 Found 195 hits with Last Name = 'zalewska' and Initial = 't'
Found 195 hits with Last Name = 'zalewska' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(MOUSE) | BDBM50061295

((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H47FN6O8S2/c1-38(2)31(45-33(49)27(41)18-23-12-16-26(47)17-13-23)35(51)42-21-30(48)43-28(19-24-10-14-25(40)15-11-24)34(50)46-32(39(3,4)56-55-38)36(52)44-29(37(53)54)20-22-8-6-5-7-9-22/h5-17,27-29,31-32,47H,18-21,41H2,1-4H3,(H,42,51)(H,43,48)(H,44,52)(H,45,49)(H,46,50)(H,53,54)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory potency against Opioid receptor delta 1 was determined in electrically induced smooth muscle contractions of mouse vas deferens (MVD) |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50043724
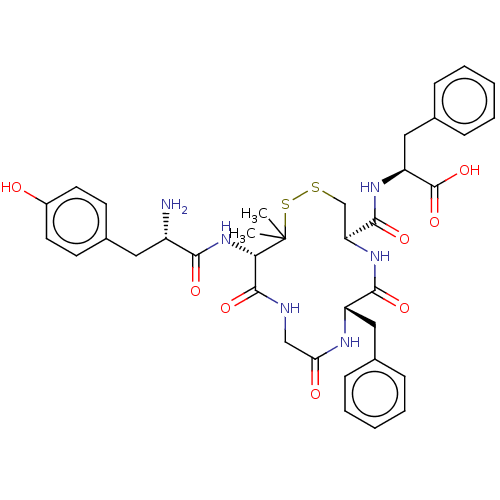
((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H44N6O8S2/c1-37(2)31(43-32(46)26(38)17-24-13-15-25(44)16-14-24)35(49)39-20-30(45)40-27(18-22-9-5-3-6-10-22)33(47)42-29(21-52-53-37)34(48)41-28(36(50)51)19-23-11-7-4-8-12-23/h3-16,26-29,31,44H,17-21,38H2,1-2H3,(H,39,49)(H,40,45)(H,41,48)(H,42,47)(H,43,46)(H,50,51)/t26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory potency against Opioid receptor delta 1 was determined in electrically induced smooth muscle contractions of mouse vas deferens (MVD) |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50043721
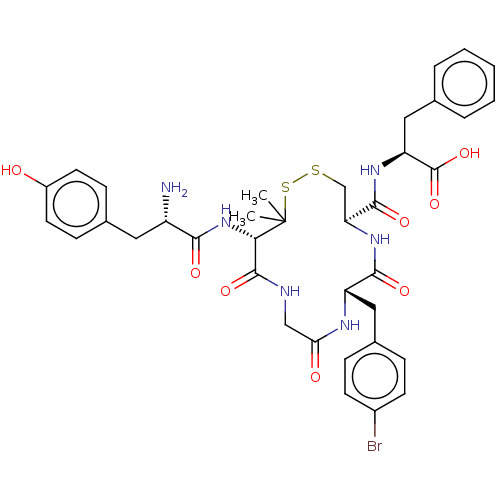
((S)-2-{[(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccc(Br)cc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H43BrN6O8S2/c1-37(2)31(44-32(47)26(39)16-22-10-14-25(45)15-11-22)35(50)40-19-30(46)41-27(17-23-8-12-24(38)13-9-23)33(48)43-29(20-53-54-37)34(49)42-28(36(51)52)18-21-6-4-3-5-7-21/h3-15,26-29,31,45H,16-20,39H2,1-2H3,(H,40,50)(H,41,46)(H,42,49)(H,43,48)(H,44,47)(H,51,52)/t26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against delta opioid receptor of electrically induced smooth muscle contraction of mouse vas deferens |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50001468
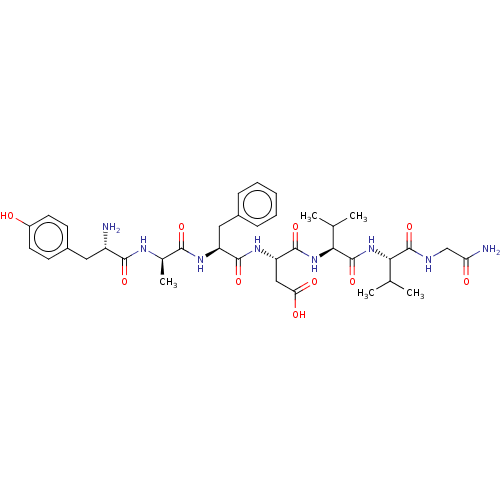
((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C37H52N8O10/c1-19(2)30(36(54)40-18-28(39)47)45-37(55)31(20(3)4)44-35(53)27(17-29(48)49)43-34(52)26(16-22-9-7-6-8-10-22)42-32(50)21(5)41-33(51)25(38)15-23-11-13-24(46)14-12-23/h6-14,19-21,25-27,30-31,46H,15-18,38H2,1-5H3,(H2,39,47)(H,40,54)(H,41,51)(H,42,50)(H,43,52)(H,44,53)(H,45,55)(H,48,49)/t21-,25+,26+,27+,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against delta opioid receptor of electrically induced smooth muscle contraction of mouse vas deferens |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50061292
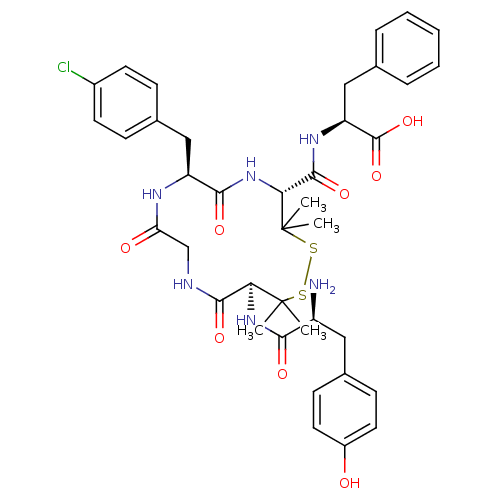
((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(Cl)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H47ClN6O8S2/c1-38(2)31(45-33(49)27(41)18-23-12-16-26(47)17-13-23)35(51)42-21-30(48)43-28(19-24-10-14-25(40)15-11-24)34(50)46-32(39(3,4)56-55-38)36(52)44-29(37(53)54)20-22-8-6-5-7-9-22/h5-17,27-29,31-32,47H,18-21,41H2,1-4H3,(H,42,51)(H,43,48)(H,44,52)(H,45,49)(H,46,50)(H,53,54)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory potency against Opioid receptor delta 1 was determined in electrically induced smooth muscle contractions of mouse vas deferens (MVD) |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50061291

