

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576898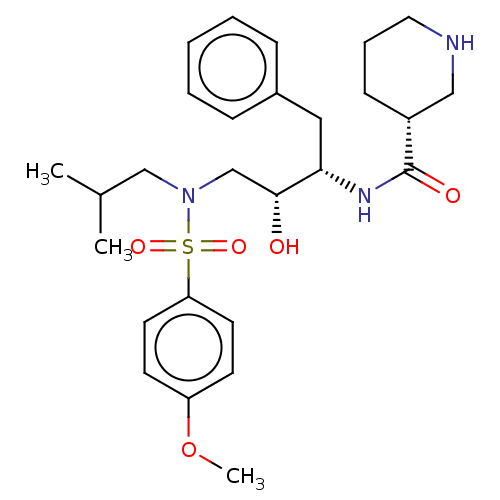 (CHEMBL4877646) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576910 (CHEMBL4862758) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50463772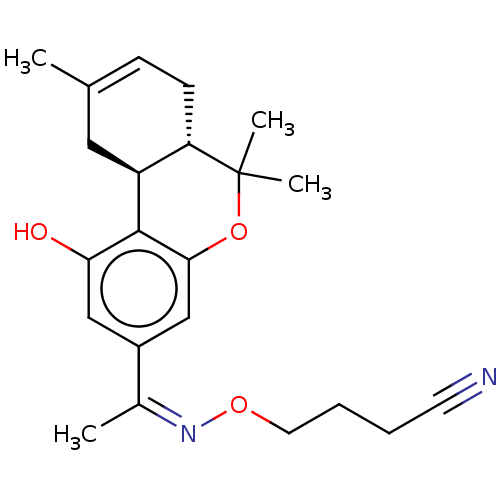 (CHEMBL4239190) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant mouse Cb2 receptor expressed in HEK293 cells | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576913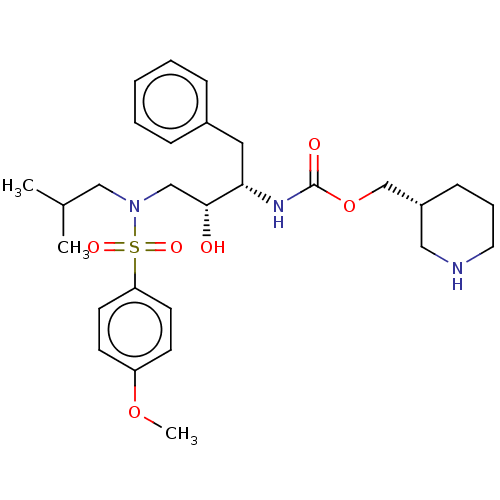 (CHEMBL4868812) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576900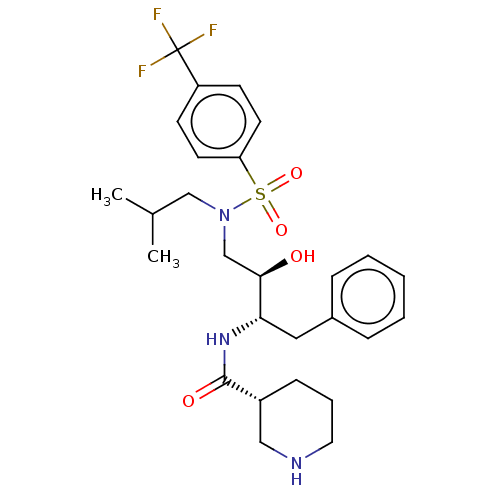 (CHEMBL4852688) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50604026 (CHEMBL5192384) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01670 BindingDB Entry DOI: 10.7270/Q2VH5SX2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50604023 (CHEMBL5192977) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01670 BindingDB Entry DOI: 10.7270/Q2VH5SX2 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50355491 (CHEMBL1835870) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of human recombinant FLT3 by radiometric assay | J Med Chem 55: 725-34 (2012) Article DOI: 10.1021/jm201198w BindingDB Entry DOI: 10.7270/Q2GQ6Z6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576912 (CHEMBL4861507) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576899 (CHEMBL4852584) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576914 (CHEMBL4866330) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8143 (N-[3-bromo-5-(trifluoromethyl)phenyl]-4-{pyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50463772 (CHEMBL4239190) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from rat brain Cb1 receptor | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50463775 (CHEMBL4240671) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from rat brain Cb1 receptor | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576916 (CHEMBL4875282) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576911 (CHEMBL4863180) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50020310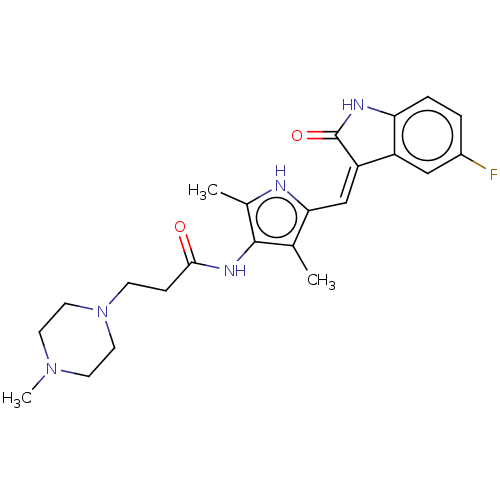 (CHEMBL3288854) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Binding affinity to VEGFR3 (unknown origin) | Eur J Med Chem 82: 139-51 (2014) Article DOI: 10.1016/j.ejmech.2014.05.051 BindingDB Entry DOI: 10.7270/Q2F1918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50020310 (CHEMBL3288854) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Binding affinity to FLT3 (unknown origin) | Eur J Med Chem 82: 139-51 (2014) Article DOI: 10.1016/j.ejmech.2014.05.051 BindingDB Entry DOI: 10.7270/Q2F1918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576904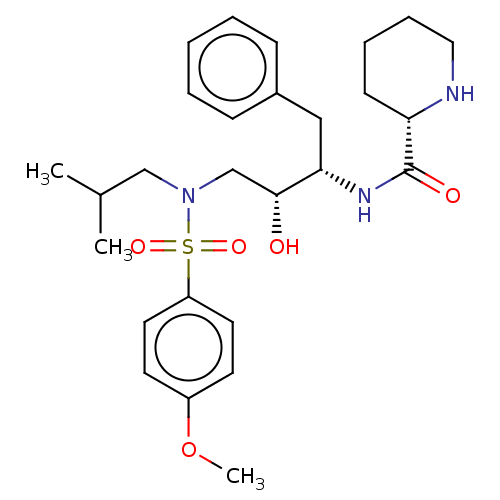 (CHEMBL4860352) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50463772 (CHEMBL4239190) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human Cb2 receptor expressed in HEK293 cells | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50463775 (CHEMBL4240671) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant mouse Cb2 receptor expressed in HEK293 cells | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50463785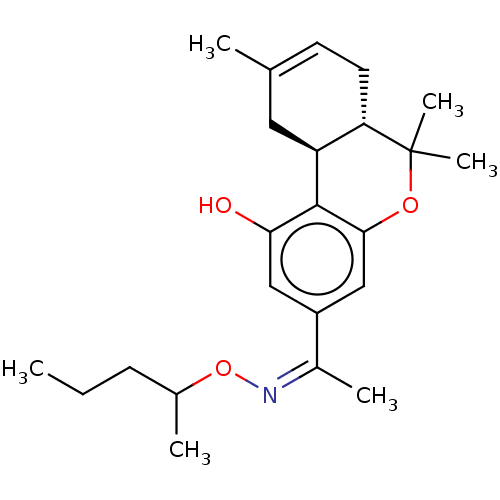 (CHEMBL4238778) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from rat brain Cb1 receptor | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576907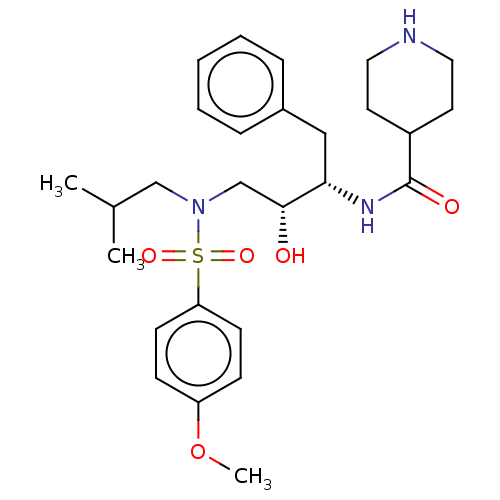 (CHEMBL4877811) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50557516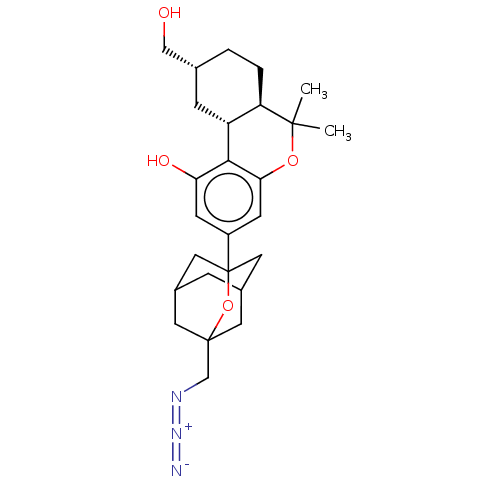 (CHEMBL4787347) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127882 BindingDB Entry DOI: 10.7270/Q2NP283T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50463776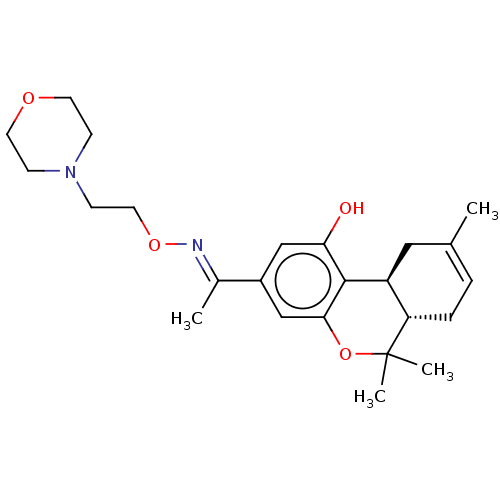 (CHEMBL4246445) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human Cb2 receptor expressed in HEK293 cells | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50287936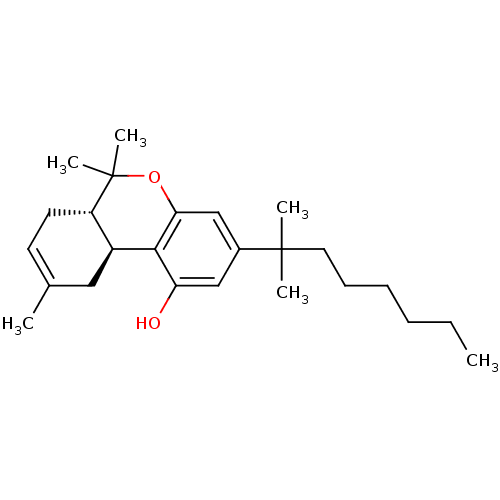 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-6,6,9-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from rat brain Cb1 receptor | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388994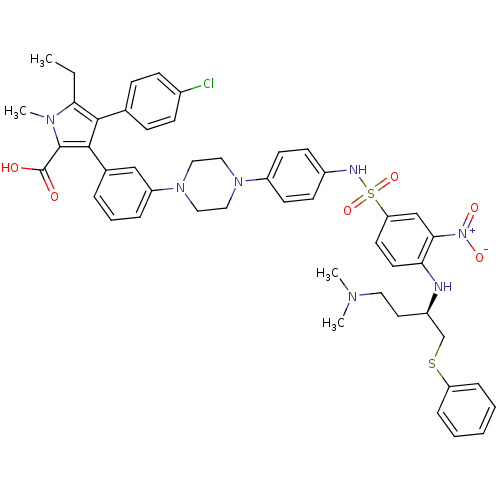 (CHEMBL2063897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50397455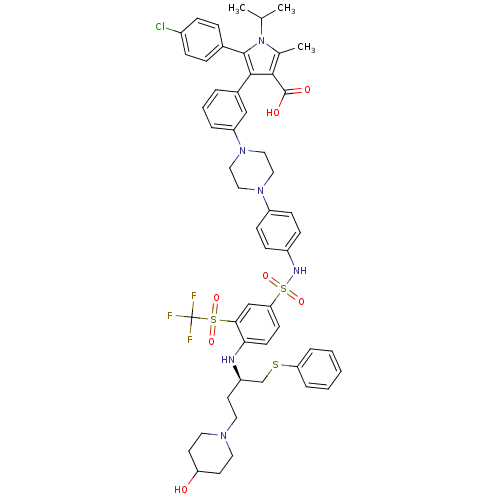 (CHEMBL2170838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminal 6xHis-tagged human Bcl-2 expressed in Escherichia coli BL21 (DE3) after 2 hrs by fluorescence polarization assay | J Med Chem 55: 8502-14 (2012) Article DOI: 10.1021/jm3010306 BindingDB Entry DOI: 