 Found 1427 hits with Last Name = 'yu' and Initial = 'g'
Found 1427 hits with Last Name = 'yu' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50557532

(CHEMBL4792421)Show SMILES COc1cc2C(=O)O\C(=C/c3ccc(O)c(c3)N3CCCCC3)c2cc1OC | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of Electrophorus electricus AChE using varying levels of acetylthiocholine iodide as substrate preincubated for 15 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116074
BindingDB Entry DOI: 10.7270/Q2HX1HBN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011322
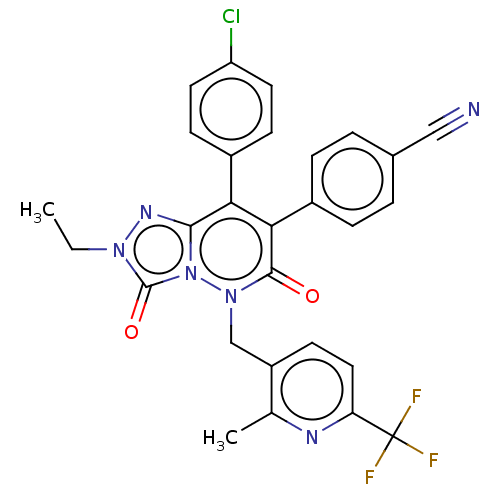
(CHEMBL3260745)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C28H20ClF3N6O2/c1-3-36-27(40)38-25(35-36)23(18-8-11-21(29)12-9-18)24(19-6-4-17(14-33)5-7-19)26(39)37(38)15-20-10-13-22(28(30,31)32)34-16(20)2/h4-13H,3,15H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011324
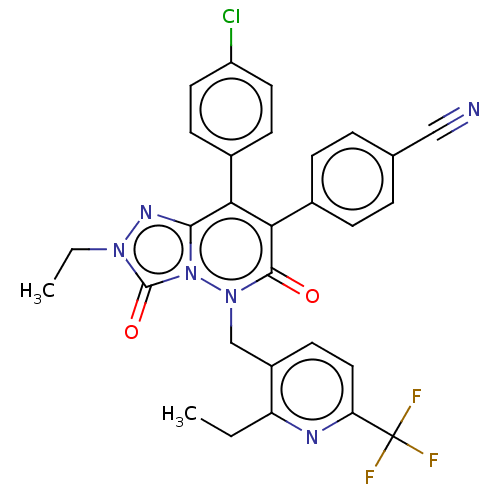
(CHEMBL3260747)Show SMILES CCc1nc(ccc1Cn1n2c(nn(CC)c2=O)c(-c2ccc(Cl)cc2)c(-c2ccc(cc2)C#N)c1=O)C(F)(F)F Show InChI InChI=1S/C29H22ClF3N6O2/c1-3-22-20(11-14-23(35-22)29(31,32)33)16-38-27(40)25(19-7-5-17(15-34)6-8-19)24(18-9-12-21(30)13-10-18)26-36-37(4-2)28(41)39(26)38/h5-14H,3-4,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011328
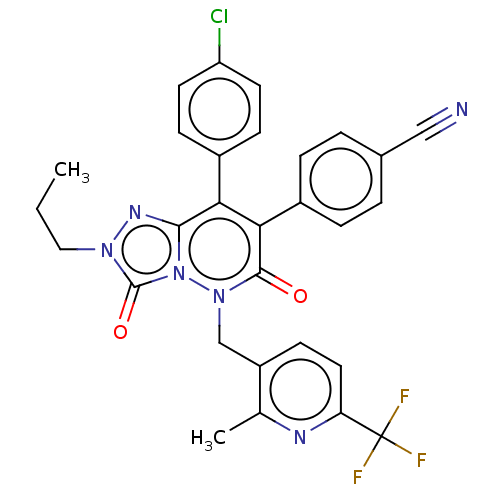
(CHEMBL3259829)Show SMILES CCCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C29H22ClF3N6O2/c1-3-14-37-28(41)39-26(36-37)24(19-8-11-22(30)12-9-19)25(20-6-4-18(15-34)5-7-20)27(40)38(39)16-21-10-13-23(29(31,32)33)35-17(21)2/h4-13H,3,14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011326
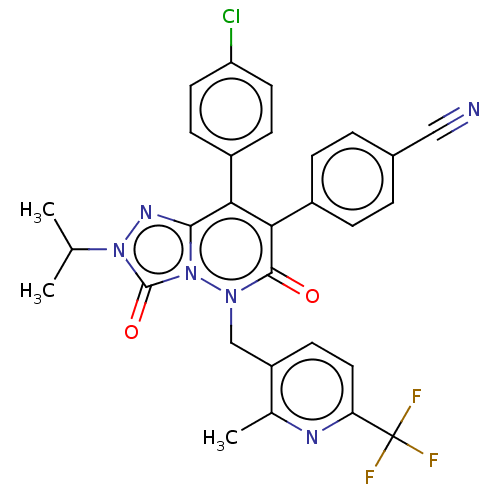
(CHEMBL3260748)Show SMILES CC(C)n1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C29H22ClF3N6O2/c1-16(2)38-28(41)39-26(36-38)24(19-8-11-22(30)12-9-19)25(20-6-4-18(14-34)5-7-20)27(40)37(39)15-21-10-13-23(29(31,32)33)35-17(21)3/h4-13,16H,15H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011330
