 Found 264 hits with Last Name = 'zhang' and Initial = 'mq'
Found 264 hits with Last Name = 'zhang' and Initial = 'mq' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligand |
J Med Chem 41: 1439-45 (1998)
Article DOI: 10.1021/jm970180w
BindingDB Entry DOI: 10.7270/Q2WH2P4P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50052024

(CHEMBL787 | montelukast)Show SMILES CC(C)(O)c1ccccc1CC[C@@H](SCC1(CC(O)=O)CC1)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 |r| Show InChI InChI=1S/C35H36ClNO3S/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39)/b15-10+/t32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligand |
J Med Chem 41: 1439-45 (1998)
Article DOI: 10.1021/jm970180w
BindingDB Entry DOI: 10.7270/Q2WH2P4P |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50064085
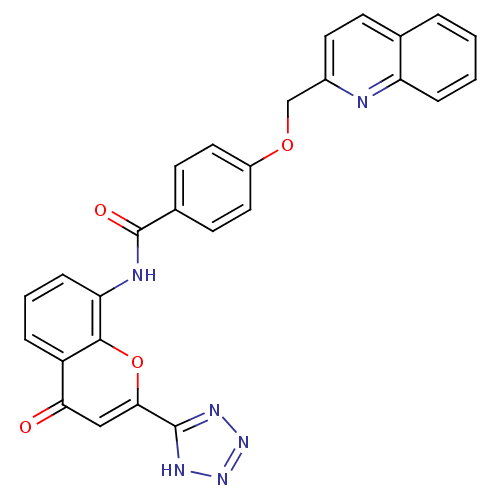
(CHEMBL285070 | N-[4-Oxo-2-(1H-tetrazol-5-yl)-4H-ch...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H18N6O4/c34-23-14-24(26-30-32-33-31-26)37-25-20(23)5-3-7-22(25)29-27(35)17-9-12-19(13-10-17)36-15-18-11-8-16-4-1-2-6-21(16)28-18/h1-14H,15H2,(H,29,35)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligand |
J Med Chem 41: 1439-45 (1998)
Article DOI: 10.1021/jm970180w
BindingDB Entry DOI: 10.7270/Q2WH2P4P |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50064083
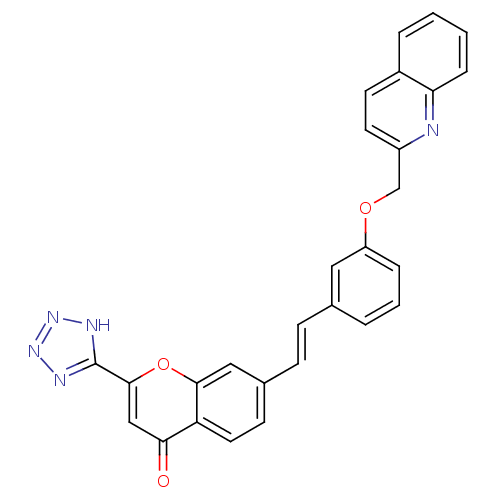
(7-{(E)-2-[3-(Quinolin-2-ylmethoxy)-phenyl]-vinyl}-...)Show SMILES O=c1cc(oc2cc(\C=C\c3cccc(OCc4ccc5ccccc5n4)c3)ccc12)-c1nnn[nH]1 Show InChI InChI=1S/C28H19N5O3/c34-25-16-27(28-30-32-33-31-28)36-26-15-19(10-13-23(25)26)9-8-18-4-3-6-22(14-18)35-17-21-12-11-20-5-1-2-7-24(20)29-21/h1-16H,17H2,(H,30,31,32,33)/b9-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligand |
J Med Chem 41: 1439-45 (1998)
Article DOI: 10.1021/jm970180w
BindingDB Entry DOI: 10.7270/Q2WH2P4P |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50064084
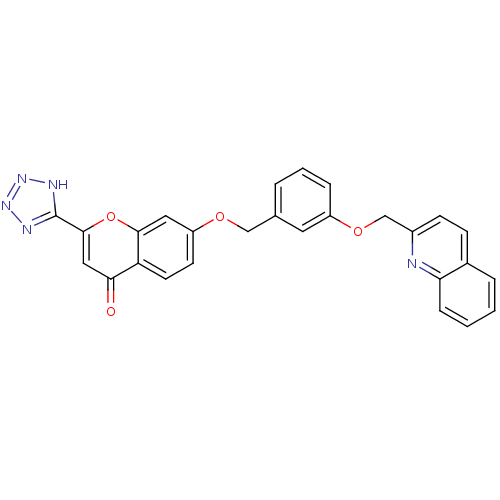
(7-[3-(Quinolin-2-ylmethoxy)-benzyloxy]-2-(1H-tetra...)Show SMILES O=c1cc(oc2cc(OCc3cccc(OCc4ccc5ccccc5n4)c3)ccc12)-c1nnn[nH]1 Show InChI InChI=1S/C27H19N5O4/c33-24-14-26(27-29-31-32-30-27)36-25-13-21(10-11-22(24)25)34-15-17-4-3-6-20(12-17)35-16-19-9-8-18-5-1-2-7-23(18)28-19/h1-14H,15-16H2,(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligand |
J Med Chem 41: 1439-45 (1998)
Article DOI: 10.1021/jm970180w
BindingDB Entry DOI: 10.7270/Q2WH2P4P |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50064086
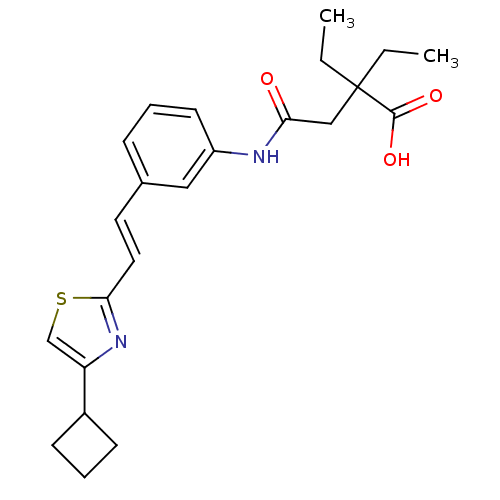
(CHEMBL283754 | Cinalukast | N-{3-[(E)-2-(4-Cyclobu...)Show SMILES CCC(CC)(CC(=O)Nc1cccc(\C=C\c2nc(cs2)C2CCC2)c1)C(O)=O Show InChI InChI=1S/C23H28N2O3S/c1-3-23(4-2,22(27)28)14-20(26)24-18-10-5-7-16(13-18)11-12-21-25-19(15-29-21)17-8-6-9-17/h5,7,10-13,15,17H,3-4,6,8-9,14H2,1-2H3,(H,24,26)(H,27,28)/b12-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligand |
J Med Chem 41: 1439-45 (1998)
Article DOI: 10.1021/jm970180w
BindingDB Entry DOI: 10.7270/Q2WH2P4P |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50023198

