

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365773 (CHEMBL1956552) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Non-competitive inhibition of Electrophorus electricus AChE assessed as hydrolysis of acetylthiocholineiodide after 15 mins incubation by spectrophot... | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM22826 (2,3-dioxo-2,3-dihydro-1H-indole-7-carboxylic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of human recombinant DNA ligase 1 using nicked DNA substrate by kinetic assay | J Med Chem 51: 4553-62 (2008) Article DOI: 10.1021/jm8001668 BindingDB Entry DOI: 10.7270/Q2639PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinate-nucleotide adenylyltransferase (Escherichia coli) | BDBM50318652 ((E)-4-(2-(anthracen-9-ylmethylene)hydrazinyl)-N-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The enzymatic assay was masured at fixed NaMN and ATP concentration (equal to two-fold km values)with various concentration of inhibitory compounds. | Chem Biol 16: 849-61 (2009) Article DOI: 10.1016/j.chembiol.2009.07.006 BindingDB Entry DOI: 10.7270/Q2N8787C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinate-nucleotide adenylyltransferase (Escherichia coli) | BDBM50318652 ((E)-4-(2-(anthracen-9-ylmethylene)hydrazinyl)-N-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 8.00E+3 | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The enzymatic assay was masured at fixed NaMN and ATP concentration (equal to two-fold km values)with various concentration of inhibitory compounds. | Chem Biol 16: 849-61 (2009) Article DOI: 10.1016/j.chembiol.2009.07.006 BindingDB Entry DOI: 