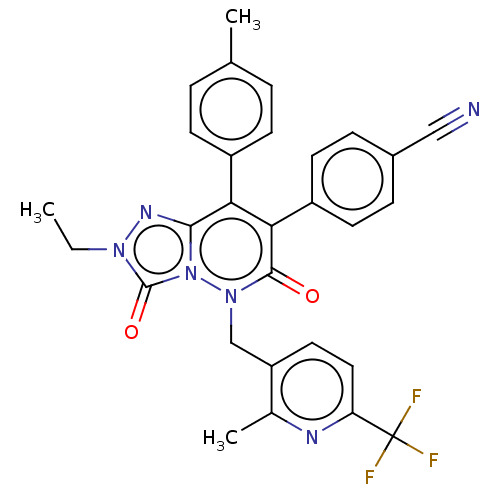
(CHEMBL3260750)Show SMILES CCn1nc2c(-c3ccc(C)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C29H23F3N6O2/c1-4-36-28(40)38-26(35-36)24(20-9-5-17(2)6-10-20)25(21-11-7-19(15-33)8-12-21)27(39)37(38)16-22-13-14-23(29(30,31)32)34-18(22)3/h5-14H,4,16H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396616
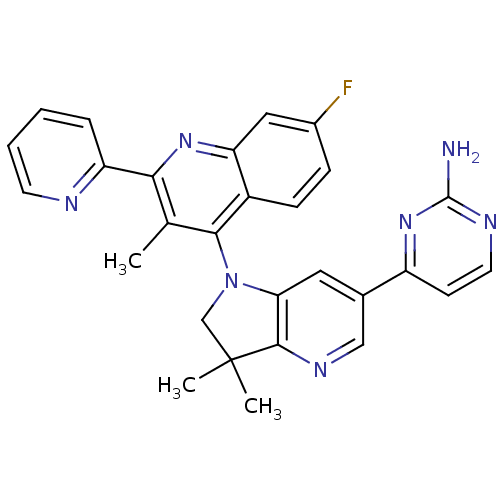
(CHEMBL2171930 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C28H24FN7/c1-16-24(21-6-4-5-10-31-21)34-22-13-18(29)7-8-19(22)25(16)36-15-28(2,3)26-23(36)12-17(14-33-26)20-9-11-32-27(30)35-20/h4-14H,15H2,1-3H3,(H2,30,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011323
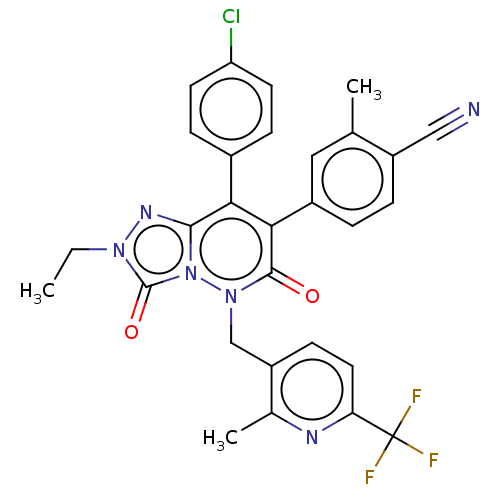
(CHEMBL3260746)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(C#N)c(C)c3)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C29H22ClF3N6O2/c1-4-37-28(41)39-26(36-37)24(18-7-10-22(30)11-8-18)25(19-5-6-20(14-34)16(2)13-19)27(40)38(39)15-21-9-12-23(29(31,32)33)35-17(21)3/h5-13H,4,15H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011334
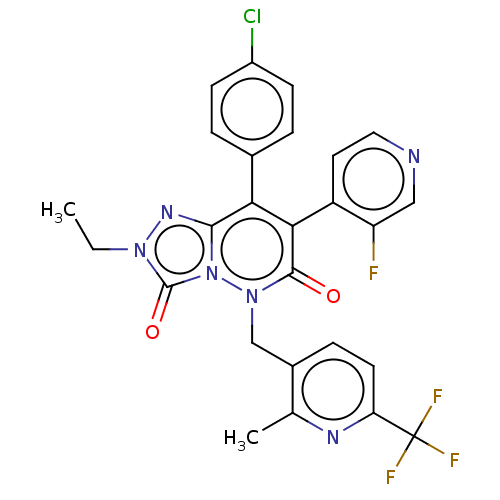
(CHEMBL3260754)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3F)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O |(14.54,-16.67,;15.31,-15.33,;14.53,-14,;13,-13.84,;12.68,-12.33,;11.35,-11.56,;10.01,-12.34,;10.02,-13.88,;8.68,-14.65,;7.35,-13.89,;6.01,-14.66,;7.35,-12.34,;8.68,-11.57,;11.34,-10.03,;10.01,-9.26,;8.68,-10.03,;7.35,-9.26,;7.34,-7.72,;8.67,-6.95,;10.01,-7.72,;11.34,-6.95,;12.68,-9.26,;12.67,-7.72,;14.01,-10.02,;15.35,-9.25,;15.34,-7.71,;14,-6.95,;13.98,-5.41,;15.32,-4.62,;16.67,-5.39,;16.67,-6.93,;18.01,-7.69,;15.31,-3.08,;16.64,-2.3,;13.97,-2.32,;15.3,-1.53,;14.01,-11.57,;15.16,-12.6,;16.66,-12.27,)| Show InChI InChI=1S/C26H19ClF4N6O2/c1-3-35-25(39)37-23(34-35)21(15-4-7-17(27)8-5-15)22(18-10-11-32-12-19(18)28)24(38)36(37)13-16-6-9-20(26(29,30)31)33-14(16)2/h4-12H,3,13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011310
