

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071729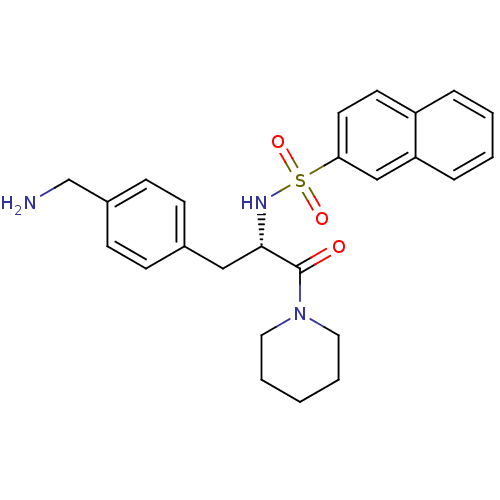 (CHEMBL313826 | Naphthalene-2-sulfonic acid [(S)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071723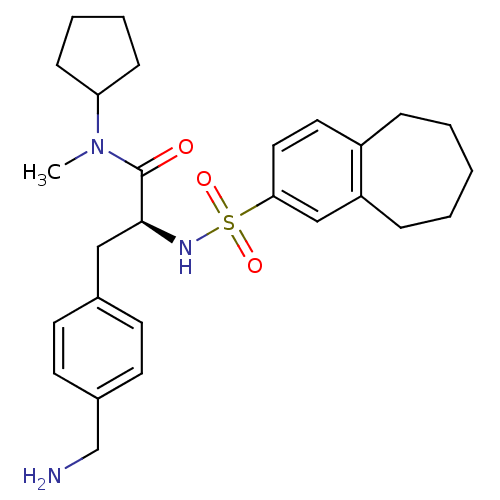 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069292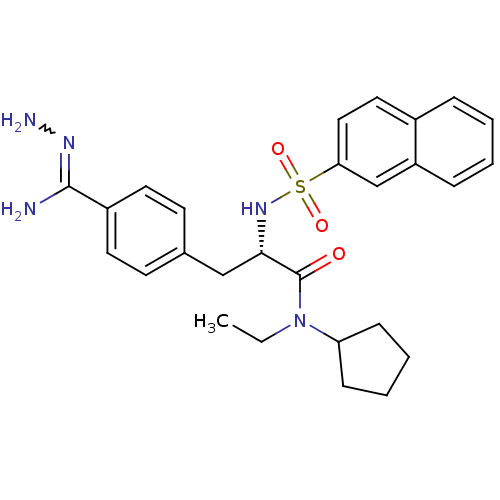 (CHEMBL156082 | N-ethyl-N-cyclopentyl-3-(4-hydrazon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22741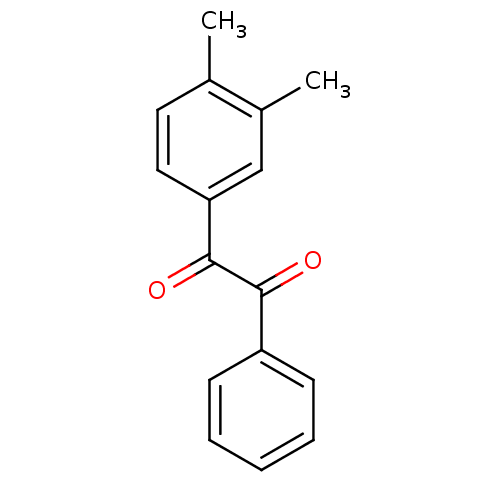 (1-(3,4-dimethylphenyl)-2-phenylethane-1,2-dione | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 4.10 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22733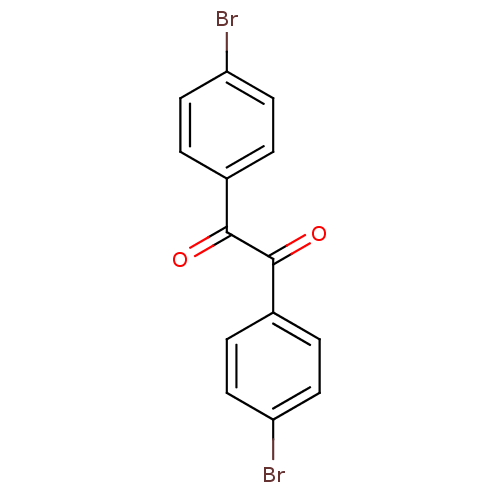 (1,2-bis(4-bromophenyl)ethane-1,2-dione | Benzil-ba...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50496304 (CHEMBL3125712) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22759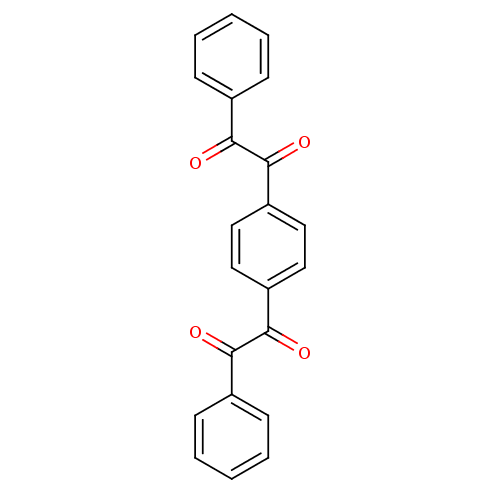 (1-[4-(2-oxo-2-phenylacetyl)phenyl]-2-phenylethane-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071725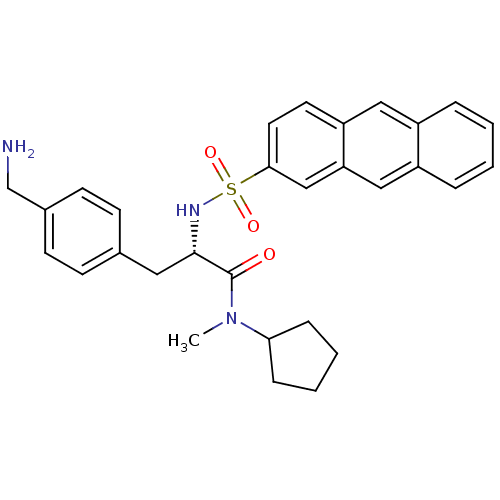 ((S)-3-(4-Aminomethyl-phenyl)-2-(anthracene-2-sulfo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22759 (1-[4-(2-oxo-2-phenylacetyl)phenyl]-2-phenylethane-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071722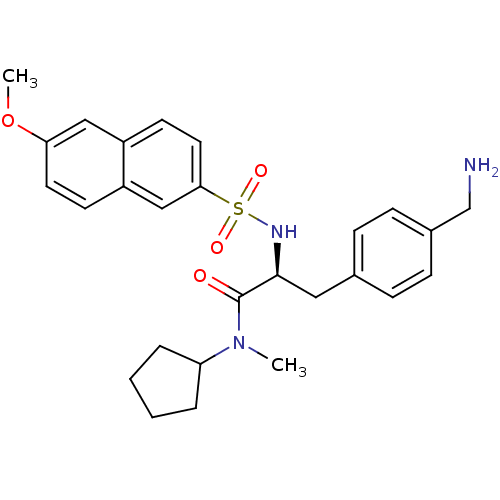 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50496310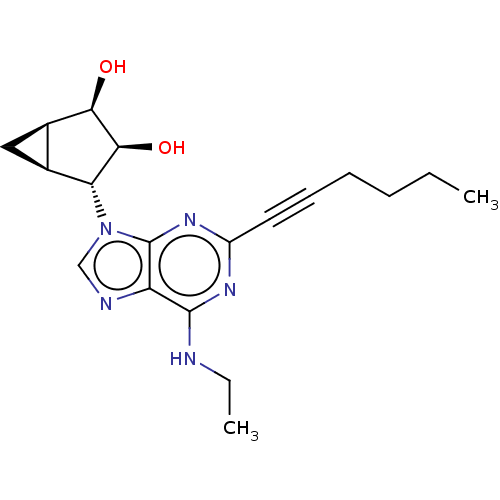 (CHEMBL3125713) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070783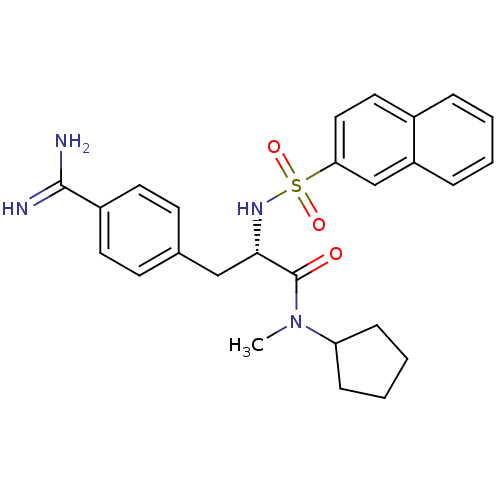 ((S)-3-(4-Carbamimidoyl-phenyl)-N-cyclopentyl-N-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50496311 (CHEMBL3125718) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22748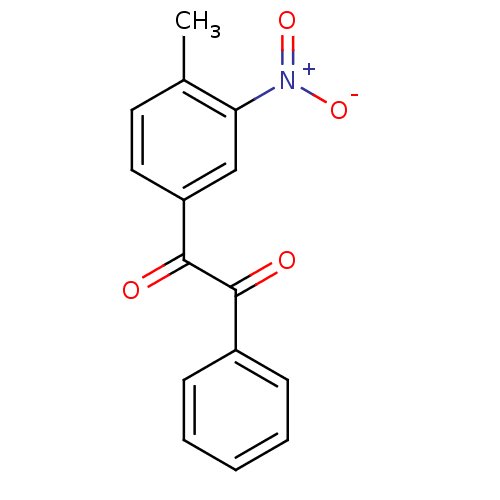 (1-(4-methyl-3-nitrophenyl)-2-phenylethane-1,2-dion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM22759 (1-[4-(2-oxo-2-phenylacetyl)phenyl]-2-phenylethane-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50496307 (CHEMBL3125717) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22747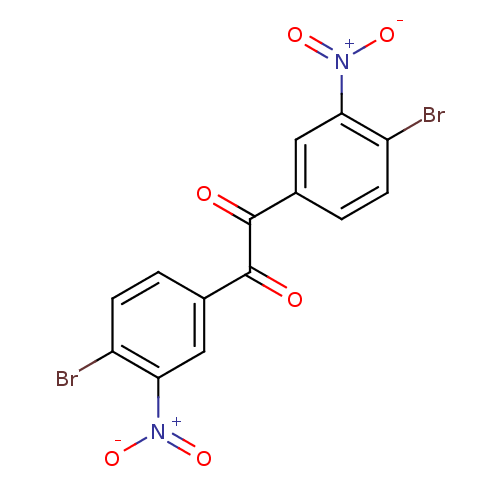 (1,2-bis(4-bromo-3-nitrophenyl)ethane-1,2-dione | B...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22745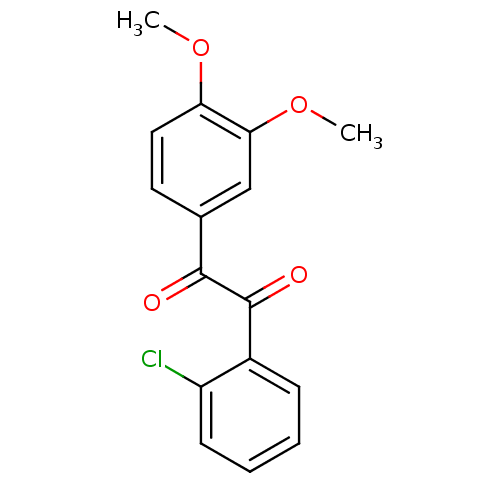 (1-(2-chlorophenyl)-2-(3,4-dimethoxyphenyl)ethane-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 8.90 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50496308 (CHEMBL3125714) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22731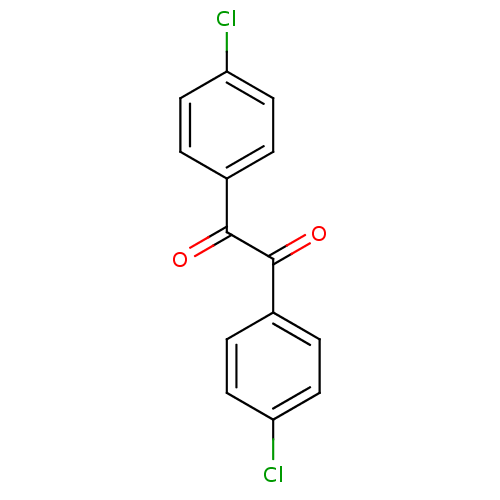 (1,2-bis(4-chlorophenyl)ethane-1,2-dione | Benzil-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069055 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071726 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22742 (1-(4-methoxyphenyl)-2-phenylethane-1,2-dione | Ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 10.3 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071724 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50496311 (CHEMBL3125718) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from rat adenosine A3 receptor expressed in RBL-2H3 cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22748 (1-(4-methyl-3-nitrophenyl)-2-phenylethane-1,2-dion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50496305 (CHEMBL3125716) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22722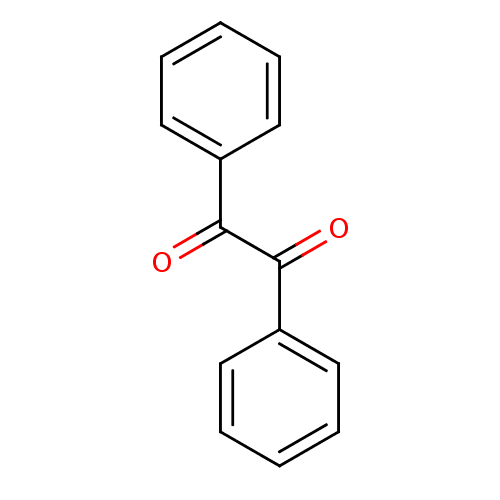 (1,2-diphenylethane-1,2-dione | Benzil | CHEMBL1898...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 14.7 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM22722 (1,2-diphenylethane-1,2-dione | Benzil | CHEMBL1898...) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 14.