10.7270/Q2N8787C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable nicotinate-nucleotide adenylyltransferase (Bacillus anthracis) | BDBM50318652 ((E)-4-(2-(anthracen-9-ylmethylene)hydrazinyl)-N-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 9.00E+3 | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The enzymatic assay was masured at fixed NaMN and ATP concentration (equal to two-fold km values)with various concentration of inhibitory compounds. | Chem Biol 16: 849-61 (2009) Article DOI: 10.1016/j.chembiol.2009.07.006 BindingDB Entry DOI: 10.7270/Q2N8787C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Probable nicotinate-nucleotide adenylyltransferase (Bacillus anthracis) | BDBM50318652 ((E)-4-(2-(anthracen-9-ylmethylene)hydrazinyl)-N-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The enzymatic assay was masured at fixed NaMN and ATP concentration (equal to two-fold km values)with various concentration of inhibitory compounds. | Chem Biol 16: 849-61 (2009) Article DOI: 10.1016/j.chembiol.2009.07.006 BindingDB Entry DOI: 10.7270/Q2N8787C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Probable nicotinate-nucleotide adenylyltransferase (Bacillus anthracis) | BDBM50318653 (3-amino-N-(3-fluorophenyl)-6-(thiophen-2-yl)thieno...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.80E+4 | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The enzymatic assay was masured at fixed NaMN and ATP concentration (equal to two-fold km values)with various concentration of inhibitory compounds. | Chem Biol 16: 849-61 (2009) Article DOI: 10.1016/j.chembiol.2009.07.006 BindingDB Entry DOI: 10.7270/Q2N8787C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinate-nucleotide adenylyltransferase (Escherichia coli) | BDBM50318653 (3-amino-N-(3-fluorophenyl)-6-(thiophen-2-yl)thieno...