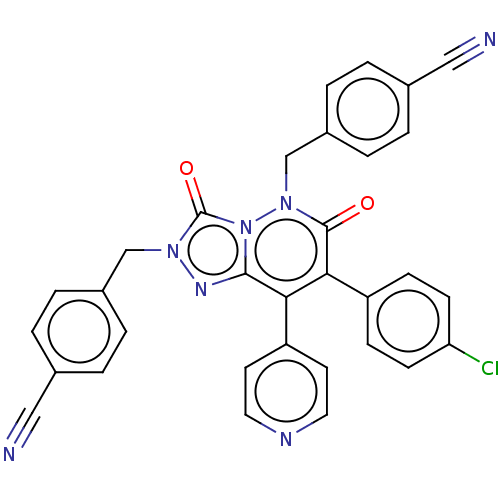
(CHEMBL3260737)Show SMILES Clc1ccc(cc1)-c1c(-c2ccncc2)c2nn(Cc3ccc(cc3)C#N)c(=O)n2n(Cc2ccc(cc2)C#N)c1=O Show InChI InChI=1S/C32H20ClN7O2/c33-27-11-9-25(10-12-27)29-28(26-13-15-36-16-14-26)30-37-38(19-23-5-1-21(17-34)2-6-23)32(42)40(30)39(31(29)41)20-24-7-3-22(18-35)4-8-24/h1-16H,19-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011316
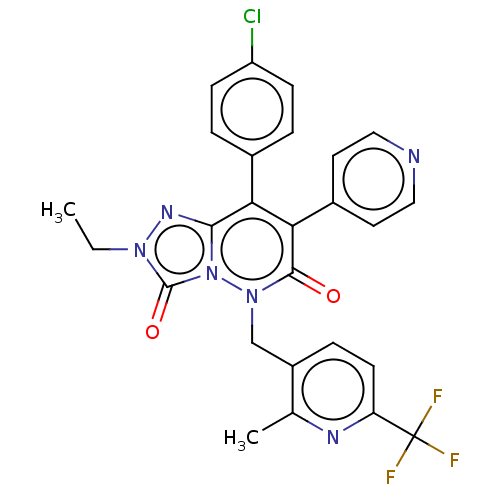
(CHEMBL3260743)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C26H20ClF3N6O2/c1-3-34-25(38)36-23(33-34)21(16-4-7-19(27)8-5-16)22(17-10-12-31-13-11-17)24(37)35(36)14-18-6-9-20(26(28,29)30)32-15(18)2/h4-13H,3,14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011333
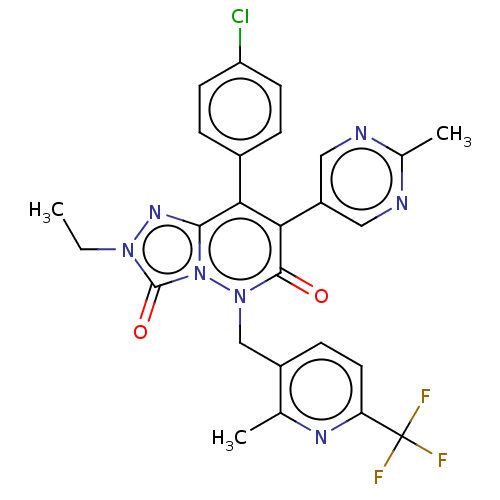
(CHEMBL3260753)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3cnc(C)nc3)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C26H21ClF3N7O2/c1-4-35-25(39)37-23(34-35)21(16-5-8-19(27)9-6-16)22(18-11-31-15(3)32-12-18)24(38)36(37)13-17-7-10-20(26(28,29)30)33-14(17)2/h5-12H,4,13H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011331
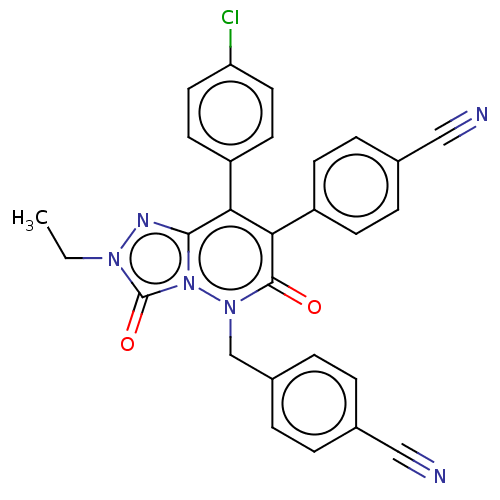
(CHEMBL3260751)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(cc3)C#N)n2c1=O Show InChI InChI=1S/C28H19ClN6O2/c1-2-33-28(37)35-26(32-33)24(21-11-13-23(29)14-12-21)25(22-9-7-19(16-31)8-10-22)27(36)34(35)17-20-5-3-18(15-30)4-6-20/h3-14H,2,17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011332
(CHEMBL3260752)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(CO)nc3)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O Show InChI InChI=1S/C27H22ClF3N6O3/c1-3-35-26(40)37-24(34-35)22(16-4-8-19(28)9-5-16)23(17-6-10-20(14-38)32-12-17)25(39)36(37)13-18-7-11-21(27(29,30)31)33-15(18)2/h4-12,38H,3,13-14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Bifunctional glutamate/proline--tRNA ligase
(Homo sapiens (Human)) | BDBM50097096