((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(Br)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H47BrN6O8S2/c1-38(2)31(45-33(49)27(41)18-23-12-16-26(47)17-13-23)35(51)42-21-30(48)43-28(19-24-10-14-25(40)15-11-24)34(50)46-32(39(3,4)56-55-38)36(52)44-29(37(53)54)20-22-8-6-5-7-9-22/h5-17,27-29,31-32,47H,18-21,41H2,1-4H3,(H,42,51)(H,43,48)(H,44,52)(H,45,49)(H,46,50)(H,53,54)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory potency against Opioid receptor delta 1 was determined in electrically induced smooth muscle contractions of mouse vas deferens (MVD) |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50061291

((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(Br)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H47BrN6O8S2/c1-38(2)31(45-33(49)27(41)18-23-12-16-26(47)17-13-23)35(51)42-21-30(48)43-28(19-24-10-14-25(40)15-11-24)34(50)46-32(39(3,4)56-55-38)36(52)44-29(37(53)54)20-22-8-6-5-7-9-22/h5-17,27-29,31-32,47H,18-21,41H2,1-4H3,(H,42,51)(H,43,48)(H,44,52)(H,45,49)(H,46,50)(H,53,54)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50453313

(CHEMBL2372329)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H35N5O7S2/c1-28(2)23(33-24(36)19(29)12-17-8-10-18(34)11-9-17)26(38)30-14-22(35)31-20(13-16-6-4-3-5-7-16)25(37)32-21(27(39)40)15-41-42-28/h3-11,19-21,23,34H,12-15,29H2,1-2H3,(H,30,38)(H,31,35)(H,32,37)(H,33,36)(H,39,40)/t19-,20-,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against delta opioid receptor of electrically induced smooth muscle contraction of mouse vas deferens |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50061292

((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(Cl)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H47ClN6O8S2/c1-38(2)31(45-33(49)27(41)18-23-12-16-26(47)17-13-23)35(51)42-21-30(48)43-28(19-24-10-14-25(40)15-11-24)34(50)46-32(39(3,4)56-55-38)36(52)44-29(37(53)54)20-22-8-6-5-7-9-22/h5-17,27-29,31-32,47H,18-21,41H2,1-4H3,(H,42,51)(H,43,48)(H,44,52)(H,45,49)(H,46,50)(H,53,54)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50061295

((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H47FN6O8S2/c1-38(2)31(45-33(49)27(41)18-23-12-16-26(47)17-13-23)35(51)42-21-30(48)43-28(19-24-10-14-25(40)15-11-24)34(50)46-32(39(3,4)56-55-38)36(52)44-29(37(53)54)20-22-8-6-5-7-9-22/h5-17,27-29,31-32,47H,18-21,41H2,1-4H3,(H,42,51)(H,43,48)(H,44,52)(H,45,49)(H,46,50)(H,53,54)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50061293