(8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-24(26-29-31-32-30-26)36-25-21(23)10-6-11-22(25)28-27(34)19-12-14-20(15-13-19)35-16-5-4-9-18-7-2-1-3-8-18/h1-3,6-8,10-15,17H,4-5,9,16H2,(H,28,34)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligand |
J Med Chem 41: 1439-45 (1998)
Article DOI: 10.1021/jm970180w
BindingDB Entry DOI: 10.7270/Q2WH2P4P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50022775

((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...)Show InChI InChI=1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant AChE |
Bioorg Med Chem Lett 12: 193-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CR5SP0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117612

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C25H23FNO3S/c1-29-22-13-19-14-25(31-24(19)15-23(22)30-2)21(28)8-5-17-9-11-27(12-10-17)16-18-3-6-20(26)7-4-18/h3-4,6-7,9-15H,5,8,16H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50366796

(CHEMBL609440)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccc(o2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C23H21N2O6S/c1-29-19-11-16-12-22(32-21(16)13-20(19)30-2)18(26)5-3-15-7-9-24(10-8-15)14-17-4-6-23(31-17)25(27)28/h4,6-13H,3,5,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117595
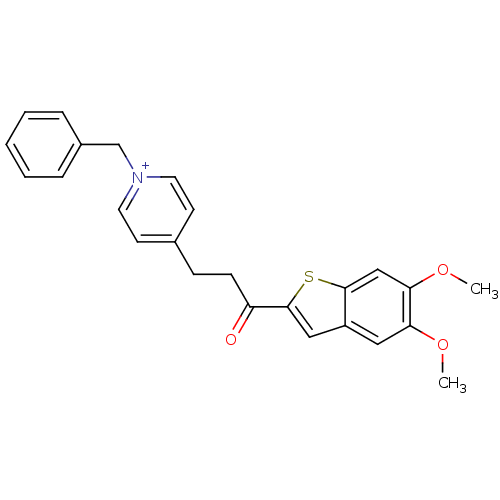
(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccccc2)cc1 Show InChI InChI=1S/C25H24NO3S/c1-28-22-14-20-15-25(30-24(20)16-23(22)29-2)21(27)9-8-18-10-12-26(13-11-18)17-19-6-4-3-5-7-19/h3-7,10-16H,8-9,17H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117614

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccsc2)cc1 Show InChI InChI=1S/C23H22NO3S2/c1-26-20-11-18-12-23(29-22(18)13-21(20)27-2)19(25)4-3-16-5-8-24(9-6-16)14-17-7-10-28-15-17/h5-13,15H,3-4,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117618

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES CCOCC[n+]1ccc(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)cc1 Show InChI InChI=1S/C22H26NO4S/c1-4-27-12-11-23-9-7-16(8-10-23)5-6-18(24)22-14-17-13-19(25-2)20(26-3)15-21(17)28-22/h7-10,13-15H,4-6,11-12H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117577
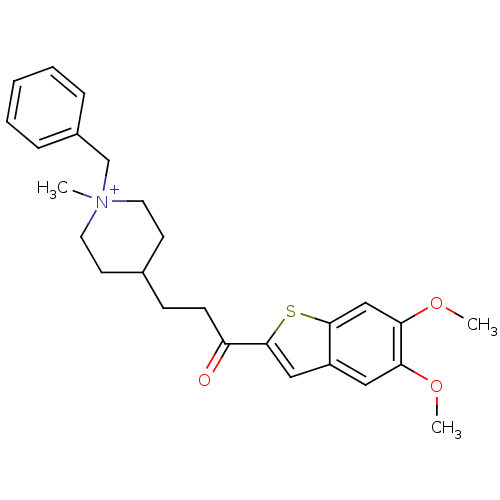
(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(.52,-5.47,;1.15,-6.88,;2.69,-7.05,;3.59,-5.8,;5.13,-5.96,;6.27,-4.92,;7.6,-5.69,;7.28,-7.21,;5.75,-7.37,;4.85,-8.61,;3.32,-8.45,;2.42,-9.7,;.89,-9.55,;9.01,-5.06,;9.17,-3.53,;10.25,-5.97,;11.67,-5.34,;12.94,-5.99,;12.93,-7.54,;14.26,-8.31,;15.6,-7.54,;16.68,-6.44,;16.93,-8.31,;18.27,-7.54,;18.26,-6.01,;19.59,-5.24,;20.93,-6.01,;20.93,-7.55,;19.6,-8.31,;15.6,-5.99,;14.27,-5.22,)| Show InChI InChI=1S/C26H32NO3S/c1-27(18-20-7-5-4-6-8-20)13-11-19(12-14-27)9-10-22(28)26-16-21-15-23(29-2)24(30-3)17-25(21)31-26/h4-8,15-17,19H,9-14,18H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117594

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(-.09,-3.27,;1.22,-2.5,;2.55,-3.27,;3.88,-2.5,;5.21,-3.27,;6.68,-2.78,;7.59,-4.04,;6.68,-5.3,;5.21,-4.81,;3.88,-5.58,;2.55,-4.81,;1.22,-5.58,;-.09,-4.81,;9.13,-4.04,;9.9,-5.37,;9.9,-2.71,;11.44,-2.71,;12.21,-1.38,;13.74,-1.22,;14.37,.18,;13.47,1.42,;12.28,2.4,;14.1,2.84,;15.64,2.99,;16.27,4.39,;17.79,4.54,;18.7,3.29,;18.07,1.88,;16.53,1.74,;11.95,1.28,;11.3,-.12,)| Show InChI InChI=1S/C26H32NO3S/c1-27(18-20-7-5-4-6-8-20)13-11-19(12-14-27)9-10-22(28)26-16-21-15-23(29-2)24(30-3)17-25(21)31-26/h4-8,15-17,19H,9-14,18H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50064072
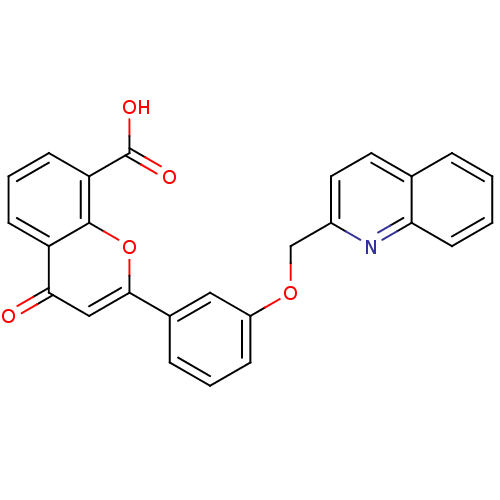
(4-Oxo-2-[3-(quinolin-2-ylmethoxy)-phenyl]-4H-chrom...)Show SMILES OC(=O)c1cccc2c1oc(cc2=O)-c1cccc(OCc2ccc3ccccc3n2)c1 Show InChI InChI=1S/C26H17NO5/c28-23-14-24(32-25-20(23)8-4-9-21(25)26(29)30)17-6-3-7-19(13-17)31-15-18-12-11-16-5-1-2-10-22(16)27-18/h1-14H,15H2,(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligand |
J Med Chem 41: 1439-45 (1998)
Article DOI: 10.1021/jm970180w
BindingDB Entry DOI: 10.7270/Q2WH2P4P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117584