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition constant against human intestinal carboxylesterase (hiCE) expressed in Sf21 cells using 3 mM o-NPA | J Med Chem 48: 5543-50 (2005) Article DOI: 10.1021/jm0504196 BindingDB Entry DOI: 10.7270/Q2FB52G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22746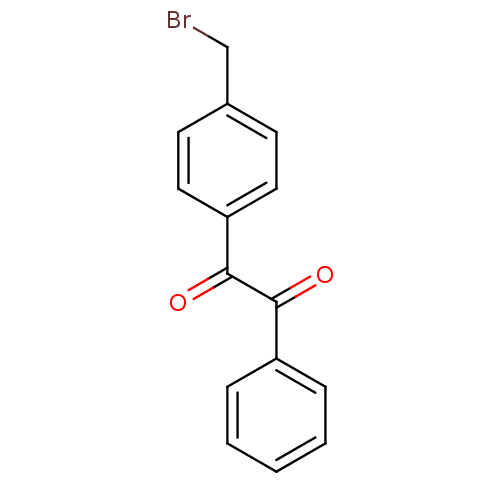 (1-[4-(bromomethyl)phenyl]-2-phenylethane-1,2-dione...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50496306 (CHEMBL3125711) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50496309 (CHEMBL3125719) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22738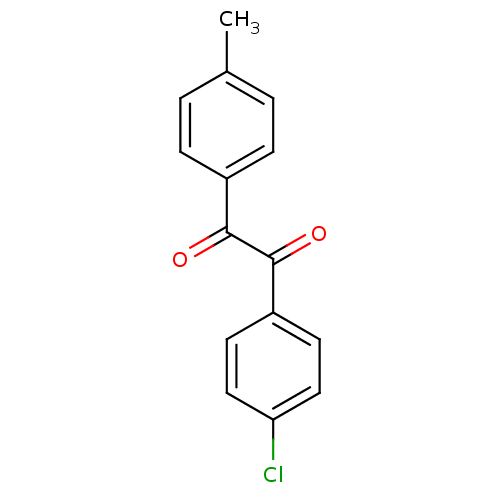 (1-(4-chlorophenyl)-2-(4-methylphenyl)ethane-1,2-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22734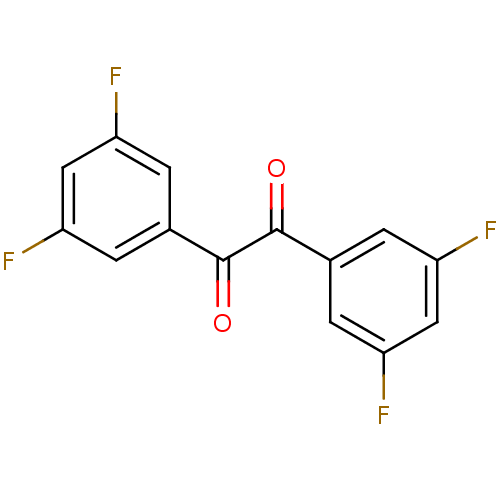 (1,2-bis(3,5-difluorophenyl)ethane-1,2-dione | Benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069053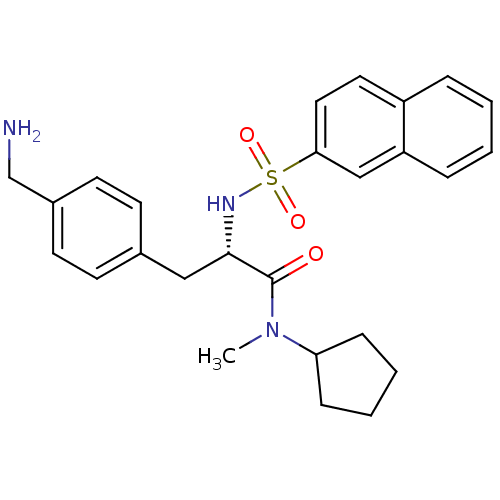 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22737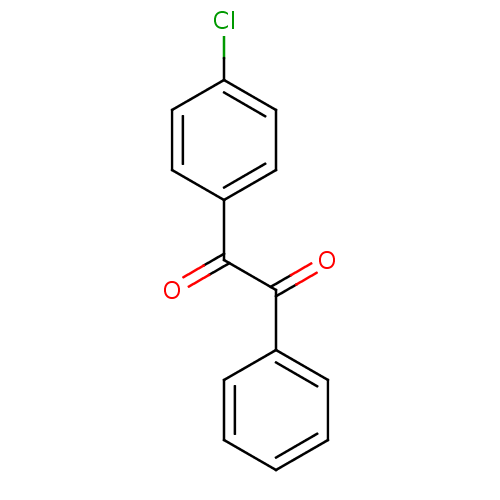 (1-(4-chlorophenyl)-2-phenylethane-1,2-dione | Benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 18.2 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071721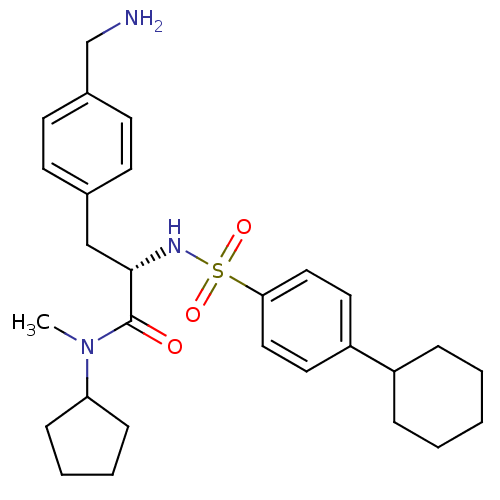 ((S)-3-(4-Aminomethyl-phenyl)-2-(4-cyclohexyl-benze...