((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(I)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H47IN6O8S2/c1-38(2)31(45-33(49)27(41)18-23-12-16-26(47)17-13-23)35(51)42-21-30(48)43-28(19-24-10-14-25(40)15-11-24)34(50)46-32(39(3,4)56-55-38)36(52)44-29(37(53)54)20-22-8-6-5-7-9-22/h5-17,27-29,31-32,47H,18-21,41H2,1-4H3,(H,42,51)(H,43,48)(H,44,52)(H,45,49)(H,46,50)(H,53,54)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50043720
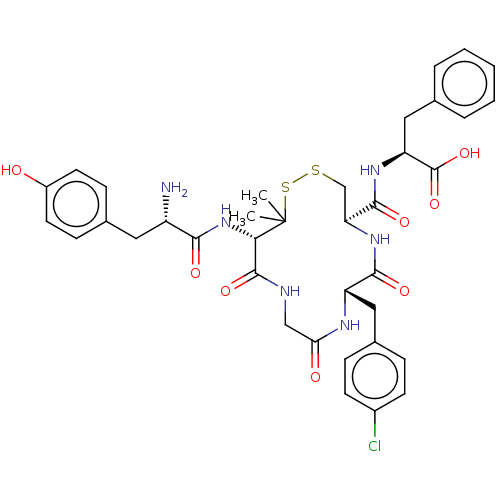
((S)-2-{[(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H43ClN6O8S2/c1-37(2)31(44-32(47)26(39)16-22-10-14-25(45)15-11-22)35(50)40-19-30(46)41-27(17-23-8-12-24(38)13-9-23)33(48)43-29(20-53-54-37)34(49)42-28(36(51)52)18-21-6-4-3-5-7-21/h3-15,26-29,31,45H,16-20,39H2,1-2H3,(H,40,50)(H,41,46)(H,42,49)(H,43,48)(H,44,47)(H,51,52)/t26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50038019
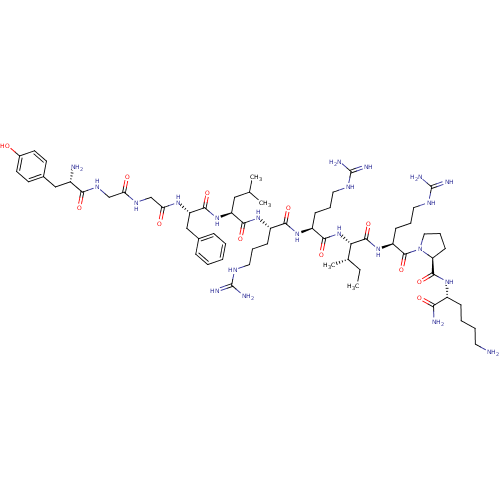
(CHEMBL411338 | Tyr-Gly-Gly-Phe-D-Leu-Arg-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42+,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at kappa opioid receptor in guinea pig brain by [3H]U-69,593 displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at kappa opioid receptor in guinea pig brain by [3H]U-69,593 displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50038424
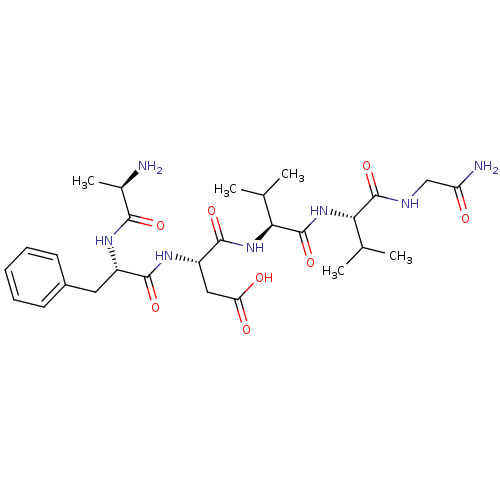
((S)-3-[(S)-2-((R)-2-Amino-propionylamino)-3-phenyl...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)N)C(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C28H43N7O8/c1-14(2)22(27(42)31-13-20(30)36)35-28(43)23(15(3)4)34-26(41)19(12-21(37)38)33-25(40)18(32-24(39)16(5)29)11-17-9-7-6-8-10-17/h6-10,14-16,18-19,22-23H,11-13,29H2,1-5H3,(H2,30,36)(H,31,42)(H,32,39)(H,33,40)(H,34,41)(H,35,43)(H,37,38)/t16-,18+,19+,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity opioid receptor |
J Med Chem 37: 1746-57 (1994)
BindingDB Entry DOI: 10.7270/Q2N58N1K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50038424

((S)-3-[(S)-2-((R)-2-Amino-propionylamino)-3-phenyl...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)N)C(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C28H43N7O8/c1-14(2)22(27(42)31-13-20(30)36)35-28(43)23(15(3)4)34-26(41)19(12-21(37)38)33-25(40)18(32-24(39)16(5)29)11-17-9-7-6-8-10-17/h6-10,14-16,18-19,22-23H,11-13,29H2,1-5H3,(H2,30,36)(H,31,42)(H,32,39)(H,33,40)(H,34,41)(H,35,43)(H,37,38)/t16-,18+,19+,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity opioid receptor |
J Med Chem 37: 1746-57 (1994)
BindingDB Entry DOI: 10.7270/Q2N58N1K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50043725
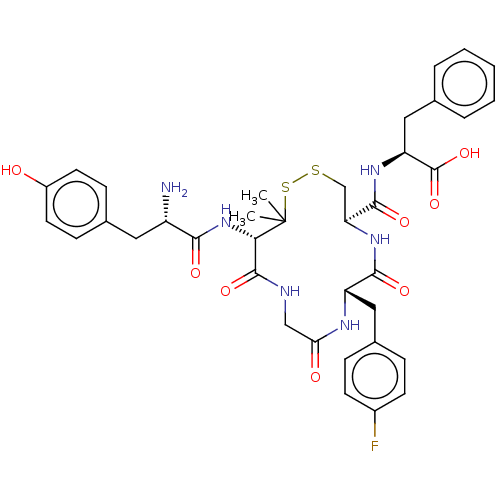
((S)-2-{[(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H43FN6O8S2/c1-37(2)31(44-32(47)26(39)16-22-10-14-25(45)15-11-22)35(50)40-19-30(46)41-27(17-23-8-12-24(38)13-9-23)33(48)43-29(20-53-54-37)34(49)42-28(36(51)52)18-21-6-4-3-5-7-21/h3-15,26-29,31,45H,16-20,39H2,1-2H3,(H,40,50)(H,41,46)(H,42,49)(H,43,48)(H,44,47)(H,51,52)/t26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50038020
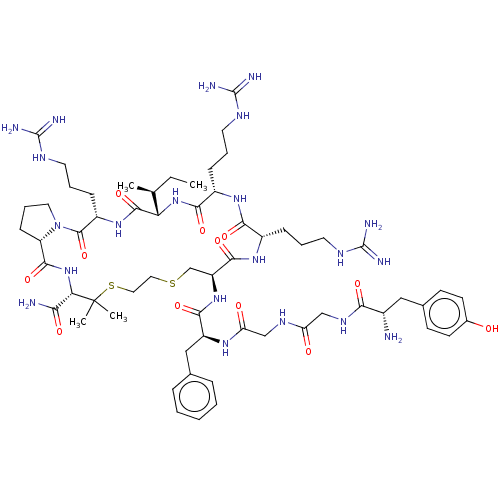
(CHEMBL2370618 | Tyr-Gly-Gly-Phe-D-Cys-Arg-Arg-Ile-...)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-[#6]-[#16]C([#6])([#6])[#6@@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C61H97N21O12S2/c1-5-34(2)47-56(93)78-41(17-11-25-72-60(68)69)57(94)82-26-12-18-44(82)55(92)81-48(49(63)86)61(3,4)96-28-27-95-33-43(54(91)77-39(15-9-23-70-58(64)65)51(88)76-40(52(89)80-47)16-10-24-71-59(66)67)79-53(90)42(30-35-13-7-6-8-14-35)75-46(85)32-73-45(84)31-74-50(87)38(62)29-36-19-21-37(83)22-20-36/h6-8,13-14,19-22,34,38-44,47-48,83H,5,9-12,15-18,23-33,62H2,1-4H3,(H2,63,86)(H,73,84)(H,74,87)(H,75,85)(H,76,88)(H,77,91)(H,78,93)(H,79,90)(H,80,89)(H,81,92)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t34-,38-,39-,40-,41-,42-,43-,44-,47-,48-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at kappa opioid receptor in guinea pig brain by [3H]U-69,593 displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50043725