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12(19)7-13-14(17(21)22)8-15(20-16(9)13)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396610
(CHEMBL2171929 | US8765940, 4-(3,3-dimethyl-6-(4-mo...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C28H28FN5O/c1-18-25(22-6-4-5-9-30-22)32-23-14-19(29)7-8-21(23)26(18)34-17-28(2,3)27-24(34)15-20(16-31-27)33-10-12-35-13-11-33/h4-9,14-16H,10-13,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011317
(CHEMBL3260744)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3C)c(=O)n(Cc3ccc(nc3C)C(F)(F)F)n2c1=O |(16.96,-15.83,;17.73,-14.5,;16.96,-13.17,;15.43,-13.01,;15.11,-11.5,;13.78,-10.73,;12.44,-11.51,;12.44,-13.05,;11.11,-13.82,;9.77,-13.05,;8.44,-13.82,;9.77,-11.51,;11.11,-10.74,;13.77,-9.2,;12.44,-8.43,;11.11,-9.2,;9.77,-8.43,;9.77,-6.89,;11.1,-6.12,;12.44,-6.89,;13.78,-5.75,;15.1,-8.42,;15.1,-6.89,;16.44,-9.19,;17.77,-8.42,;17.76,-6.87,;16.42,-6.12,;16.41,-4.58,;17.75,-3.79,;19.09,-4.55,;19.1,-6.1,;20.44,-6.86,;17.74,-2.25,;19.06,-1.47,;16.4,-1.49,;17.72,-.7,;16.44,-10.73,;17.58,-11.76,;19.09,-11.44,)| Show InChI InChI=1S/C27H22ClF3N6O2/c1-4-35-26(39)37-24(34-35)22(17-5-8-19(28)9-6-17)23(20-11-12-32-13-15(20)2)25(38)36(37)14-18-7-10-21(27(29,30)31)33-16(18)3/h5-13H,4,14H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011314
(CHEMBL3260741)Show SMILES Cc1nc(ccc1Cn1n2c(nn(C)c2=O)c(-c2ccc(Cl)cc2)c(-c2ccncc2)c1=O)C(F)(F)F Show InChI InChI=1S/C25H18ClF3N6O2/c1-14-17(5-8-19(31-14)25(27,28)29)13-34-23(36)21(16-9-11-30-12-10-16)20(15-3-6-18(26)7-4-15)22-32-33(2)24(37)35(22)34/h3-12H,13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011307
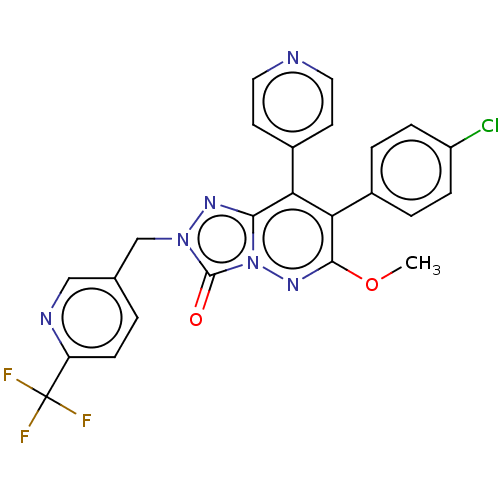
(CHEMBL3260734)Show SMILES COc1nn2c(nn(Cc3ccc(nc3)C(F)(F)F)c2=O)c(-c2ccncc2)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H16ClF3N6O2/c1-36-22-20(15-3-5-17(25)6-4-15)19(16-8-10-29-11-9-16)21-31-33(23(35)34(21)32-22)13-14-2-7-18(30-12-14)24(26,27)28/h2-12H,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396611
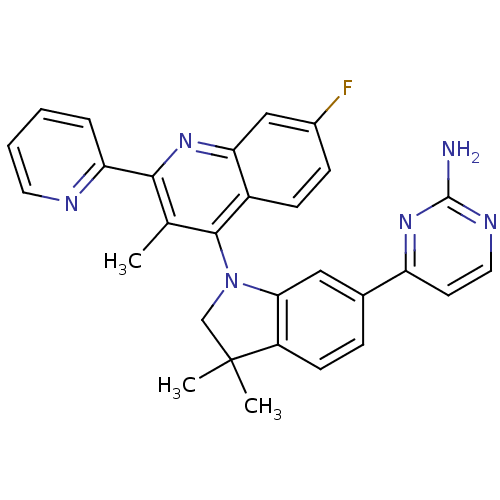
(CHEMBL2171927 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ccc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C29H25FN6/c1-17-26(23-6-4-5-12-32-23)34-24-15-19(30)8-9-20(24)27(17)36-16-29(2,3)21-10-7-18(14-25(21)36)22-11-13-33-28(31)35-22/h4-15H,16H2,1-3H3,(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396614
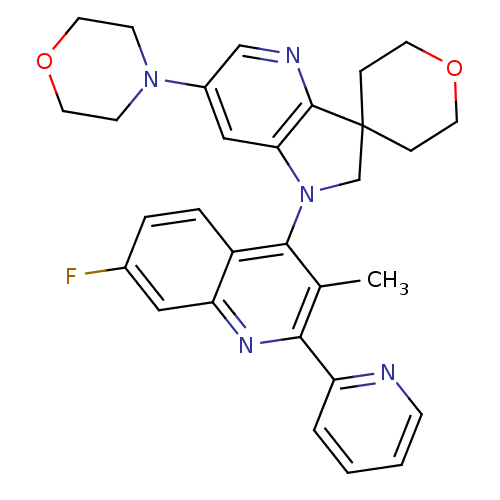
(CHEMBL2171931 | US8765940, 1'-(7-fluoro-3-meth...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CCOCC2)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C30H30FN5O2/c1-20-27(24-4-2-3-9-32-24)34-25-16-21(31)5-6-23(25)28(20)36-19-30(7-12-37-13-8-30)29-26(36)17-22(18-33-29)35-10-14-38-15-11-35/h2-6,9,16-18H,7-8,10-15,19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50356013
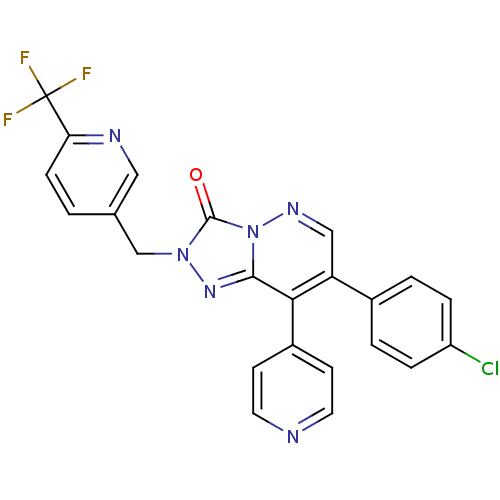
(CHEMBL1911374 | CHEMBL1911375)Show SMILES FC(F)(F)c1ccc(Cn2nc3c(-c4ccncc4)c(cnn3c2=O)-c2ccc(Cl)cc2)cn1 Show InChI InChI=1S/C23H14ClF3N6O/c24-17-4-2-15(3-5-17)18-12-30-33-21(20(18)16-7-9-28-10-8-16)31-32(22(33)34)13-14-1-6-19(29-11-14)23(25,26)27/h1-12H,13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011329
(CHEMBL3260749)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccc(cc3)C#N)c(=O)n(Cc3ccc(C)cc3)n2c1=O Show InChI InChI=1S/C28H22ClN5O2/c1-3-32-28(36)34-26(31-32)24(21-12-14-23(29)15-13-21)25(22-10-8-19(16-30)9-11-22)27(35)33(34)17-20-6-4-18(2)5-7-20/h4-15H,3,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Bifunctional glutamate/proline--tRNA ligase
(Homo sapiens (Human)) | BDBM50097096