(1-Benzyl-4-[3-(2,3-dihydro-benzofuran-5-yl)-3-oxo-...)Show SMILES C[N+]1(Cc2ccccc2)CCC(CCC(=O)c2ccc3OCCc3c2)CC1 |(20.14,-5.28,;19.05,-6.38,;20.38,-7.15,;21.72,-6.38,;21.71,-4.84,;23.04,-4.07,;24.38,-4.84,;24.38,-6.38,;23.05,-7.15,;19.05,-4.84,;17.74,-4.07,;16.4,-4.83,;15.14,-4.18,;13.73,-4.81,;12.48,-3.9,;12.65,-2.36,;11.07,-4.53,;10.92,-6.05,;9.52,-6.68,;8.24,-5.79,;6.73,-6.1,;5.96,-4.74,;7.01,-3.59,;8.43,-4.25,;9.83,-3.62,;16.39,-6.37,;17.72,-7.15,)| Show InChI InChI=1S/C24H30NO2/c1-25(18-20-5-3-2-4-6-20)14-11-19(12-15-25)7-9-23(26)21-8-10-24-22(17-21)13-16-27-24/h2-6,8,10,17,19H,7,9,11-16,18H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50064074
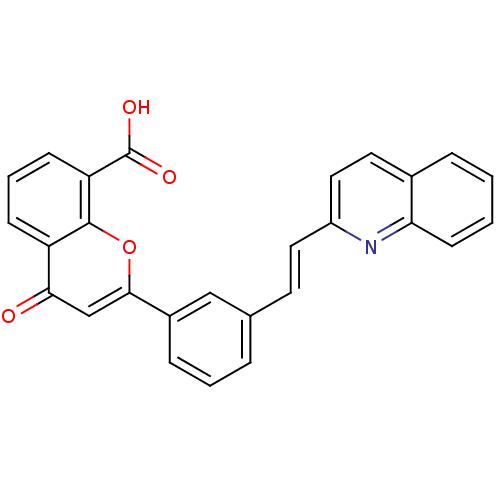
(4-Oxo-2-[3-((E)-2-quinolin-2-yl-vinyl)-phenyl]-4H-...)Show SMILES OC(=O)c1cccc2c1oc(cc2=O)-c1cccc(\C=C\c2ccc3ccccc3n2)c1 Show InChI InChI=1S/C27H17NO4/c29-24-16-25(32-26-21(24)8-4-9-22(26)27(30)31)19-7-3-5-17(15-19)11-13-20-14-12-18-6-1-2-10-23(18)28-20/h1-16H,(H,30,31)/b13-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligand |
J Med Chem 41: 1439-45 (1998)
Article DOI: 10.1021/jm970180w
BindingDB Entry DOI: 10.7270/Q2WH2P4P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117574

(1-Benzyl-1-methyl-4-[3-(5-methyl-1-phenyl-1H-pyraz...)Show SMILES Cc1cc(nn1-c1ccccc1)C(=O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(5.62,-.38,;6.31,-1.76,;7.82,-2.01,;8.06,-3.52,;6.68,-4.21,;5.61,-3.13,;4.08,-3.34,;3.13,-2.15,;1.59,-2.36,;1.03,-3.81,;2.01,-5.02,;3.53,-4.78,;9.47,-2.9,;9.64,-1.36,;10.72,-3.8,;12.12,-3.17,;13.38,-3.81,;13.38,-5.37,;14.71,-6.14,;16.04,-5.37,;17.13,-4.27,;17.37,-6.14,;18.7,-5.37,;18.7,-3.83,;20.03,-3.06,;21.37,-3.83,;21.37,-5.37,;20.03,-6.14,;16.05,-3.83,;14.72,-3.06,)| Show InChI InChI=1S/C26H32N3O/c1-21-19-25(27-28(21)24-11-7-4-8-12-24)26(30)14-13-22-15-17-29(2,18-16-22)20-23-9-5-3-6-10-23/h3-12,19,22H,13-18,20H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117583