((S)-2-{[(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H43FN6O8S2/c1-37(2)31(44-32(47)26(39)16-22-10-14-25(45)15-11-22)35(50)40-19-30(46)41-27(17-23-8-12-24(38)13-9-23)33(48)43-29(20-53-54-37)34(49)42-28(36(51)52)18-21-6-4-3-5-7-21/h3-15,26-29,31,45H,16-20,39H2,1-2H3,(H,40,50)(H,41,46)(H,42,49)(H,43,48)(H,44,47)(H,51,52)/t26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against delta opioid receptor of electrically induced smooth muscle contraction of mouse vas deferens |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21146

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C55H66N10O13/c1-3-4-19-45(54(77)64-43(29-48(70)71)53(76)61-40(49(57)72)24-32-13-7-5-8-14-32)65(2)55(78)44(27-35-30-58-39-18-12-11-17-37(35)39)60-46(67)31-59-51(74)41(25-33-15-9-6-10-16-33)63-52(75)42(26-34-20-22-36(66)23-21-34)62-50(73)38(56)28-47(68)69/h5-18,20-23,30,38,40-45,58,66H,3-4,19,24-29,31,56H2,1-2H3,(H2,57,72)(H,59,74)(H,60,67)(H,61,76)(H,62,73)(H,63,75)(H,64,77)(H,68,69)(H,70,71)/t38-,40-,41+,42-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor was determined in guinea pig cortex using [3H]SNF8702 as radioligand |
J Med Chem 39: 4120-4 (1996)
Article DOI: 10.1021/jm960078j
BindingDB Entry DOI: 10.7270/Q2P55P5Z |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50061293

((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(I)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H47IN6O8S2/c1-38(2)31(45-33(49)27(41)18-23-12-16-26(47)17-13-23)35(51)42-21-30(48)43-28(19-24-10-14-25(40)15-11-24)34(50)46-32(39(3,4)56-55-38)36(52)44-29(37(53)54)20-22-8-6-5-7-9-22/h5-17,27-29,31-32,47H,18-21,41H2,1-4H3,(H,42,51)(H,43,48)(H,44,52)(H,45,49)(H,46,50)(H,53,54)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory potency against Opioid receptor delta 1 was determined in electrically induced smooth muscle contractions of mouse vas deferens (MVD) |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50043721