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12(19)7-13-14(17(21)22)8-15(20-16(9)13)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011315
(CHEMBL3260742)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3)c(=O)n(Cc3ccc(nc3)C(F)(F)F)n2c1=O Show InChI InChI=1S/C25H18ClF3N6O2/c1-2-33-24(37)35-22(32-33)20(16-4-6-18(26)7-5-16)21(17-9-11-30-12-10-17)23(36)34(35)14-15-3-8-19(31-13-15)25(27,28)29/h3-13H,2,14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011309
(CHEMBL3260736)Show SMILES Cn1n2c(nn(Cc3ccc(nc3)C(F)(F)F)c2=O)c(-c2ccncc2)c(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C24H16ClF3N6O2/c1-32-22(35)20(15-3-5-17(25)6-4-15)19(16-8-10-29-11-9-16)21-31-33(23(36)34(21)32)13-14-2-7-18(30-12-14)24(26,27)28/h2-12H,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396616

(CHEMBL2171930 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C28H24FN7/c1-16-24(21-6-4-5-10-31-21)34-22-13-18(29)7-8-19(22)25(16)36-15-28(2,3)26-23(36)12-17(14-33-26)20-9-11-32-27(30)35-20/h4-14H,15H2,1-3H3,(H2,30,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011313
(CHEMBL3260740)Show SMILES Cn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3)c(=O)n(Cc3ccc(nc3)C(F)(F)F)n2c1=O Show InChI InChI=1S/C24H16ClF3N6O2/c1-32-23(36)34-21(31-32)19(15-3-5-17(25)6-4-15)20(16-8-10-29-11-9-16)22(35)33(34)13-14-2-7-18(30-12-14)24(26,27)28/h2-12H,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396616