(1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylmethyl)-...)Show SMILES COc1cc2CC(CC3CC[N+](C)(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C25H32NO3/c1-26(17-19-7-5-4-6-8-19)11-9-18(10-12-26)13-21-14-20-15-23(28-2)24(29-3)16-22(20)25(21)27/h4-8,15-16,18,21H,9-14,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117609
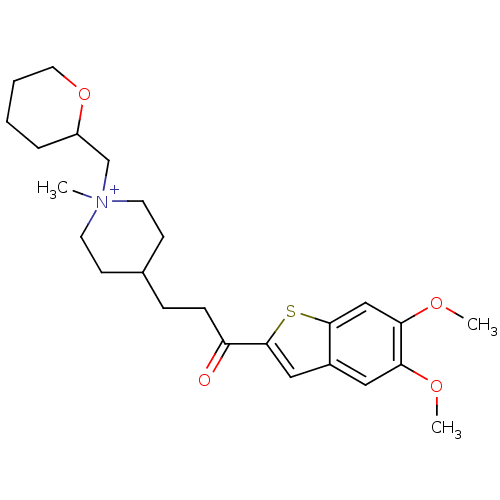
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(CC2CCCCO2)CC1 |(-1.63,-4.95,;-.3,-4.18,;1.03,-4.95,;2.36,-4.18,;3.69,-4.95,;5.16,-4.46,;6.07,-5.72,;5.16,-6.98,;3.69,-6.49,;2.36,-7.26,;1.03,-6.49,;-.3,-7.26,;-1.63,-6.49,;7.61,-5.72,;8.38,-7.05,;8.38,-4.37,;9.92,-4.37,;10.69,-3.04,;9.78,-1.8,;10.41,-.4,;11.95,-.24,;10.97,.95,;13.45,.11,;13.89,1.58,;12.84,2.7,;13.3,4.18,;14.8,4.53,;15.85,3.4,;15.41,1.93,;12.84,-1.49,;12.21,-2.89,)| Show InChI InChI=1S/C25H36NO4S/c1-26(17-20-6-4-5-13-30-20)11-9-18(10-12-26)7-8-21(27)25-15-19-14-22(28-2)23(29-3)16-24(19)31-25/h14-16,18,20H,4-13,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117620
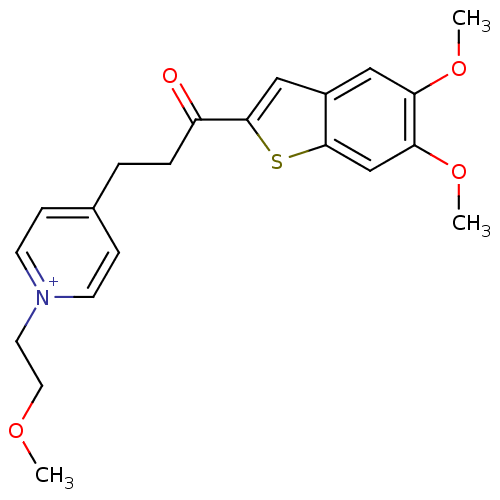
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COCC[n+]1ccc(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)cc1 Show InChI InChI=1S/C21H24NO4S/c1-24-11-10-22-8-6-15(7-9-22)4-5-17(23)21-13-16-12-18(25-2)19(26-3)14-20(16)27-21/h6-9,12-14H,4-5,10-11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50199518
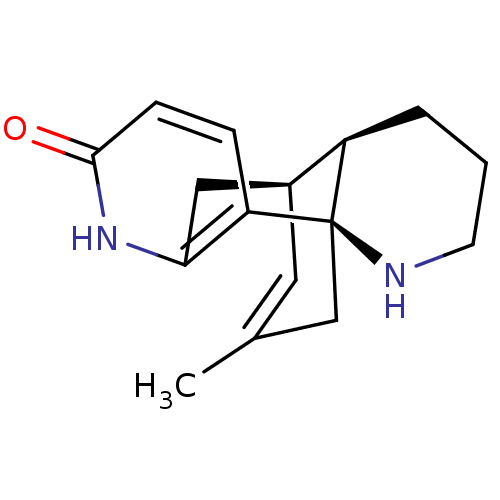
((-)-huperzine B | CHEMBL245079 | huperzine B)Show SMILES CC1=C[C@H]2Cc3[nH]c(=O)ccc3[C@]3(C1)NCCC[C@H]23 |r,t:1,THB:0:1:4.5.11:18| Show InChI InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11-,12+,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117622
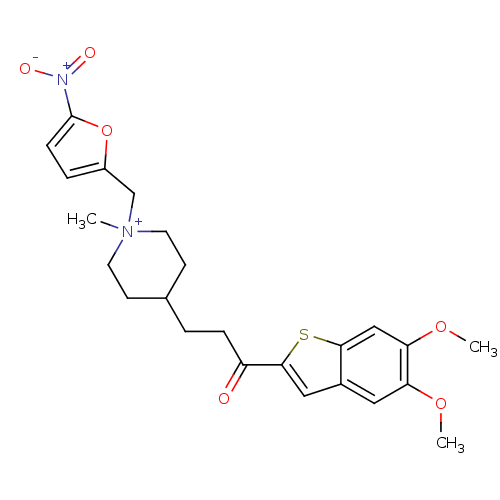
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(Cc2ccc(o2)[N+]([O-])=O)CC1 |(-1.63,-4.96,;-.3,-4.19,;1.04,-4.96,;2.36,-4.19,;3.69,-4.96,;5.18,-4.47,;6.09,-5.73,;5.18,-6.99,;3.69,-6.51,;2.37,-7.27,;1.04,-6.5,;-.3,-7.27,;-1.63,-6.5,;7.63,-5.73,;8.4,-7.06,;8.4,-4.38,;9.95,-4.38,;10.71,-3.05,;9.8,-1.81,;10.44,-.4,;11.98,-.24,;11,.95,;13.48,.11,;13.92,1.58,;12.98,2.81,;13.87,4.08,;15.35,3.64,;15.39,2.1,;16.56,4.58,;17.99,3.97,;16.37,6.09,;12.87,-1.5,;12.24,-2.9,)| Show InChI InChI=1S/C24H29N2O6S/c1-26(15-18-5-7-24(32-18)25(28)29)10-8-16(9-11-26)4-6-19(27)23-13-17-12-20(30-2)21(31-3)14-22(17)33-23/h5,7,12-14,16H,4,6,8-11,15H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117623

(1-Cyclobutylmethyl-4-[3-(5,6-dimethoxy-benzo[b]thi...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](CC2CCC2)cc1 Show InChI InChI=1S/C23H26NO3S/c1-26-20-12-18-13-23(28-22(18)14-21(20)27-2)19(25)7-6-16-8-10-24(11-9-16)15-17-4-3-5-17/h8-14,17H,3-7,15H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117599

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.47,-2.29,;15.47,.37,;16.99,.27,;17.86,1.54,;17.18,2.93,;16.18,4.08,;18.04,4.2,;19.58,4.1,;20.25,2.71,;21.78,2.61,;22.64,3.89,;21.96,5.27,;20.42,5.37,;15.64,3.03,;14.78,1.75,)| Show InChI InChI=1S/C26H32NO4S/c1-27(17-18-7-5-4-6-8-18)11-9-19(10-12-27)21(28)15-22(29)26-14-20-13-23(30-2)24(31-3)16-25(20)32-26/h4-8,13-14,16,19,21,28H,9-12,15,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117576
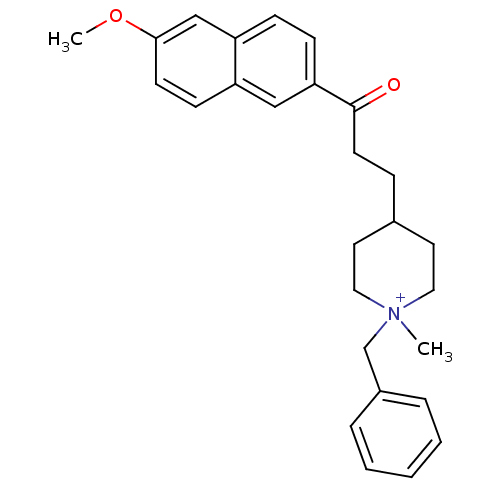
(1-Benzyl-4-[3-(6-methoxy-naphthalen-2-yl)-3-oxo-pr...)Show SMILES COc1ccc2cc(ccc2c1)C(=O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(-3.21,-6.68,;-1.98,-7.59,;-.58,-6.98,;-.39,-5.42,;1.03,-4.81,;2.27,-5.74,;3.67,-5.12,;4.91,-6.03,;4.76,-7.54,;3.36,-8.17,;2.08,-7.28,;.68,-7.89,;6.32,-5.4,;6.49,-3.86,;7.57,-6.3,;8.98,-5.68,;10.24,-6.32,;11.58,-5.56,;12.9,-6.33,;12.89,-7.87,;13.98,-6.77,;14.22,-8.64,;15.56,-7.87,;16.89,-8.64,;18.22,-7.87,;18.22,-6.33,;16.88,-5.56,;15.55,-6.33,;11.56,-8.64,;10.23,-7.86,)| Show InChI InChI=1S/C27H32NO2/c1-28(20-22-6-4-3-5-7-22)16-14-21(15-17-28)8-13-27(29)25-10-9-24-19-26(30-2)12-11-23(24)18-25/h3-7,9-12,18-19,21H,8,13-17,20H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117602