((S)-2-{[(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccc(Br)cc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H43BrN6O8S2/c1-37(2)31(44-32(47)26(39)16-22-10-14-25(45)15-11-22)35(50)40-19-30(46)41-27(17-23-8-12-24(38)13-9-23)33(48)43-29(20-53-54-37)34(49)42-28(36(51)52)18-21-6-4-3-5-7-21/h3-15,26-29,31,45H,16-20,39H2,1-2H3,(H,40,50)(H,41,46)(H,42,49)(H,43,48)(H,44,47)(H,51,52)/t26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50038016
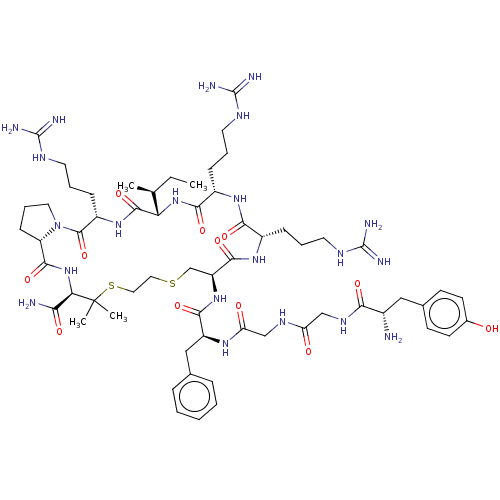
(CHEMBL2370611 | Tyr-Gly-Gly-Phe-D-Cys-Arg-Arg-Ile-...)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-[#6]-[#16]C([#6])([#6])[#6@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C61H97N21O12S2/c1-5-34(2)47-56(93)78-41(17-11-25-72-60(68)69)57(94)82-26-12-18-44(82)55(92)81-48(49(63)86)61(3,4)96-28-27-95-33-43(54(91)77-39(15-9-23-70-58(64)65)51(88)76-40(52(89)80-47)16-10-24-71-59(66)67)79-53(90)42(30-35-13-7-6-8-14-35)75-46(85)32-73-45(84)31-74-50(87)38(62)29-36-19-21-37(83)22-20-36/h6-8,13-14,19-22,34,38-44,47-48,83H,5,9-12,15-18,23-33,62H2,1-4H3,(H2,63,86)(H,73,84)(H,74,87)(H,75,85)(H,76,88)(H,77,91)(H,78,93)(H,79,90)(H,80,89)(H,81,92)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t34-,38-,39-,40-,41-,42-,43-,44-,47-,48+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at kappa opioid receptor in guinea pig brain by [3H]U-69,593 displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50453445
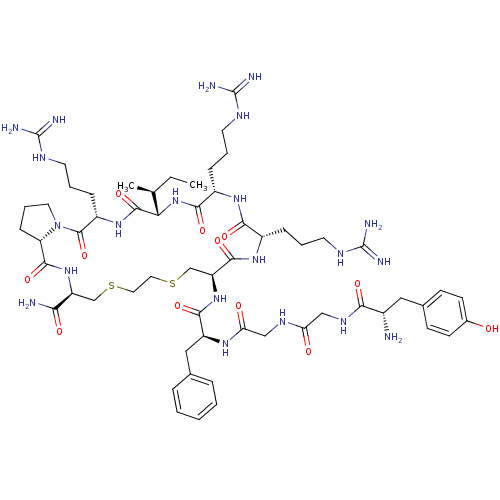
(CHEMBL2112652)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C59H93N21O12S2/c1-3-33(2)47-55(91)76-40(15-9-23-70-59(66)67)56(92)80-24-10-16-44(80)54(90)77-42(48(61)84)31-93-25-26-94-32-43(53(89)75-38(13-7-21-68-57(62)63)50(86)74-39(51(87)79-47)14-8-22-69-58(64)65)78-52(88)41(28-34-11-5-4-6-12-34)73-46(83)30-71-45(82)29-72-49(85)37(60)27-35-17-19-36(81)20-18-35/h4-6,11-12,17-20,33,37-44,47,81H,3,7-10,13-16,21-32,60H2,1-2H3,(H2,61,84)(H,71,82)(H,72,85)(H,73,83)(H,74,86)(H,75,89)(H,76,91)(H,77,90)(H,78,88)(H,79,87)(H4,62,63,68)(H4,64,65,69)(H4,66,67,70)/t33-,37-,38-,39-,40-,41-,42-,43-,44-,47-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at kappa opioid receptor in guinea pig brain by [3H]U-69,593 displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50038012
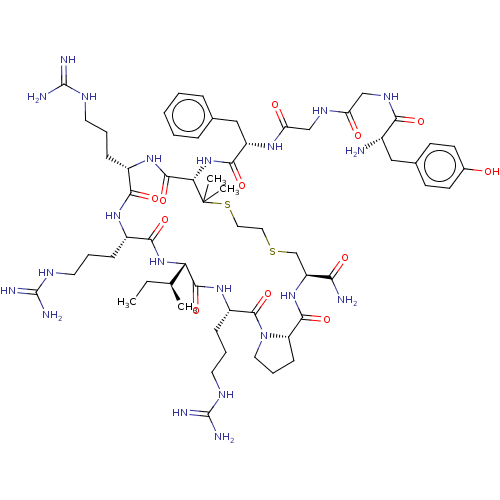
(CHEMBL2370629 | Tyr-Gly-Gly-Phe-D-Pen-Arg-Arg-Ile-...)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)C([#6])([#6])[#16]-[#6]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C61H97N21O12S2/c1-5-34(2)47-55(92)78-41(17-11-25-72-60(68)69)57(94)82-26-12-18-44(82)54(91)79-43(49(63)86)33-95-27-28-96-61(3,4)48(56(93)77-39(15-9-23-70-58(64)65)51(88)76-40(52(89)80-47)16-10-24-71-59(66)67)81-53(90)42(30-35-13-7-6-8-14-35)75-46(85)32-73-45(84)31-74-50(87)38(62)29-36-19-21-37(83)22-20-36/h6-8,13-14,19-22,34,38-44,47-48,83H,5,9-12,15-18,23-33,62H2,1-4H3,(H2,63,86)(H,73,84)(H,74,87)(H,75,85)(H,76,88)(H,77,93)(H,78,92)(H,79,91)(H,80,89)(H,81,90)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t34-,38-,39-,40-,41-,42-,43-,44-,47-,48-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at kappa opioid receptor in guinea pig brain by [3H]U-69,593 displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50043723
((S)-2-{[(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccc(I)cc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H43IN6O8S2/c1-37(2)31(44-32(47)26(39)16-22-10-14-25(45)15-11-22)35(50)40-19-30(46)41-27(17-23-8-12-24(38)13-9-23)33(48)43-29(20-53-54-37)34(49)42-28(36(51)52)18-21-6-4-3-5-7-21/h3-15,26-29,31,45H,16-20,39H2,1-2H3,(H,40,50)(H,41,46)(H,42,49)(H,43,48)(H,44,47)(H,51,52)/t26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50043724

((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H44N6O8S2/c1-37(2)31(43-32(46)26(38)17-24-13-15-25(44)16-14-24)35(49)39-20-30(45)40-27(18-22-9-5-3-6-10-22)33(47)42-29(21-52-53-37)34(48)41-28(36(50)51)19-23-11-7-4-8-12-23/h3-16,26-29,31,44H,17-21,38H2,1-2H3,(H,39,49)(H,40,45)(H,41,48)(H,42,47)(H,43,46)(H,50,51)/t26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50043724

((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H44N6O8S2/c1-37(2)31(43-32(46)26(38)17-24-13-15-25(44)16-14-24)35(49)39-20-30(45)40-27(18-22-9-5-3-6-10-22)33(47)42-29(21-52-53-37)34(48)41-28(36(50)51)19-23-11-7-4-8-12-23/h3-16,26-29,31,44H,17-21,38H2,1-2H3,(H,39,49)(H,40,45)(H,41,48)(H,42,47)(H,43,46)(H,50,51)/t26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50001683