(CHEMBL2171930 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C28H24FN7/c1-16-24(21-6-4-5-10-31-21)34-22-13-18(29)7-8-19(22)25(16)36-15-28(2,3)26-23(36)12-17(14-33-26)20-9-11-32-27(30)35-20/h4-14H,15H2,1-3H3,(H2,30,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011312
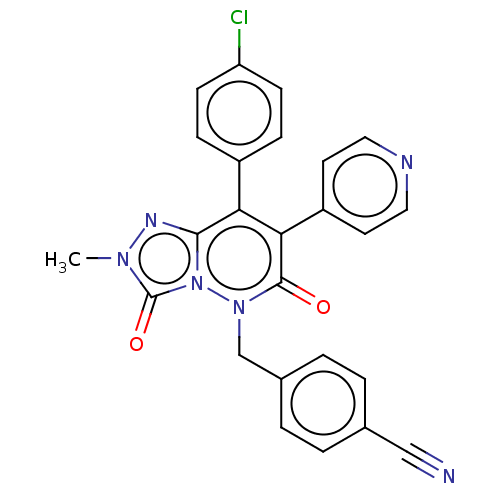
(CHEMBL3260739)Show SMILES Cn1nc2c(-c3ccc(Cl)cc3)c(-c3ccncc3)c(=O)n(Cc3ccc(cc3)C#N)n2c1=O Show InChI InChI=1S/C25H17ClN6O2/c1-30-25(34)32-23(29-30)21(18-6-8-20(26)9-7-18)22(19-10-12-28-13-11-19)24(33)31(32)15-17-4-2-16(14-27)3-5-17/h2-13H,15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396610

(CHEMBL2171929 | US8765940, 4-(3,3-dimethyl-6-(4-mo...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C28H28FN5O/c1-18-25(22-6-4-5-9-30-22)32-23-14-19(29)7-8-21(23)26(18)34-17-28(2,3)27-24(34)15-20(16-31-27)33-10-12-35-13-11-33/h4-9,14-16H,10-13,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396614

(CHEMBL2171931 | US8765940, 1'-(7-fluoro-3-meth...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CCOCC2)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C30H30FN5O2/c1-20-27(24-4-2-3-9-32-24)34-25-16-21(31)5-6-23(25)28(20)36-19-30(7-12-37-13-8-30)29-26(36)17-22(18-33-29)35-10-14-38-15-11-35/h2-6,9,16-18H,7-8,10-15,19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396611

(CHEMBL2171927 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ccc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C29H25FN6/c1-17-26(23-6-4-5-12-32-23)34-24-15-19(30)8-9-20(24)27(17)36-16-29(2,3)21-10-7-18(14-25(21)36)22-11-13-33-28(31)35-22/h4-15H,16H2,1-3H3,(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM403460

(US10335399, Example 2-5 | US10806724, Example 2-5)Show InChI InChI=1S/C17H16F2N2O2/c1-17(2)6-5-11-12(10-4-3-9(18)7-13(10)19)8-14(15(20)22)21-16(11)23-17/h3-4,7-8H,5-6H2,1-2H3,(H2,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM403460