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COCC[N+]1(C)CCC(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)CC1 |(15.88,3.41,;15.42,1.94,;13.92,1.58,;13.48,.11,;11.98,-.24,;10.79,.74,;10.44,-.4,;9.8,-1.81,;10.71,-3.05,;9.95,-4.38,;8.4,-4.38,;7.63,-5.73,;8.4,-7.06,;6.09,-5.73,;5.18,-4.47,;3.69,-4.96,;2.36,-4.19,;1.04,-4.96,;-.3,-4.19,;-1.63,-4.96,;1.04,-6.5,;-.3,-7.27,;-1.63,-6.5,;2.37,-7.27,;3.69,-6.51,;5.18,-6.99,;12.24,-2.9,;12.87,-1.5,)| Show InChI InChI=1S/C22H32NO4S/c1-23(11-12-25-2)9-7-16(8-10-23)5-6-18(24)22-14-17-13-19(26-3)20(27-4)15-21(17)28-22/h13-16H,5-12H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117597
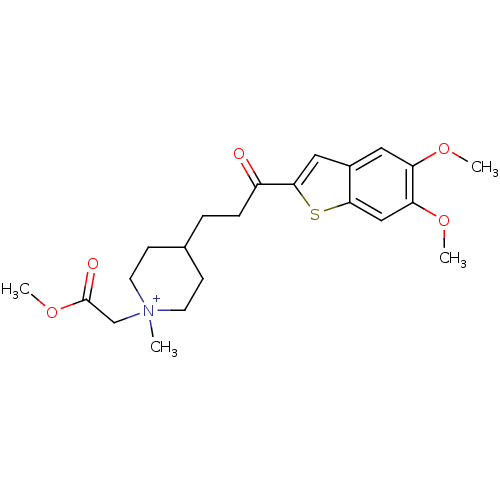
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COC(=O)C[N+]1(C)CCC(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)CC1 |(15.88,3.41,;15.42,1.94,;13.92,1.58,;12.87,2.71,;13.48,.11,;11.98,-.24,;11,.95,;12.87,-1.5,;12.24,-2.9,;10.71,-3.05,;9.95,-4.38,;8.4,-4.38,;7.63,-5.73,;8.4,-7.06,;6.09,-5.73,;5.18,-4.47,;3.69,-4.96,;2.36,-4.19,;1.04,-4.96,;-.3,-4.19,;-1.63,-4.96,;1.04,-6.5,;-.3,-7.27,;-1.63,-6.5,;2.37,-7.27,;3.69,-6.51,;5.18,-6.99,;9.8,-1.81,;10.44,-.4,)| Show InChI InChI=1S/C22H30NO5S/c1-23(14-22(25)28-4)9-7-15(8-10-23)5-6-17(24)21-12-16-11-18(26-2)19(27-3)13-20(16)29-21/h11-13,15H,5-10,14H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117588

(1-Benzyl-4-[3-(2,3-dihydro-benzofuran-5-yl)-3-oxo-...)Show SMILES C[N@+]1(Cc2ccccc2)CC[C@H](CCC(=O)c2ccc3OCCc3c2)CC1 |wU:11.12,1.0,wD:1.1,(9.94,-2.22,;9.95,-.68,;11.28,-1.45,;12.62,-.68,;13.95,-1.45,;15.28,-.68,;15.28,.86,;13.94,1.63,;12.61,.86,;9.95,.86,;8.64,1.63,;7.3,.88,;6.04,1.52,;4.63,.9,;3.38,1.8,;3.55,3.34,;1.97,1.17,;1.82,-.35,;.42,-.98,;-.86,-.09,;-2.37,-.4,;-3.14,.96,;-2.09,2.12,;-.67,1.46,;.73,2.08,;7.29,-.66,;8.62,-1.45,)| Show InChI InChI=1S/C24H30NO2/c1-25(18-20-5-3-2-4-6-20)14-11-19(12-15-25)7-9-23(26)21-8-10-24-22(17-21)13-16-27-24/h2-6,8,10,17,19H,7,9,11-16,18H2,1H3/q+1/t19-,25- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117611
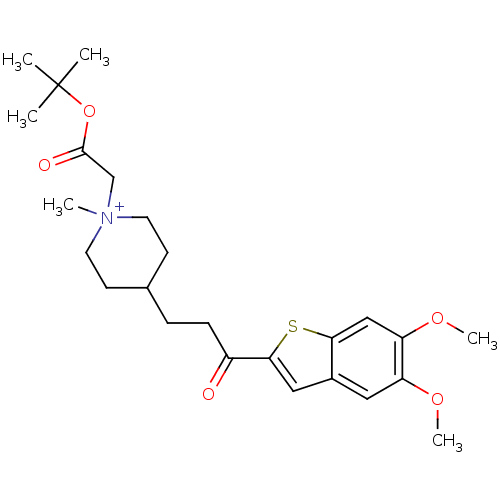
(1-tert-Butoxycarbonylmethyl-4-[3-(5,6-dimethoxy-be...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(CC(=O)OC(C)(C)C)CC1 |(-4.12,-6.7,;-2.8,-5.93,;-1.47,-6.7,;-.16,-5.93,;1.17,-6.7,;2.66,-6.21,;3.57,-7.47,;2.66,-8.73,;1.17,-8.24,;-.14,-9.01,;-1.47,-8.24,;-2.8,-9.01,;-4.12,-8.24,;5.11,-7.47,;5.88,-8.8,;5.88,-6.14,;7.42,-6.14,;8.19,-4.81,;7.28,-3.55,;7.92,-2.15,;9.45,-2.01,;8.47,-.82,;10.95,-1.64,;11.39,-.17,;10.34,.95,;12.89,.18,;13.33,1.65,;11.84,2.05,;13.73,3.14,;14.82,1.25,;10.34,-3.25,;9.71,-4.65,)| Show InChI InChI=1S/C25H36NO5S/c1-25(2,3)31-24(28)16-26(4)11-9-17(10-12-26)7-8-19(27)23-14-18-13-20(29-5)21(30-6)15-22(18)32-23/h13-15,17H,7-12,16H2,1-6H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117608