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity opioid receptor |
J Med Chem 37: 1746-57 (1994)
BindingDB Entry DOI: 10.7270/Q2N58N1K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50061290
((S)-2-({(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H48N6O8S2/c1-38(2)31(44-33(48)27(40)19-25-15-17-26(46)18-16-25)35(50)41-22-30(47)42-28(20-23-11-7-5-8-12-23)34(49)45-32(39(3,4)55-54-38)36(51)43-29(37(52)53)21-24-13-9-6-10-14-24/h5-18,27-29,31-32,46H,19-22,40H2,1-4H3,(H,41,50)(H,42,47)(H,43,51)(H,44,48)(H,45,49)(H,52,53)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory potency against Opioid receptor delta 1 was determined in electrically induced smooth muscle contractions of mouse vas deferens (MVD) |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50280901
((S)-3-({1-[(S)-2-(2-Amino-acetylamino)-3-(S)-1H-in...)Show SMILES CCCC1C[C@H](N(C1)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H43N7O7/c1-2-8-21-14-28(33(47)40-26(16-30(43)44)32(46)39-25(31(36)45)13-20-9-4-3-5-10-20)41(19-21)34(48)27(38-29(42)17-35)15-22-18-37-24-12-7-6-11-23(22)24/h3-7,9-12,18,21,25-28,37H,2,8,13-17,19,35H2,1H3,(H2,36,45)(H,38,42)(H,39,46)(H,40,47)(H,43,44)/t21?,25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) |
Bioorg Med Chem Lett 3: 2011-2016 (1993)
Article DOI: 10.1016/S0960-894X(01)81005-2
BindingDB Entry DOI: 10.7270/Q2B27V63 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50038014
(CHEMBL2370622 | Tyr-Gly-Gly-Phe-Cys-Arg-Arg-Ile-Ar...)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)C([#6])([#6])[#16]-[#6]-[#6]-[#16]C([#6])([#6])[#6@@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C63H101N21O12S2/c1-7-35(2)47-56(94)80-42(19-13-27-74-61(70)71)58(96)84-28-14-20-44(84)55(93)82-48(50(65)88)62(3,4)97-29-30-98-63(5,6)49(57(95)79-40(17-11-25-72-59(66)67)52(90)78-41(53(91)81-47)18-12-26-73-60(68)69)83-54(92)43(32-36-15-9-8-10-16-36)77-46(87)34-75-45(86)33-76-51(89)39(64)31-37-21-23-38(85)24-22-37/h8-10,15-16,21-24,35,39-44,47-49,85H,7,11-14,17-20,25-34,64H2,1-6H3,(H2,65,88)(H,75,86)(H,76,89)(H,77,87)(H,78,90)(H,79,95)(H,80,94)(H,81,91)(H,82,93)(H,83,92)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t35-,39-,40-,41-,42-,43-,44-,47-,48-,49-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at kappa opioid receptor in guinea pig brain by [3H]U-69,593 displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50038010
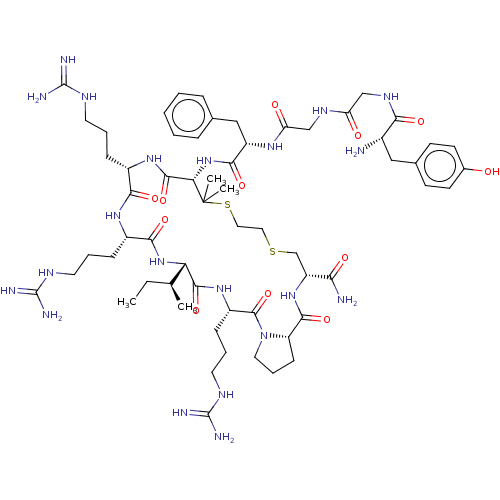
(CHEMBL2370627 | Tyr-Gly-Gly-Phe-Pen-Arg-Arg-Ile-Ar...)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)C([#6])([#6])[#16]-[#6]-[#6]-[#16]-[#6]-[#6@@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C61H97N21O12S2/c1-5-34(2)47-55(92)78-41(17-11-25-72-60(68)69)57(94)82-26-12-18-44(82)54(91)79-43(49(63)86)33-95-27-28-96-61(3,4)48(56(93)77-39(15-9-23-70-58(64)65)51(88)76-40(52(89)80-47)16-10-24-71-59(66)67)81-53(90)42(30-35-13-7-6-8-14-35)75-46(85)32-73-45(84)31-74-50(87)38(62)29-36-19-21-37(83)22-20-36/h6-8,13-14,19-22,34,38-44,47-48,83H,5,9-12,15-18,23-33,62H2,1-4H3,(H2,63,86)(H,73,84)(H,74,87)(H,75,85)(H,76,88)(H,77,93)(H,78,92)(H,79,91)(H,80,89)(H,81,90)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t34-,38-,39-,40-,41-,42-,43+,44-,47-,48-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at kappa opioid receptor in guinea pig brain by [3H]U-69,593 displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50001683

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory potency against Opioid receptor delta 1 was determined in electrically induced smooth muscle contractions of mouse vas deferens (MVD) |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50038425