(US10335399, Example 2-5 | US10806724, Example 2-5)Show InChI InChI=1S/C17H16F2N2O2/c1-17(2)6-5-11-12(10-4-3-9(18)7-13(10)19)8-14(15(20)22)21-16(11)23-17/h3-4,7-8H,5-6H2,1-2H3,(H2,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011336
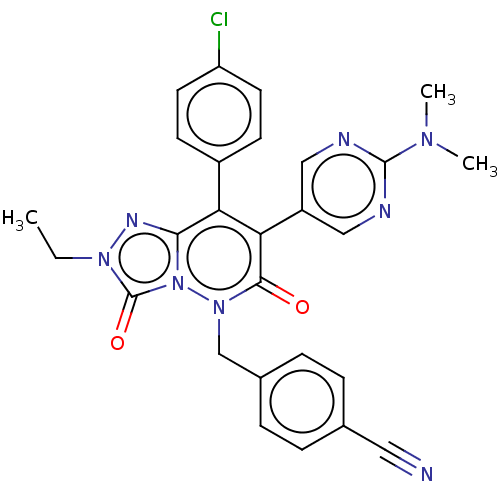
(CHEMBL3260756)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3cnc(nc3)N(C)C)c(=O)n(Cc3ccc(cc3)C#N)n2c1=O Show InChI InChI=1S/C27H23ClN8O2/c1-4-34-27(38)36-24(32-34)22(19-9-11-21(28)12-10-19)23(20-14-30-26(31-15-20)33(2)3)25(37)35(36)16-18-7-5-17(13-29)6-8-18/h5-12,14-15H,4,16H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396613

(CHEMBL2171924 | US8765940, 1-(7-fluoro-3-methyl-2-...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CS(=O)(=O)C2)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H27FN4O3S/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-38(35,36)18-29)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396611

(CHEMBL2171927 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ccc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C29H25FN6/c1-17-26(23-6-4-5-12-32-23)34-24-15-19(30)8-9-20(24)27(17)36-16-29(2,3)21-10-7-18(14-25(21)36)22-11-13-33-28(31)35-22/h4-15H,16H2,1-3H3,(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011335
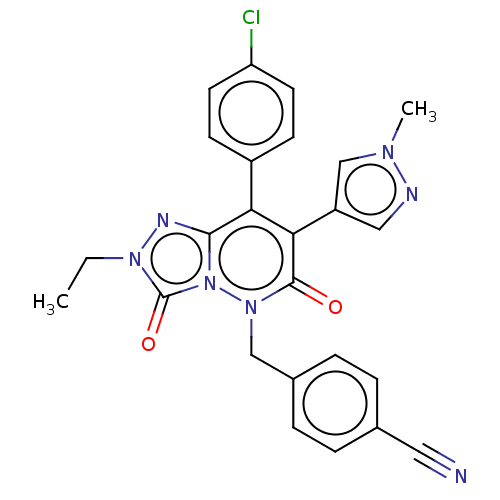
(CHEMBL3260755)Show SMILES CCn1nc2c(-c3ccc(Cl)cc3)c(-c3cnn(C)c3)c(=O)n(Cc3ccc(cc3)C#N)n2c1=O Show InChI InChI=1S/C25H20ClN7O2/c1-3-31-25(35)33-23(29-31)21(18-8-10-20(26)11-9-18)22(19-13-28-30(2)15-19)24(34)32(33)14-17-6-4-16(12-27)5-7-17/h4-11,13,15H,3,14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396610

(CHEMBL2171929 | US8765940, 4-(3,3-dimethyl-6-(4-mo...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C28H28FN5O/c1-18-25(22-6-4-5-9-30-22)32-23-14-19(29)7-8-21(23)26(18)34-17-28(2,3)27-24(34)15-20(16-31-27)33-10-12-35-13-11-33/h4-9,14-16H,10-13,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596341

(CHEMBL5176418)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(ccc1O)N(C)C)Cc1ccccc1N(C)C | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396617

(CHEMBL2171925 | US8765940, (1-(7-fluoro-3-methyl-2...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(CO)(CO)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H29FN4O3/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-35,18-36)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15,35-36H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441

