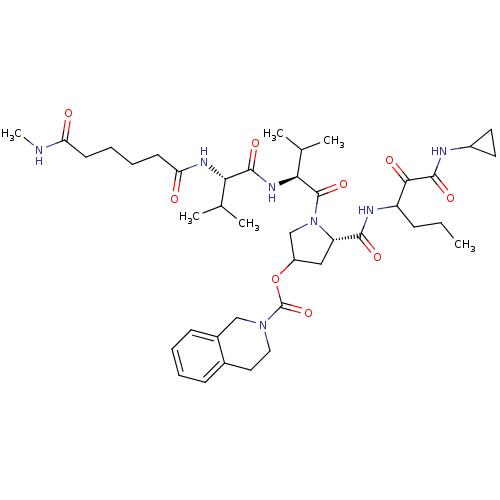 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137730 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity of the compound towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137736 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity of the compound towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137729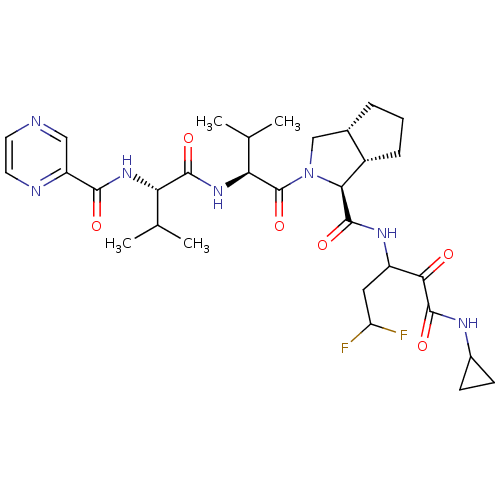 ((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HCV protease using replicon assay in rats | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50137732 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin K | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150590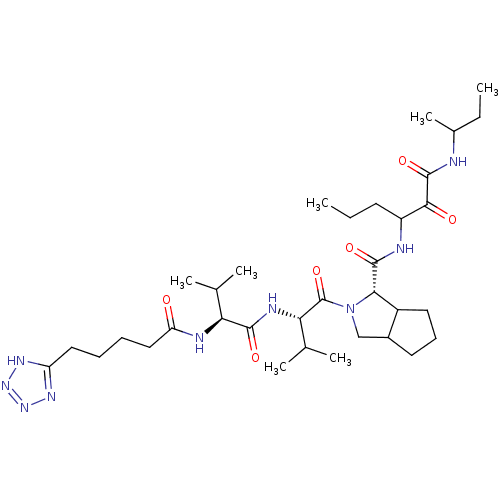 ((S)-2-{(S)-3-Methyl-2-[(S)-3-methyl-2-(5-1H-tetraz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50152758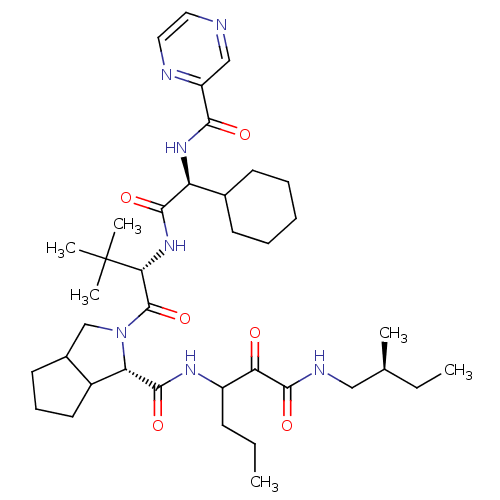 ((S)-2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HCV NS3 protease in the pNA based inhibition assay | Bioorg Med Chem Lett 14: 5007-11 (2004) Article DOI: 10.1016/j.bmcl.2004.07.007 BindingDB Entry DOI: 10.7270/Q29P314N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150597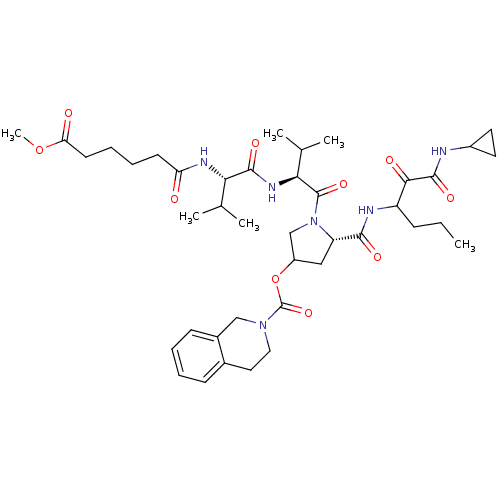 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137717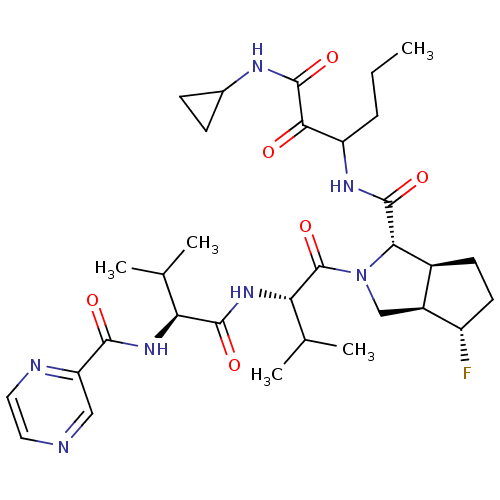 ((1S,5S,6R)-4-Fluoro-2-((S)-3-methyl-2-{(S)-3-methy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50137730 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 303 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Chymotrypsin | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 178 total ) | Next | Last >> |