(1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC1(O)CC[N+](C)(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H30NO4S.BrH/c1-26(17-18-7-5-4-6-8-18)11-9-25(28,10-12-26)16-20(27)24-14-19-13-21(29-2)22(30-3)15-23(19)31-24;/h4-8,13-15,28H,9-12,16-17H2,1-3H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117578
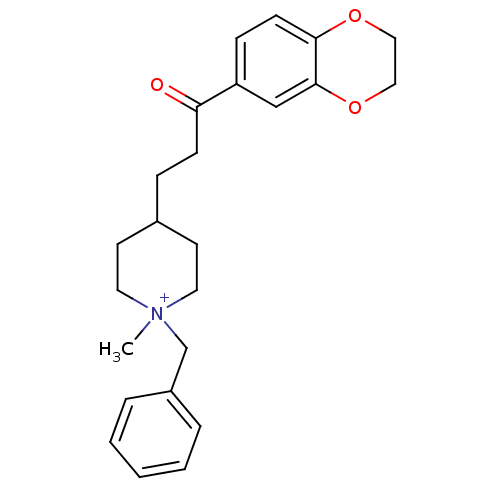
(1-Benzyl-4-[3-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-...)Show SMILES C[N+]1(Cc2ccccc2)CCC(CCC(=O)c2ccc3OCCOc3c2)CC1 |(20.14,-5.28,;19.05,-6.38,;20.38,-7.15,;21.72,-6.38,;23.05,-7.15,;24.38,-6.38,;24.38,-4.84,;23.04,-4.07,;21.71,-4.84,;19.05,-4.84,;17.74,-4.07,;16.4,-4.83,;15.14,-4.18,;13.73,-4.81,;12.48,-3.9,;12.65,-2.36,;11.07,-4.53,;10.92,-6.05,;9.52,-6.68,;8.26,-5.79,;6.84,-6.42,;5.58,-5.49,;5.75,-3.94,;7.19,-3.32,;8.43,-4.25,;9.83,-3.62,;16.39,-6.37,;17.72,-7.15,)| Show InChI InChI=1S/C24H30NO3/c1-25(18-20-5-3-2-4-6-20)13-11-19(12-14-25)7-9-22(26)21-8-10-23-24(17-21)28-16-15-27-23/h2-6,8,10,17,19H,7,9,11-16,18H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117596

(1-Cyclopropylmethyl-4-[3-(5,6-dimethoxy-benzo[b]th...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](CC2CC2)cc1 Show InChI InChI=1S/C22H24NO3S/c1-25-19-11-17-12-22(27-21(17)13-20(19)26-2)18(24)6-5-15-7-9-23(10-8-15)14-16-3-4-16/h7-13,16H,3-6,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117606

(1-Benzyl-4-[5-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCCCC1CC[N+](C)(Cc2ccccc2)CC1 |(-.74,-2.58,;.58,-1.81,;1.92,-2.58,;3.23,-1.81,;4.56,-2.58,;6.05,-2.09,;6.96,-3.35,;6.05,-4.61,;4.56,-4.12,;3.25,-4.89,;1.92,-4.12,;.58,-4.89,;-.74,-4.12,;8.5,-3.35,;9.27,-4.68,;9.27,-2.01,;10.82,-2.01,;11.59,-.68,;13.13,-.68,;13.88,.67,;15.4,.9,;15.95,2.34,;14.98,3.54,;13.74,4.44,;15.54,4.98,;17.06,5.22,;17.6,6.65,;19.12,6.91,;20.1,5.7,;19.54,4.27,;18.02,4.02,;13.46,3.29,;12.9,1.85,)| Show InChI InChI=1S/C28H36NO3S/c1-29(20-22-10-5-4-6-11-22)15-13-21(14-16-29)9-7-8-12-24(30)28-18-23-17-25(31-2)26(32-3)19-27(23)33-28/h4-6,10-11,17-19,21H,7-9,12-16,20H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117585
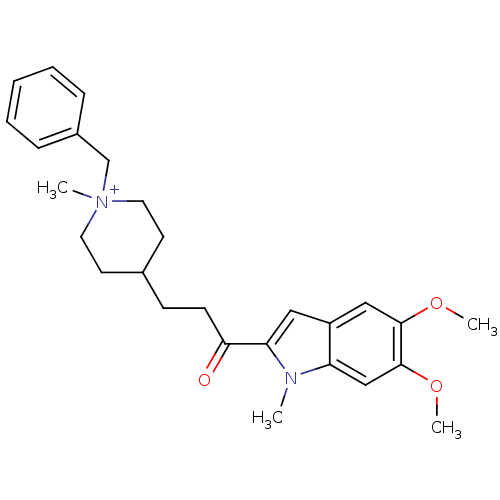
(1-Benzyl-4-[3-(5,6-dimethoxy-1-methyl-1H-indol-2-y...)Show SMILES COc1cc2cc(C(=O)CCC3CC[N+](C)(Cc4ccccc4)CC3)n(C)c2cc1OC |(-2.15,-5.79,;-1.52,-7.21,;.01,-7.37,;.92,-6.12,;2.45,-6.28,;3.58,-5.26,;4.91,-6.03,;6.32,-5.4,;6.49,-3.86,;7.57,-6.3,;8.98,-5.68,;10.24,-6.32,;11.58,-5.56,;12.9,-6.33,;12.89,-7.87,;13.98,-6.77,;14.22,-8.64,;15.56,-7.87,;16.89,-8.64,;18.22,-7.87,;18.22,-6.33,;16.88,-5.56,;15.55,-6.33,;11.56,-8.64,;10.23,-7.86,;4.6,-7.54,;5.63,-8.68,;3.06,-7.68,;2.17,-8.94,;.64,-8.78,;-.25,-10.02,;-1.79,-9.87,)| Show InChI InChI=1S/C27H35N2O3/c1-28-23-18-27(32-4)26(31-3)17-22(23)16-24(28)25(30)11-10-20-12-14-29(2,15-13-20)19-21-8-6-5-7-9-21/h5-9,16-18,20H,10-15,19H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117615
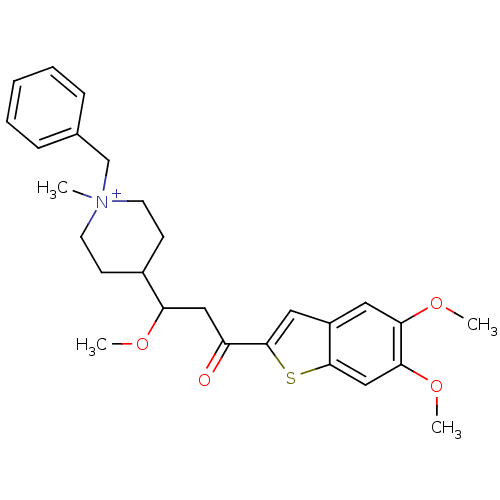
(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COC(CC(=O)c1cc2cc(OC)c(OC)cc2s1)C1CC[N+](C)(Cc2ccccc2)CC1 |(11.32,-7.89,;12.1,-6.55,;11.32,-5.22,;9.78,-5.22,;9.01,-6.55,;9.78,-7.89,;7.47,-6.55,;6.55,-5.29,;5.07,-5.78,;3.74,-5.01,;2.42,-5.78,;1.08,-5.01,;-.23,-5.78,;2.42,-7.33,;1.08,-8.1,;-.23,-7.33,;3.75,-8.1,;5.07,-7.33,;6.55,-7.82,;12.1,-3.89,;13.63,-3.98,;14.5,-2.72,;13.82,-1.32,;12.81,-.17,;14.68,-.05,;16.23,-.15,;17.07,1.11,;18.61,1.02,;19.29,-.38,;18.42,-1.64,;16.89,-1.55,;12.28,-1.22,;11.41,-2.51,)| Show InChI InChI=1S/C27H34NO4S/c1-28(18-19-8-6-5-7-9-19)12-10-20(11-13-28)23(30-2)16-22(29)27-15-21-14-24(31-3)25(32-4)17-26(21)33-27/h5-9,14-15,17,20,23H,10-13,16,18H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117579
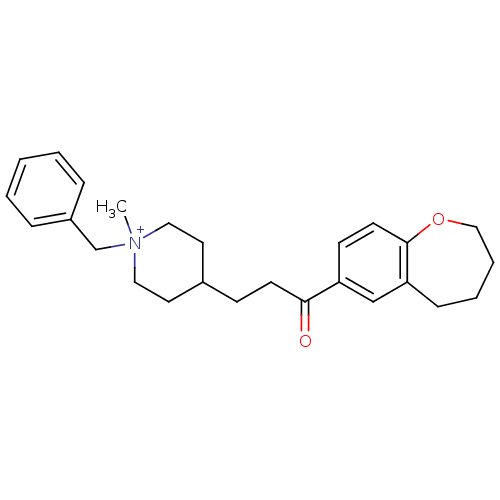
(1-Benzyl-1-methyl-4-[3-oxo-3-(2,3,4,5-tetrahydro-b...)Show SMILES C[N+]1(Cc2ccccc2)CCC(CCC(=O)c2ccc3OCCCCc3c2)CC1 |(13.98,-6.77,;12.89,-7.87,;14.22,-8.64,;15.56,-7.87,;15.55,-6.33,;16.88,-5.56,;18.22,-6.33,;18.22,-7.87,;16.89,-8.64,;11.56,-8.64,;10.23,-7.86,;10.24,-6.32,;8.98,-5.68,;7.57,-6.3,;6.32,-5.4,;6.49,-3.86,;4.91,-6.03,;4.76,-7.54,;3.36,-8.17,;2.08,-7.28,;.75,-8.13,;-.79,-7.68,;-1.38,-6.11,;-.53,-4.72,;1.08,-4.53,;2.27,-5.74,;3.67,-5.12,;11.58,-5.56,;12.9,-6.33,)| Show InChI InChI=1S/C26H34NO2/c1-27(20-22-7-3-2-4-8-22)16-14-21(15-17-27)10-12-25(28)23-11-13-26-24(19-23)9-5-6-18-29-26/h2-4,7-8,11,13,19,21H,5-6,9-10,12,14-18,20H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117599