10.7270/Q2V69KQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50463786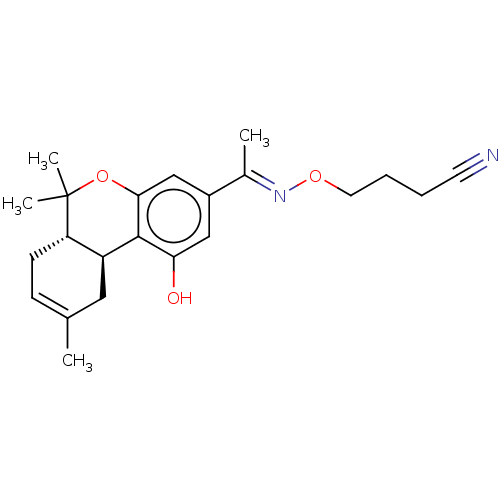 (CHEMBL4249273) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from rat brain Cb1 receptor | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8145 (N-(3,5-dichlorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8138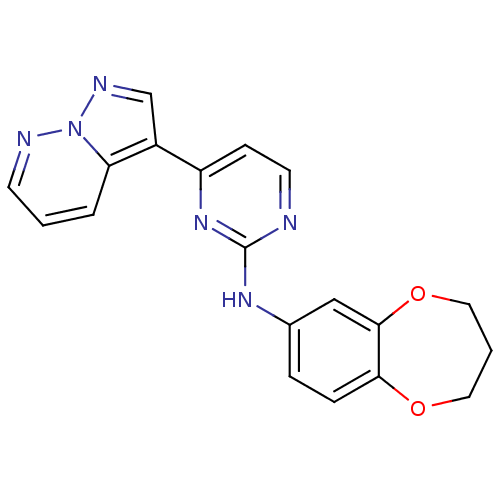 (N-(3,4-dihydro-2H-1,5-benzodioxepin-7-yl)-4-{pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of FAM-Bid binding to human BCL2 expressed in Escherichia coli BL21 after 30 mins by fluorescence polarization assay | ACS Med Chem Lett 7: 1185-1190 (2016) Article DOI: 10.1021/acsmedchemlett.6b00302 BindingDB Entry DOI: 10.7270/Q25T3NGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50551956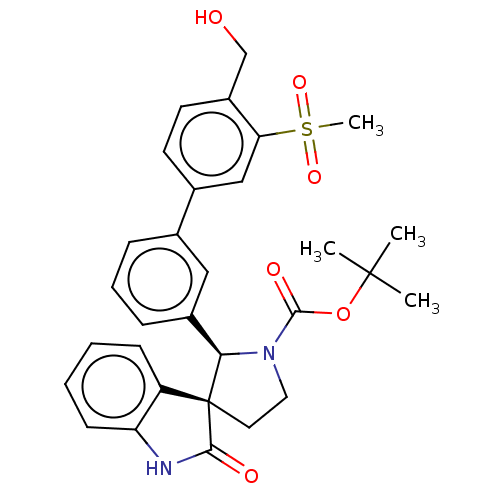 (CHEMBL4756418) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of hyodeoxycholicacid-based fluorescent tracer from recombinant human LXRbeta LBD (215 to 461 residues) expressed in Escherichia coli BL... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112240 BindingDB Entry DOI: 10.7270/Q2MG7T4D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576917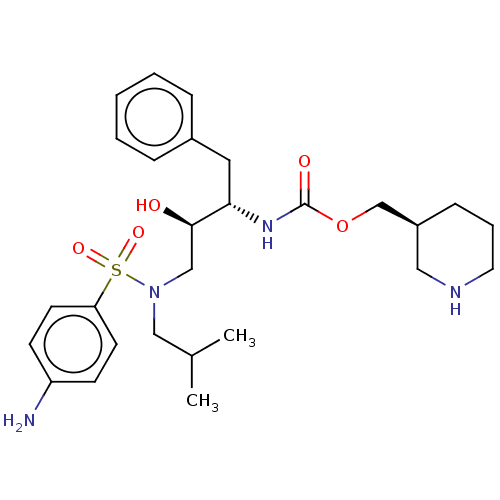 (CHEMBL4868304) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50393836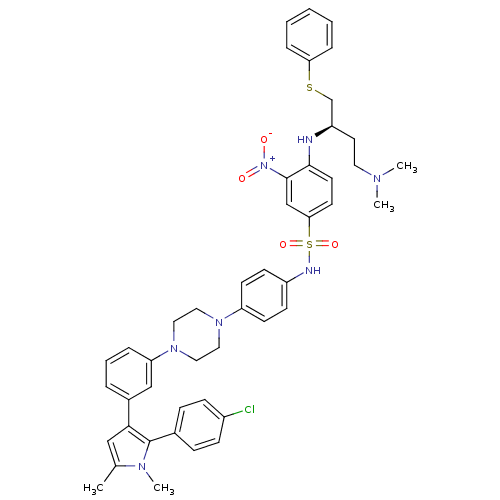 (CHEMBL2159738) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human N-terminal His8-tagged Bcl-XL expressed in Escherichia coli BL21(DE3) assessed as inhibition of Fluorescein-labeled BAK binding a... | J