((S)-3-((S)-2-{(R)-2-[(2S,3S)-2-Amino-3-(4-hydroxy-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)[C@@H](C)c1c(C)cc(O)cc1C)C(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C40H58N8O10/c1-19(2)33(39(57)43-18-29(41)50)48-40(58)34(20(3)4)47-37(55)28(17-30(51)52)46-36(54)27(16-25-12-10-9-11-13-25)45-35(53)24(8)44-38(56)32(42)23(7)31-21(5)14-26(49)15-22(31)6/h9-15,19-20,23-24,27-28,32-34,49H,16-18,42H2,1-8H3,(H2,41,50)(H,43,57)(H,44,56)(H,45,53)(H,46,54)(H,47,55)(H,48,58)(H,51,52)/t23-,24+,27-,28-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity opioid receptor |
J Med Chem 37: 1746-57 (1994)
BindingDB Entry DOI: 10.7270/Q2N58N1K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50038011
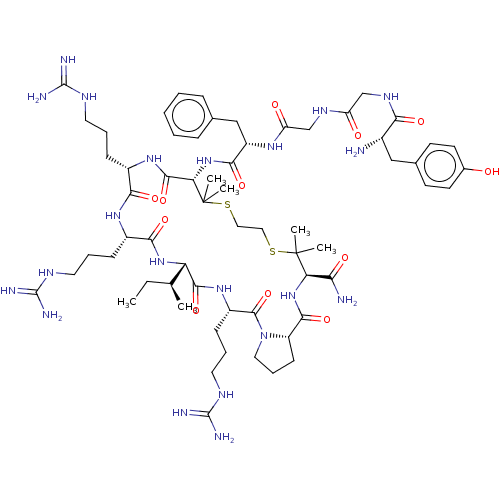
(CHEMBL2370612 | Tyr-Gly-Gly-Phe-Pen-Arg-Arg-Ile-Ar...)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)C([#6])([#6])[#16]-[#6]-[#6]-[#16]C([#6])([#6])[#6@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C63H101N21O12S2/c1-7-35(2)47-56(94)80-42(19-13-27-74-61(70)71)58(96)84-28-14-20-44(84)55(93)82-48(50(65)88)62(3,4)97-29-30-98-63(5,6)49(57(95)79-40(17-11-25-72-59(66)67)52(90)78-41(53(91)81-47)18-12-26-73-60(68)69)83-54(92)43(32-36-15-9-8-10-16-36)77-46(87)34-75-45(86)33-76-51(89)39(64)31-37-21-23-38(85)24-22-37/h8-10,15-16,21-24,35,39-44,47-49,85H,7,11-14,17-20,25-34,64H2,1-6H3,(H2,65,88)(H,75,86)(H,76,89)(H,77,87)(H,78,90)(H,79,95)(H,80,94)(H,81,91)(H,82,93)(H,83,92)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t35-,39-,40-,41-,42-,43-,44-,47-,48+,49-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at kappa opioid receptor in guinea pig brain by [3H]U-69,593 displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50092394
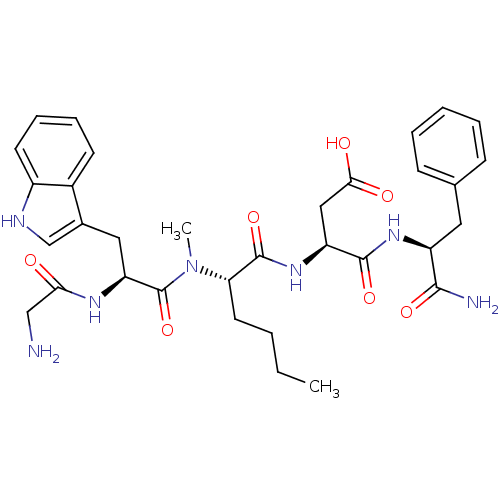
((S)-3-((S)-2-{[(S)-2-(2-Amino-acetylamino)-3-(S)-1...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C33H43N7O7/c1-3-4-14-27(40(2)33(47)26(37-28(41)18-34)16-21-19-36-23-13-9-8-12-22(21)23)32(46)39-25(17-29(42)43)31(45)38-24(30(35)44)15-20-10-6-5-7-11-20/h5-13,19,24-27,36H,3-4,14-18,34H2,1-2H3,(H2,35,44)(H,37,41)(H,38,45)(H,39,46)(H,42,43)/t24-,25-,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards Cholecystokinin type B receptor in cortical membranes (CNS) |
Bioorg Med Chem Lett 3: 2011-2016 (1993)
Article DOI: 10.1016/S0960-894X(01)81005-2
BindingDB Entry DOI: 10.7270/Q2B27V63 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50061290

((S)-2-({(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C39H48N6O8S2/c1-38(2)31(44-33(48)27(40)19-25-15-17-26(46)18-16-25)35(50)41-22-30(47)42-28(20-23-11-7-5-8-12-23)34(49)45-32(39(3,4)55-54-38)36(51)43-29(37(52)53)21-24-13-9-6-10-14-24/h5-18,27-29,31-32,46H,19-22,40H2,1-4H3,(H,41,50)(H,42,47)(H,43,51)(H,44,48)(H,45,49)(H,52,53)/t27-,28-,29-,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001683

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 39: 4120-4 (1996)
Article DOI: 10.1021/jm960078j
BindingDB Entry DOI: 10.7270/Q2P55P5Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50038014

(CHEMBL2370622 | Tyr-Gly-Gly-Phe-Cys-Arg-Arg-Ile-Ar...)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)C([#6])([#6])[#16]-[#6]-[#6]-[#16]C([#6])([#6])[#6@@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C63H101N21O12S2/c1-7-35(2)47-56(94)80-42(19-13-27-74-61(70)71)58(96)84-28-14-20-44(84)55(93)82-48(50(65)88)62(3,4)97-29-30-98-63(5,6)49(57(95)79-40(17-11-25-72-59(66)67)52(90)78-41(53(91)81-47)18-12-26-73-60(68)69)83-54(92)43(32-36-15-9-8-10-16-36)77-46(87)34-75-45(86)33-76-51(89)39(64)31-37-21-23-38(85)24-22-37/h8-10,15-16,21-24,35,39-44,47-49,85H,7,11-14,17-20,25-34,64H2,1-6H3,(H2,65,88)(H,75,86)(H,76,89)(H,77,87)(H,78,90)(H,79,95)(H,80,94)(H,81,91)(H,82,93)(H,83,92)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t35-,39-,40-,41-,42-,43-,44-,47-,48-,49-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor in guinea pig brain by [3H]-DAMGO displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50038020

(CHEMBL2370618 | Tyr-Gly-Gly-Phe-D-Cys-Arg-Arg-Ile-...)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-[#6]-[#16]C([#6])([#6])[#6@@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C61H97N21O12S2/c1-5-34(2)47-56(93)78-41(17-11-25-72-60(68)69)57(94)82-26-12-18-44(82)55(92)81-48(49(63)86)61(3,4)96-28-27-95-33-43(54(91)77-39(15-9-23-70-58(64)65)51(88)76-40(52(89)80-47)16-10-24-71-59(66)67)79-53(90)42(30-35-13-7-6-8-14-35)75-46(85)32-73-45(84)31-74-50(87)38(62)29-36-19-21-37(83)22-20-36/h6-8,13-14,19-22,34,38-44,47-48,83H,5,9-12,15-18,23-33,62H2,1-4H3,(H2,63,86)(H,73,84)(H,74,87)(H,75,85)(H,76,88)(H,77,91)(H,78,93)(H,79,90)(H,80,89)(H,81,92)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t34-,38-,39-,40-,41-,42-,43-,44-,47-,48-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor in guinea pig brain by [3H]-DAMGO displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50038019