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.47,-2.29,;15.47,.37,;16.99,.27,;17.86,1.54,;17.18,2.93,;16.18,4.08,;18.04,4.2,;19.58,4.1,;20.25,2.71,;21.78,2.61,;22.64,3.89,;21.96,5.27,;20.42,5.37,;15.64,3.03,;14.78,1.75,)| Show InChI InChI=1S/C26H32NO4S/c1-27(17-18-7-5-4-6-8-18)11-9-19(10-12-27)21(28)15-22(29)26-14-20-13-23(30-2)24(31-3)16-25(20)32-26/h4-8,13-14,16,19,21,28H,9-12,15,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117599

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.47,-2.29,;15.47,.37,;16.99,.27,;17.86,1.54,;17.18,2.93,;16.18,4.08,;18.04,4.2,;19.58,4.1,;20.25,2.71,;21.78,2.61,;22.64,3.89,;21.96,5.27,;20.42,5.37,;15.64,3.03,;14.78,1.75,)| Show InChI InChI=1S/C26H32NO4S/c1-27(17-18-7-5-4-6-8-18)11-9-19(10-12-27)21(28)15-22(29)26-14-20-13-23(30-2)24(31-3)16-25(20)32-26/h4-8,13-14,16,19,21,28H,9-12,15,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117591
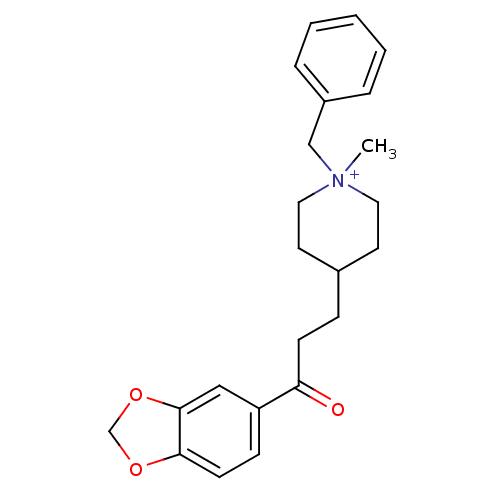
(4-(3-Benzo[1,3]dioxol-5-yl-3-oxo-propyl)-1-benzyl-...)Show SMILES C[N+]1(Cc2ccccc2)CCC(CCC(=O)c2ccc3OCOc3c2)CC1 |(20.14,-5.28,;19.05,-6.38,;20.38,-7.15,;21.72,-6.38,;21.71,-4.84,;23.04,-4.07,;24.38,-4.84,;24.38,-6.38,;23.05,-7.15,;17.72,-7.15,;16.39,-6.37,;16.4,-4.83,;15.14,-4.18,;13.73,-4.81,;12.48,-3.9,;12.65,-2.36,;11.07,-4.53,;10.92,-6.05,;9.52,-6.68,;8.26,-5.79,;6.73,-6.1,;5.97,-4.74,;7.01,-3.6,;8.43,-4.25,;9.83,-3.62,;17.74,-4.07,;19.05,-4.84,)| Show InChI InChI=1S/C23H28NO3/c1-24(16-19-5-3-2-4-6-19)13-11-18(12-14-24)7-9-21(25)20-8-10-22-23(15-20)27-17-26-22/h2-6,8,10,15,18H,7,9,11-14,16-17H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117605

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES CC[n+]1ccc(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)cc1 Show InChI InChI=1S/C20H22NO3S/c1-4-21-9-7-14(8-10-21)5-6-16(22)20-12-15-11-17(23-2)18(24-3)13-19(15)25-20/h7-13H,4-6H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50108395
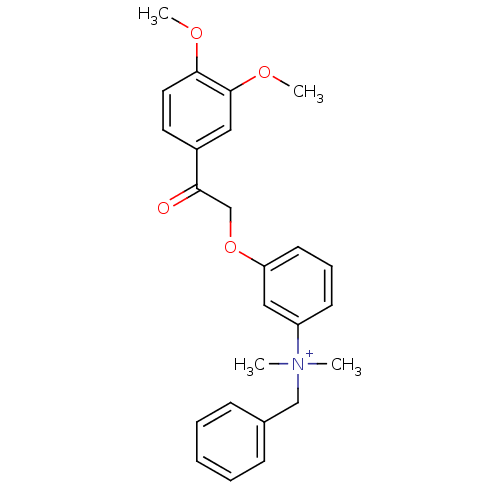
(Benzyl-{3-[2-(3,4-dimethoxy-phenyl)-2-oxo-ethoxy]-...)Show SMILES COc1ccc(cc1OC)C(=O)COc1cccc(c1)[N+](C)(C)Cc1ccccc1 Show InChI InChI=1S/C25H28NO4/c1-26(2,17-19-9-6-5-7-10-19)21-11-8-12-22(16-21)30-18-23(27)20-13-14-24(28-3)25(15-20)29-4/h5-16H,17-18H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant AChE |
Bioorg Med Chem Lett 12: 193-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CR5SP0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117600