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The enzymatic assay was masured at fixed NaMN and ATP concentration (equal to two-fold km values)with various concentration of inhibitory compounds. | Chem Biol 16: 849-61 (2009) Article DOI: 10.1016/j.chembiol.2009.07.006 BindingDB Entry DOI: 10.7270/Q2N8787C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinate-nucleotide adenylyltransferase (Escherichia coli) | BDBM50318653 (3-amino-N-(3-fluorophenyl)-6-(thiophen-2-yl)thieno...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.50E+4 | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The enzymatic assay was masured at fixed NaMN and ATP concentration (equal to two-fold km values)with various concentration of inhibitory compounds. | Chem Biol 16: 849-61 (2009) Article DOI: 10.1016/j.chembiol.2009.07.006 BindingDB Entry DOI: 10.7270/Q2N8787C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable nicotinate-nucleotide adenylyltransferase (Bacillus anthracis) | BDBM50318653 (3-amino-N-(3-fluorophenyl)-6-(thiophen-2-yl)thieno...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description The enzymatic assay was masured at fixed NaMN and ATP concentration (equal to two-fold km values)with various concentration of inhibitory compounds. | Chem Biol 16: 849-61 (2009) Article DOI: 10.1016/j.chembiol.2009.07.006 BindingDB Entry DOI: 10.7270/Q2N8787C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353684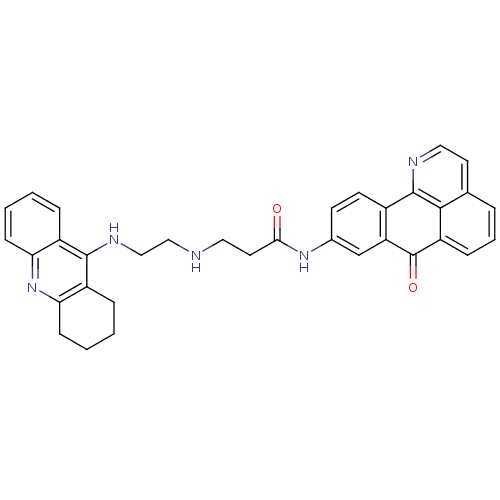 (CHEMBL1830627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573585 (CHEMBL4846412) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573586 (CHEMBL4867169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573584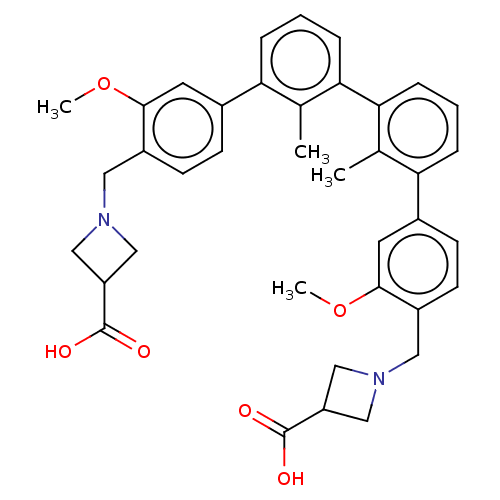 (CHEMBL4845782) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573550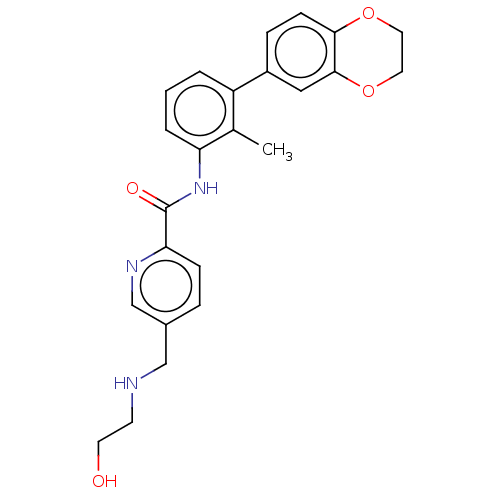 (CHEMBL4869046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM120996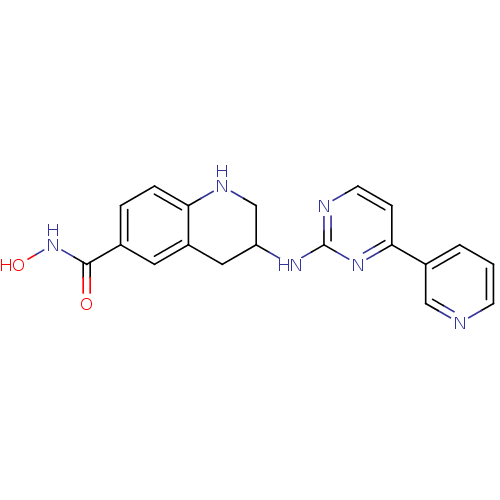 (US8716285, 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573570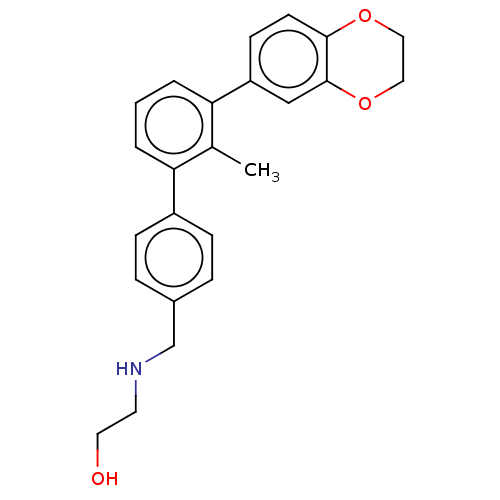 (CHEMBL4862483) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50078748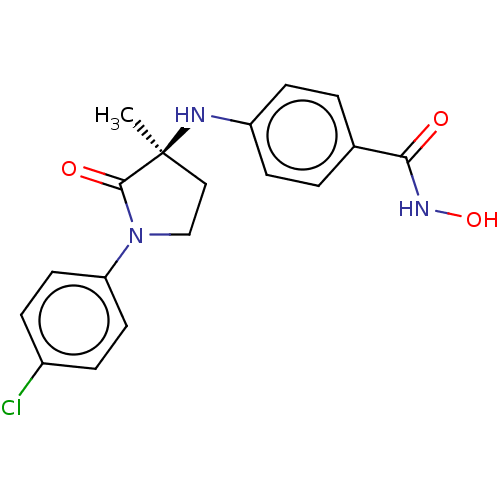 (CHEMBL3415629) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50078747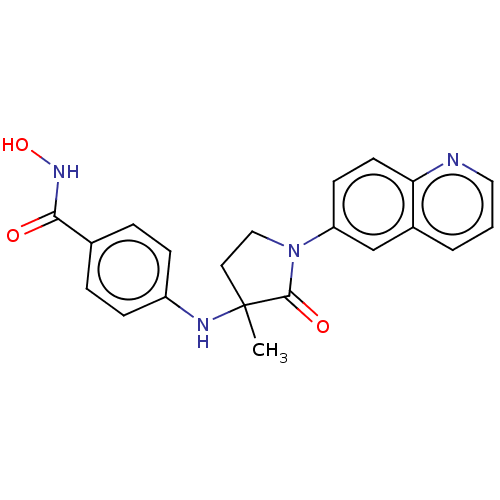 (CHEMBL3415628) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573573 (CHEMBL4861661) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573569 (CHEMBL4862708) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50078695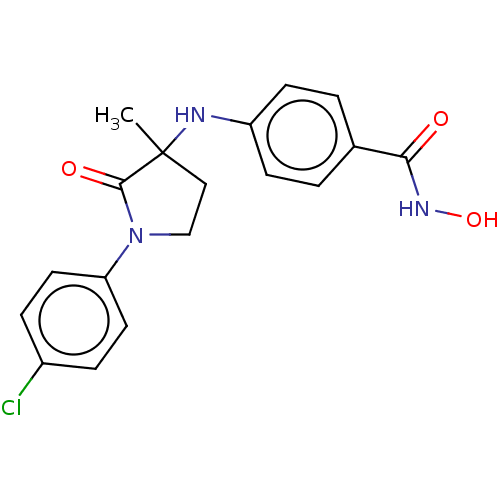 (CHEMBL3415627) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353680 (CHEMBL1830632) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573579 (CHEMBL4856653) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573580 (CHEMBL4866385) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353685 (CHEMBL1830626) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573574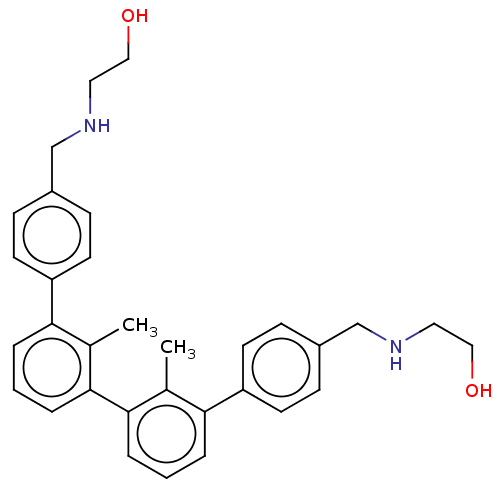 (CHEMBL4858813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50027662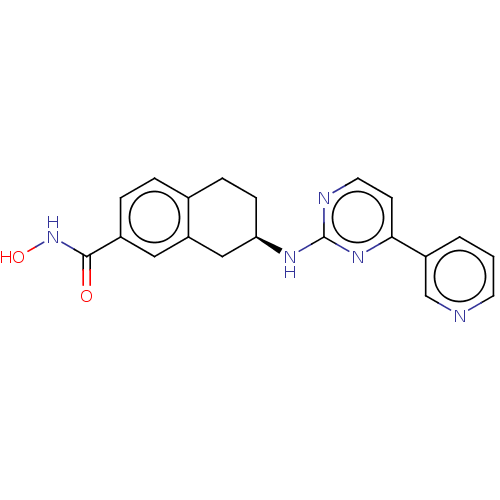 (CHEMBL3338418) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573576 (CHEMBL4857423) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50573577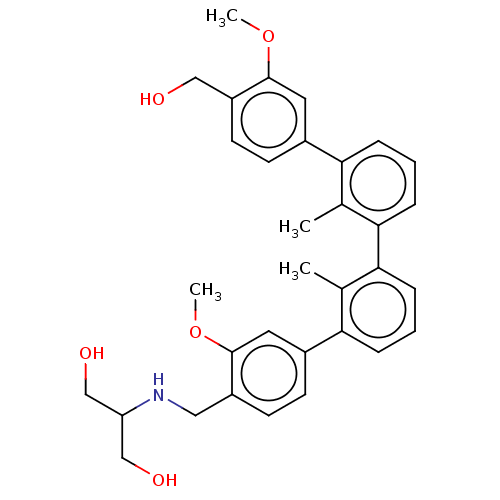 (CHEMBL4873371) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag2-PD1/Tag1-PD-L1 (unknown origin) protein-protein interaction preincubated for 15 mins followed by addition of anti-Tag1-Eu3+ and an... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113637 BindingDB Entry DOI: 10.7270/Q2P55S90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353681 (CHEMBL1830631) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353683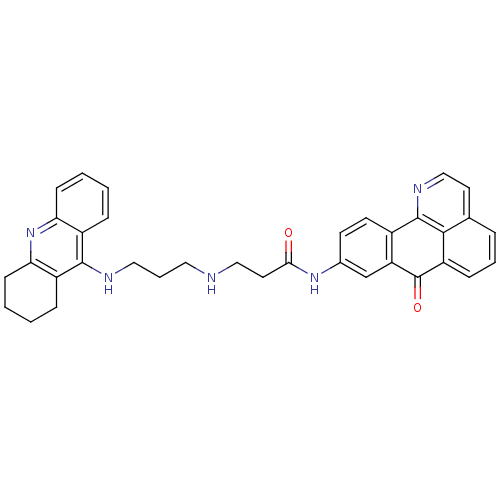 (CHEMBL1830628) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353689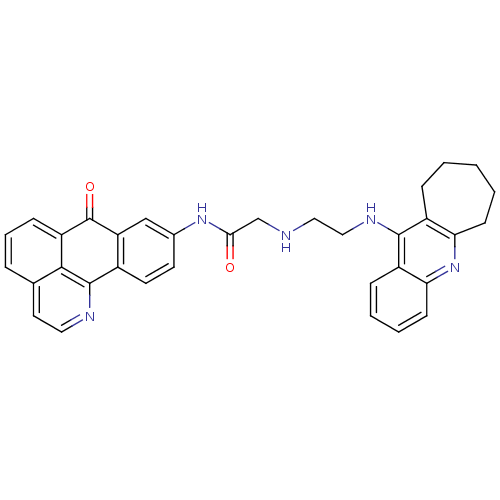 (CHEMBL1830630) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50078687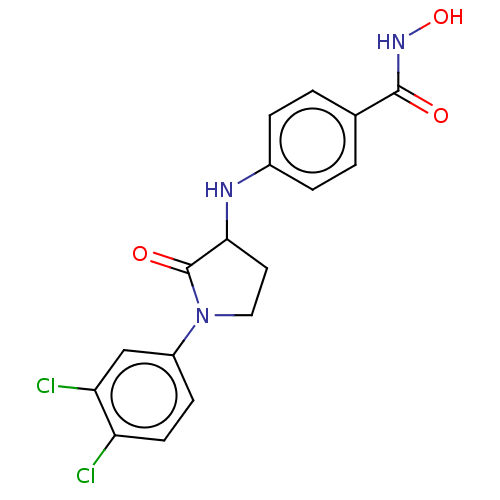 (CHEMBL3415619) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor Jun (Homo sapiens (Human)) | BDBM50213931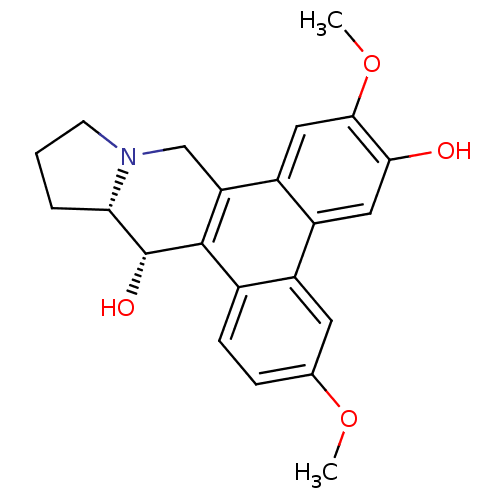 (CHEMBL250854 | tylophorinidine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of AP1-mediated gene transcription in HepG2 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 17: 4338-42 (2007) Article DOI: 10.1016/j.bmcl.2007.05.021 BindingDB Entry DOI: 10.7270/Q2HD7VBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353681 (CHEMBL1830631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor Jun (Homo sapiens (Human)) | BDBM50213932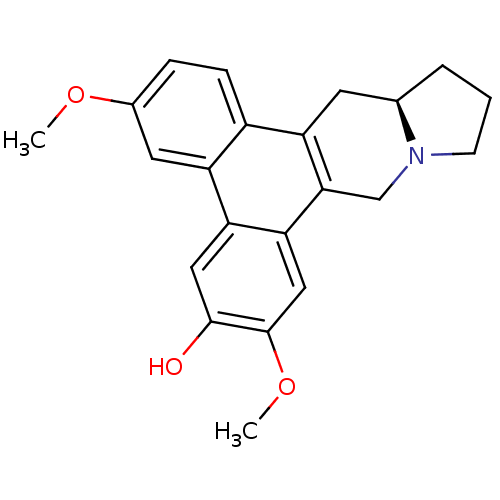 (CHEMBL250474 | [(R)-(+)-deoxytylophorinidine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of