((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...)Show SMILES C\C=C1/C2Cc3[nH]c(=O)ccc3C1(N)CC(C)=C2 |c:18,TLB:10:11:2:17.15.14,THB:1:2:4.5.11:17.15.14| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 175 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... |
Biochemistry 41: 10810-8 (2002)
Article DOI: 10.1021/bi020151+
BindingDB Entry DOI: 10.7270/Q2HQ3X52 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011306
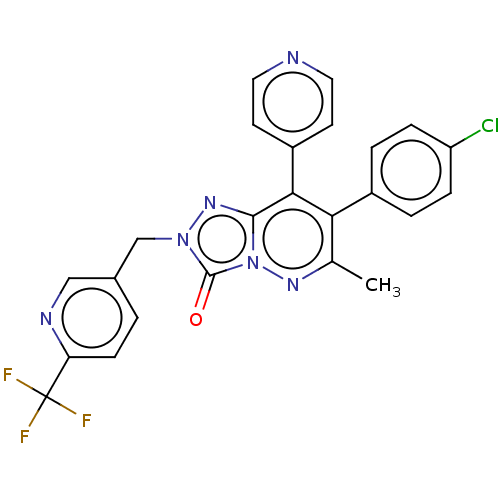
(CHEMBL3260733)Show SMILES Cc1nn2c(nn(Cc3ccc(nc3)C(F)(F)F)c2=O)c(-c2ccncc2)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H16ClF3N6O/c1-14-20(16-3-5-18(25)6-4-16)21(17-8-10-29-11-9-17)22-32-33(23(35)34(22)31-14)13-15-2-7-19(30-12-15)24(26,27)28/h2-12H,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50011311
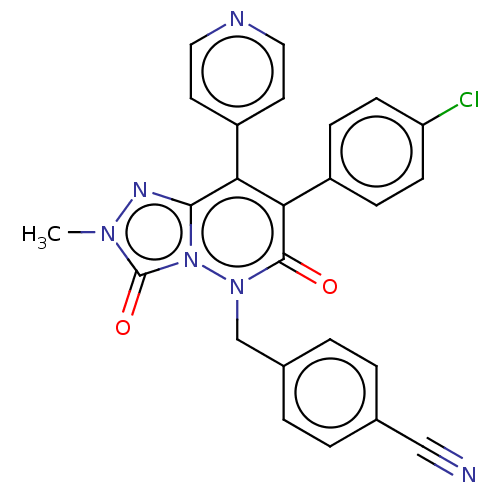
(CHEMBL3260738)Show SMILES Cn1nc2c(-c3ccncc3)c(-c3ccc(Cl)cc3)c(=O)n(Cc3ccc(cc3)C#N)n2c1=O Show InChI InChI=1S/C25H17ClN6O2/c1-30-25(34)32-23(29-30)21(19-10-12-28-13-11-19)22(18-6-8-20(26)9-7-18)24(33)31(32)15-17-4-2-16(14-27)3-5-17/h2-13H,15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis |
J Med Chem 56: 9586-600 (2014)
Article DOI: 10.1021/jm4010835
BindingDB Entry DOI: 10.7270/Q24X59B8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396617

(CHEMBL2171925 | US8765940, (1-(7-fluoro-3-methyl-2...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(CO)(CO)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H29FN4O3/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-35,18-36)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15,35-36H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50131680
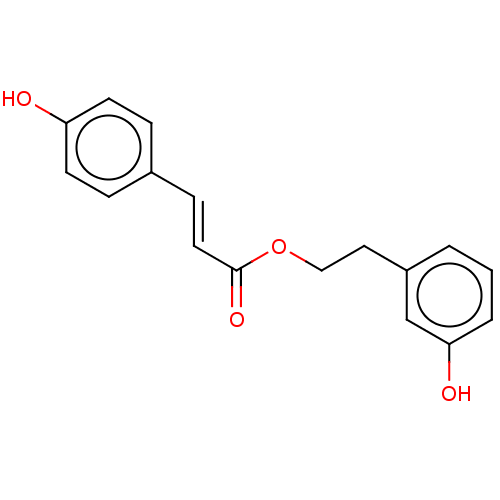
(CHEMBL3628212)Show InChI InChI=1S/C17H16O4/c18-15-7-4-13(5-8-15)6-9-17(20)21-11-10-14-2-1-3-16(19)12-14/h1-9,12,18-19H,10-11H2/b9-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 14 preincubated for 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem 23: 7181-8 (2015)
Article DOI: 10.1016/j.bmc.2015.10.014
BindingDB Entry DOI: 10.7270/Q29W0HB2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10632
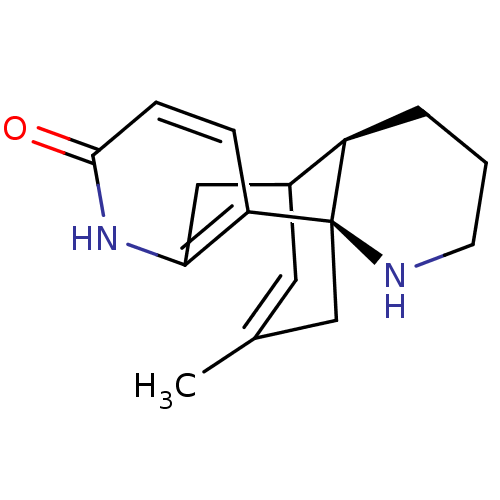
((-)-Huperzine B | (1R,10R)-16-methyl-6,14-diazatet...)Show SMILES CC1=CC2Cc3[nH]c(=O)ccc3[C@]3(C1)NCCC[C@H]23 |r,t:1,TLB:17:18:4.5.11:2.1.13,THB:10:11:18:2.1.13| Show InChI InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11?,12-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 334 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... |
Biochemistry 41: 10810-8 (2002)
Article DOI: 10.1021/bi020151+
BindingDB Entry DOI: 10.7270/Q2HQ3X52 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data