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22746 (1-[4-(bromomethyl)phenyl]-2-phenylethane-1,2-dione...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 21.3 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22749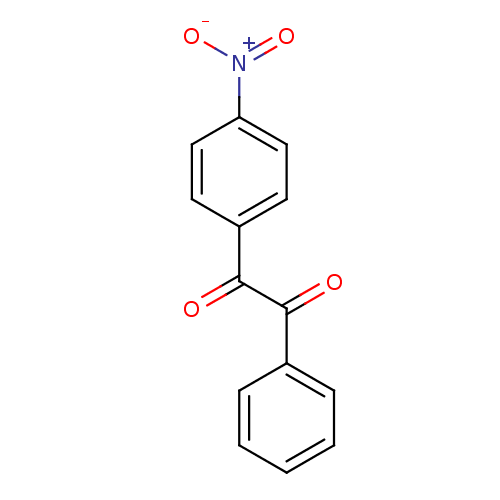 (1-(4-nitrophenyl)-2-phenylethane-1,2-dione | Benzi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22738 (1-(4-chlorophenyl)-2-(4-methylphenyl)ethane-1,2-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 22.8 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22734 (1,2-bis(3,5-difluorophenyl)ethane-1,2-dione | Benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23.4 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22737 (1-(4-chlorophenyl)-2-phenylethane-1,2-dione | Benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 23.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50496305 (CHEMBL3125716) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [125I]-I-AB-MECA from rat adenosine A3 receptor expressed in RBL-2H3 cells after 60 mins by gamma counting analysis | J Med Chem 57: 1344-54 (2014) Article DOI: 10.1021/jm4015313 BindingDB Entry DOI: 