(CHEMBL411338 | Tyr-Gly-Gly-Phe-D-Leu-Arg-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42+,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor in guinea pig brain by [3H]-DAMGO displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50001683

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50038010

(CHEMBL2370627 | Tyr-Gly-Gly-Phe-Pen-Arg-Arg-Ile-Ar...)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)C([#6])([#6])[#16]-[#6]-[#6]-[#16]-[#6]-[#6@@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C61H97N21O12S2/c1-5-34(2)47-55(92)78-41(17-11-25-72-60(68)69)57(94)82-26-12-18-44(82)54(91)79-43(49(63)86)33-95-27-28-96-61(3,4)48(56(93)77-39(15-9-23-70-58(64)65)51(88)76-40(52(89)80-47)16-10-24-71-59(66)67)81-53(90)42(30-35-13-7-6-8-14-35)75-46(85)32-73-45(84)31-74-50(87)38(62)29-36-19-21-37(83)22-20-36/h6-8,13-14,19-22,34,38-44,47-48,83H,5,9-12,15-18,23-33,62H2,1-4H3,(H2,63,86)(H,73,84)(H,74,87)(H,75,85)(H,76,88)(H,77,93)(H,78,92)(H,79,91)(H,80,89)(H,81,90)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t34-,38-,39-,40-,41-,42-,43+,44-,47-,48-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor in guinea pig brain by [3H]-DAMGO displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor in guinea pig brain by [3H]-DAMGO displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50001683

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain |
J Med Chem 40: 3957-62 (1998)
Article DOI: 10.1021/jm9704762
BindingDB Entry DOI: 10.7270/Q23R0TJ7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50453445

(CHEMBL2112652)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C59H93N21O12S2/c1-3-33(2)47-55(91)76-40(15-9-23-70-59(66)67)56(92)80-24-10-16-44(80)54(90)77-42(48(61)84)31-93-25-26-94-32-43(53(89)75-38(13-7-21-68-57(62)63)50(86)74-39(51(87)79-47)14-8-22-69-58(64)65)78-52(88)41(28-34-11-5-4-6-12-34)73-46(83)30-71-45(82)29-72-49(85)37(60)27-35-17-19-36(81)20-18-35/h4-6,11-12,17-20,33,37-44,47,81H,3,7-10,13-16,21-32,60H2,1-2H3,(H2,61,84)(H,71,82)(H,72,85)(H,73,83)(H,74,86)(H,75,89)(H,76,91)(H,77,90)(H,78,88)(H,79,87)(H4,62,63,68)(H4,64,65,69)(H4,66,67,70)/t33-,37-,38-,39-,40-,41-,42-,43-,44-,47-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor in guinea pig brain by [3H]-DAMGO displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50041669

(2-Amino-N-(7-benzyl-4-dihydroxymethyl-3,3,10,14,14...)Show SMILES CC1NC(=O)C(NC(=O)C(N)Cc2ccc(O)cc2)C(C)(C)SSC(C)(C)C(NC(=O)C(Cc2ccccc2)NC1=O)C(O)O Show InChI InChI=1S/C31H43N5O7S2/c1-17-25(38)34-22(16-18-9-7-6-8-10-18)27(40)36-24(29(42)43)31(4,5)45-44-30(2,3)23(28(41)33-17)35-26(39)21(32)15-19-11-13-20(37)14-12-19/h6-14,17,21-24,29,37,42-43H,15-16,32H2,1-5H3,(H,33,41)(H,34,38)(H,35,39)(H,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory activity of the electrically induced smooth muscle contraction of mouse vas deferens |
J Med Chem 37: 1572-7 (1994)
BindingDB Entry DOI: 10.7270/Q2DR2W47 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50453313

(CHEMBL2372329)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H35N5O7S2/c1-28(2)23(33-24(36)19(29)12-17-8-10-18(34)11-9-17)26(38)30-14-22(35)31-20(13-16-6-4-3-5-7-16)25(37)32-21(27(39)40)15-41-42-28/h3-11,19-21,23,34H,12-15,29H2,1-2H3,(H,30,38)(H,31,35)(H,32,37)(H,33,36)(H,39,40)/t19-,20-,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 37: 146-50 (1994)
BindingDB Entry DOI: 10.7270/Q2TX3G09 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50453445

(CHEMBL2112652)Show SMILES [H][C@@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]2=O)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C59H93N21O12S2/c1-3-33(2)47-55(91)76-40(15-9-23-70-59(66)67)56(92)80-24-10-16-44(80)54(90)77-42(48(61)84)31-93-25-26-94-32-43(53(89)75-38(13-7-21-68-57(62)63)50(86)74-39(51(87)79-47)14-8-22-69-58(64)65)78-52(88)41(28-34-11-5-4-6-12-34)73-46(83)30-71-45(82)29-72-49(85)37(60)27-35-17-19-36(81)20-18-35/h4-6,11-12,17-20,33,37-44,47,81H,3,7-10,13-16,21-32,60H2,1-2H3,(H2,61,84)(H,71,82)(H,72,85)(H,73,83)(H,74,86)(H,75,89)(H,76,91)(H,77,90)(H,78,88)(H,79,87)(H4,62,63,68)(H4,64,65,69)(H4,66,67,70)/t33-,37-,38-,39-,40-,41-,42-,43-,44-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity at delta opioid receptor in guinea pig brain by [3H]c[D-Pen2, p-Cl-Phe4, D-Pen5]-enkephalin displacement. |
J Med Chem 37: 3910-7 (1994)
BindingDB Entry DOI: 10.7270/Q22N51BK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