(1-Benzyl-4-[5-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(-.74,-2.58,;.58,-1.81,;1.92,-2.58,;3.23,-1.81,;4.56,-2.58,;6.05,-2.09,;6.96,-3.35,;6.05,-4.61,;4.56,-4.12,;3.25,-4.89,;1.92,-4.12,;.58,-4.89,;-.74,-4.12,;8.5,-3.35,;9.27,-4.68,;9.27,-2.01,;10.82,-2.01,;11.59,-3.35,;11.59,-.68,;13.13,-.68,;13.88,.67,;15.4,.9,;15.95,2.34,;14.98,3.54,;13.74,4.44,;15.54,4.98,;17.06,5.22,;18.02,4.02,;19.54,4.27,;20.1,5.7,;19.12,6.91,;17.6,6.65,;13.46,3.29,;12.9,1.85,)| Show InChI InChI=1S/C28H36NO4S/c1-29(19-21-7-5-4-6-8-21)13-11-20(12-14-29)9-10-23(30)17-24(31)28-16-22-15-25(32-2)26(33-3)18-27(22)34-28/h4-8,15-16,18,20,23,30H,9-14,17,19H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117613
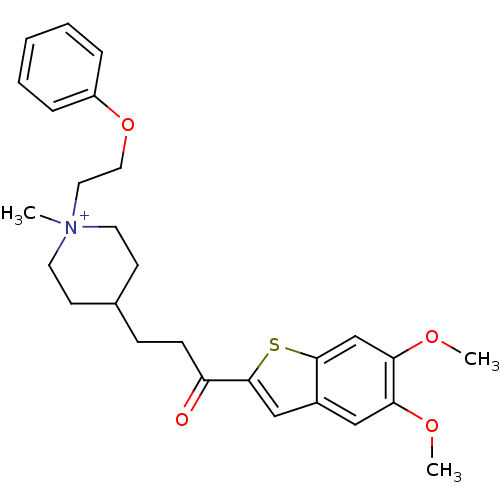
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(CCOc2ccccc2)CC1 |(-4.12,-6.71,;-2.8,-5.94,;-1.47,-6.71,;-.16,-5.94,;1.18,-6.71,;2.66,-6.22,;3.57,-7.48,;2.66,-8.74,;1.18,-8.25,;-.14,-9.02,;-1.47,-8.25,;-2.8,-9.02,;-4.12,-8.25,;5.11,-7.48,;5.88,-8.81,;5.88,-6.14,;7.42,-6.14,;8.19,-4.81,;7.28,-3.56,;7.92,-2.16,;9.45,-2.01,;8.47,-.82,;10.96,-1.64,;11.4,-.17,;12.89,.18,;13.34,1.65,;12.29,2.77,;12.72,4.24,;14.22,4.6,;15.29,3.48,;14.85,2,;10.35,-3.25,;9.72,-4.65,)| Show InChI InChI=1S/C27H34NO4S/c1-28(15-16-32-22-7-5-4-6-8-22)13-11-20(12-14-28)9-10-23(29)27-18-21-17-24(30-2)25(31-3)19-26(21)33-27/h4-8,17-20H,9-16H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50037157

(3-(1-Benzyl-piperidin-4-yl)-1-(2,3,4,5-tetrahydro-...)Show InChI InChI=1S/C25H32N2O/c28-25(23-11-10-22-8-4-5-15-26-24(22)18-23)12-9-20-13-16-27(17-14-20)19-21-6-2-1-3-7-21/h1-3,6-7,10-11,18,20,26H,4-5,8-9,12-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117580

(1-Benzyl-1-methyl-4-[3-oxo-3-(2,3,4,5-tetrahydro-1...)Show SMILES C[N+]1(Cc2ccccc2)CCC(CCC(=O)c2ccc3CCCCNc3c2)CC1 |(5.9,-6.04,;5.9,-4.5,;4.57,-5.27,;3.23,-4.5,;1.9,-5.27,;.57,-4.5,;.57,-2.95,;1.9,-2.18,;3.23,-2.95,;5.9,-2.95,;7.23,-2.18,;8.57,-2.95,;9.92,-2.18,;11.25,-2.95,;12.59,-2.18,;12.59,-.64,;13.92,-2.95,;15.25,-2.18,;16.59,-2.95,;16.59,-4.5,;18.04,-5.08,;18.48,-6.55,;17.61,-7.82,;16.09,-7.91,;15.02,-6.81,;15.25,-5.27,;13.92,-4.5,;8.57,-4.5,;7.23,-5.27,)| Show InChI InChI=1S/C26H35N2O/c1-28(20-22-7-3-2-4-8-22)17-14-21(15-18-28)10-13-26(29)24-12-11-23-9-5-6-16-27-25(23)19-24/h2-4,7-8,11-12,19,21,27H,5-6,9-10,13-18,20H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117616

(1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)C=C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.1,-2.43,;16.59,-2.82,;17.67,-1.73,;18.43,-.38,;19.16,-2.11,;19.58,-3.59,;21.05,-3.97,;21.47,-5.44,;20.4,-6.56,;18.91,-6.17,;18.49,-4.69,;17.25,-.24,;15.76,.14,)| Show InChI InChI=1S/C25H28NO3S/c1-26(17-19-7-5-4-6-8-19)11-9-18(10-12-26)13-21(27)25-15-20-14-22(28-2)23(29-3)16-24(20)30-25/h4-8,13-16H,9-12,17H2,1-3H3/q+1/b18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117603
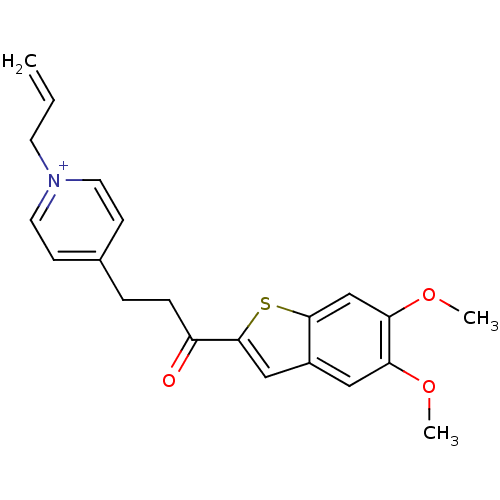
(1-Allyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl)...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](CC=C)cc1 Show InChI InChI=1S/C21H22NO3S/c1-4-9-22-10-7-15(8-11-22)5-6-17(23)21-13-16-12-18(24-2)19(25-3)14-20(16)26-21/h4,7-8,10-14H,1,5-6,9H2,2-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data