10.7270/Q26976JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50171925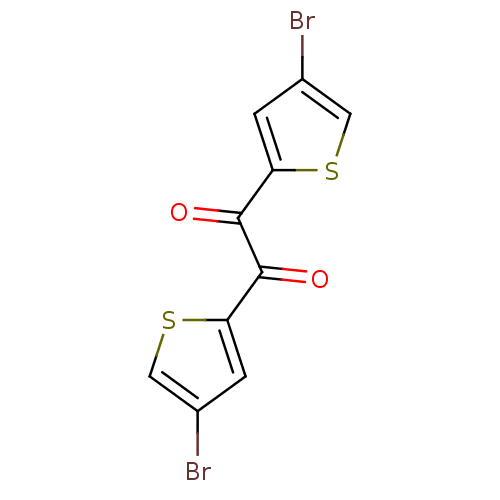 (1,2-Bis-(4-bromo-thiophen-2-yl)-ethane-1,2-dione |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition constant against human liver carboxylesterase (hCE1) expressed in Sf21 cells using 3 mM o-NPA | J Med Chem 48: 5543-50 (2005) Article DOI: 10.1021/jm0504196 BindingDB Entry DOI: 10.7270/Q2FB52G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50171925 (1,2-Bis-(4-bromo-thiophen-2-yl)-ethane-1,2-dione |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition constant against human liver carboxylesterase (hCE1) expressed in Sf21 cells using 3 mM o-NPA | J Med Chem 48: 5543-50 (2005) Article DOI: 10.1021/jm0504196 BindingDB Entry DOI: 10.7270/Q2FB52G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22749 (1-(4-nitrophenyl)-2-phenylethane-1,2-dione | Benzi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 30.6 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM22739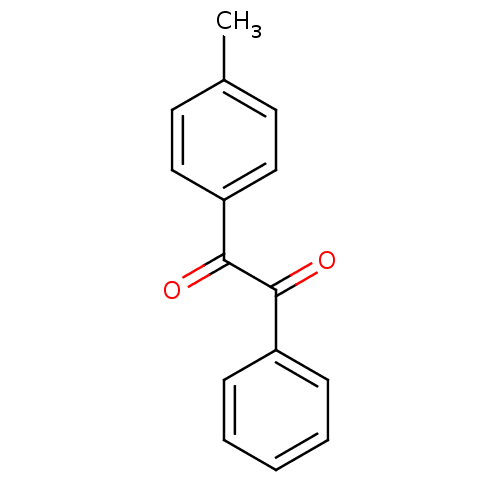 (1-(4-methylphenyl)-2-phenylethane-1,2-dione | Benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 33.3 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2258 total ) | Next | Last >> |