AP1-mediated gene transcription in HepG2 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 17: 4338-42 (2007) Article DOI: 10.1016/j.bmcl.2007.05.021 BindingDB Entry DOI: 10.7270/Q2HD7VBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50078747 (CHEMBL3415628) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50027662 (CHEMBL3338418) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50078680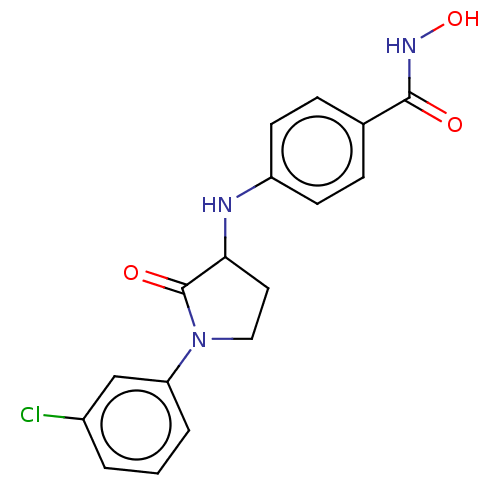 (CHEMBL3415454) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50078690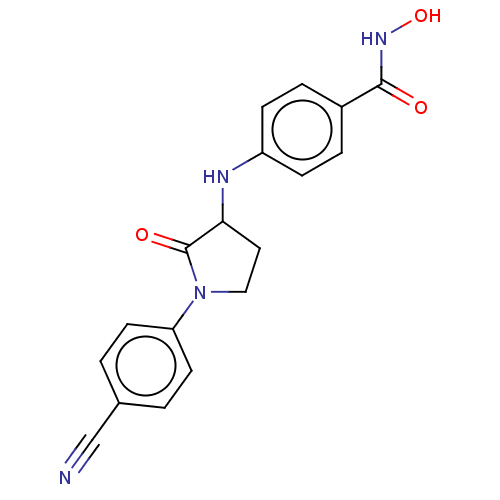 (CHEMBL3415621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353684 (CHEMBL1830627) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor Jun (Homo sapiens (Human)) | BDBM50213934 (CHEMBL398325 | tylophorinine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of AP1-mediated gene transcription in HepG2 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 17: 4338-42 (2007) Article DOI: 10.1016/j.bmcl.2007.05.021 BindingDB Entry DOI: 10.7270/Q2HD7VBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50078682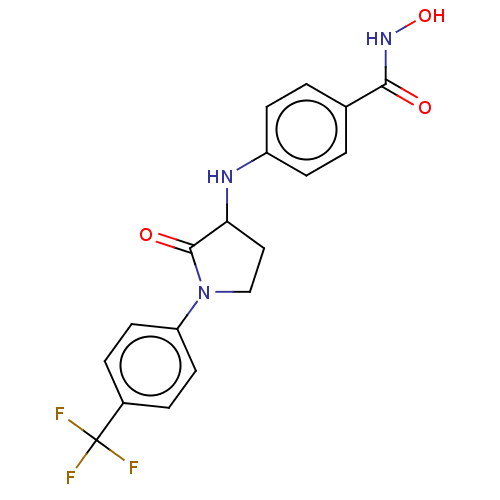 (CHEMBL3415618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50078757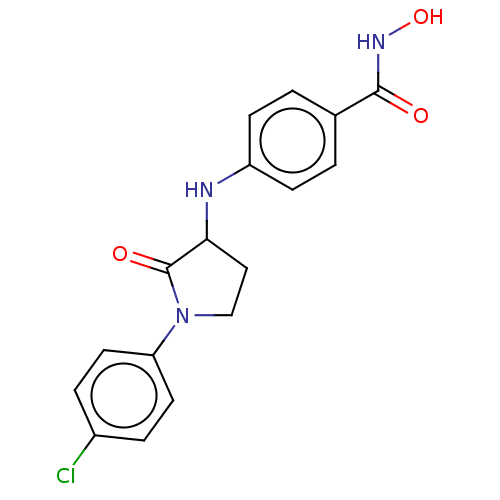 (CHEMBL3415453) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50078689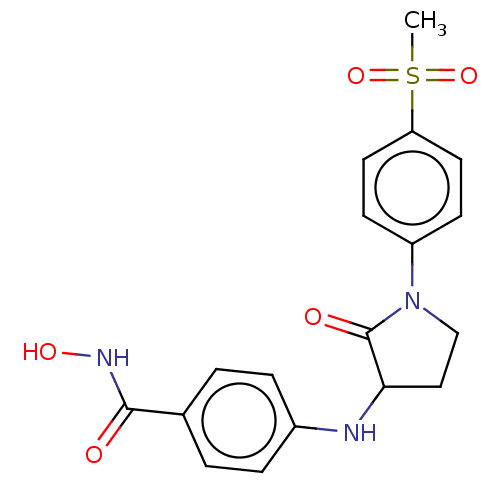 (CHEMBL3415620) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate | J Med Chem 58: 2809-20 (2015) Article DOI: 10.1021/jm502011f BindingDB Entry DOI: 10.7270/Q2B56MF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 306 total ) | Next | Last >> |