Med Chem 55: 6149-61 (2012) Article DOI: 10.1021/jm300608w BindingDB Entry DOI: 10.7270/Q2J10485 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50463777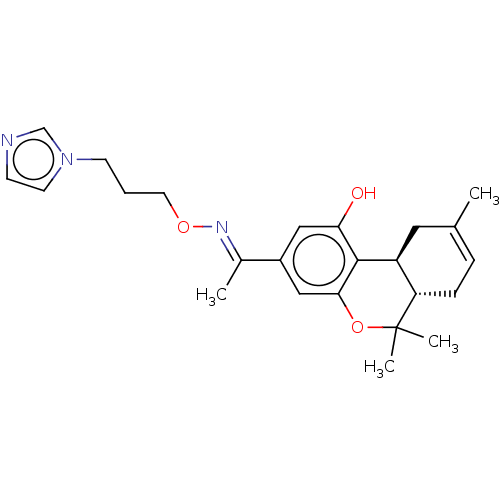 (CHEMBL4238224) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from rat brain Cb1 receptor | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388990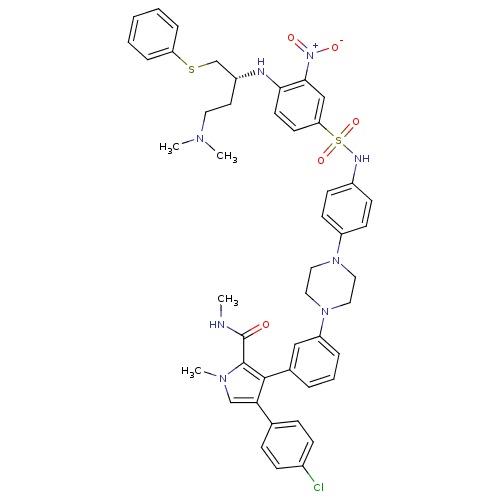 (CHEMBL2063893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50397456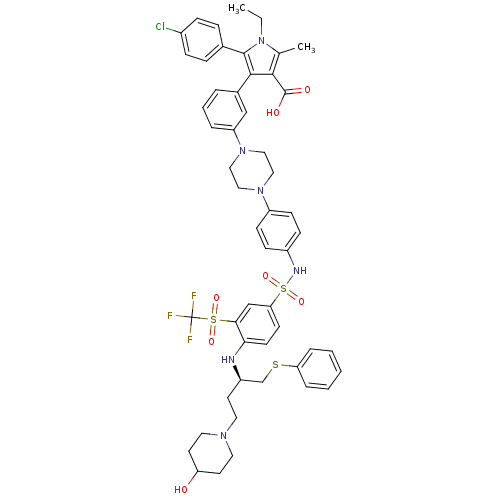 (CHEMBL2170837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminal 6xHis-tagged human Bcl-2 expressed in Escherichia coli BL21 (DE3) after 2 hrs by fluorescence polarization assay | J Med Chem 55: 8502-14 (2012) Article DOI: 10.1021/jm3010306 BindingDB Entry DOI: 10.7270/Q2V69KQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50463786 (CHEMBL4249273) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human Cb2 receptor expressed in HEK293 cells | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Binding affinity to recombinant human LXRbeta-LBD expressed in Escherichia coli BL21 (DE3) assessed as inhibitory constant incubated for 30 mins by f... | Eur J Med Chem 178: 458-467 (2019) Article DOI: 10.1016/j.ejmech.2019.06.011 BindingDB Entry DOI: 10.7270/Q2BC42WH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50459322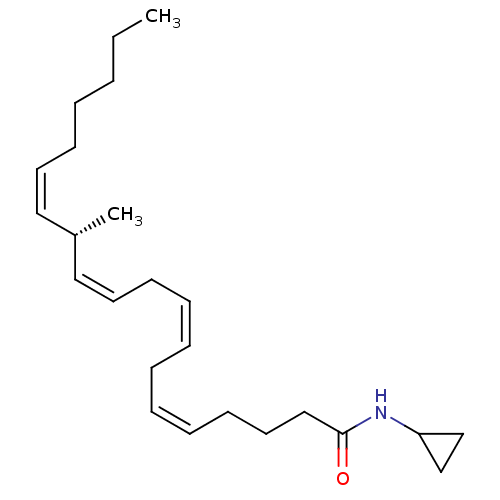 (CHEMBL4215369) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes in presence of FAAH inhibitor PMSF by radioligand binding assay | J Med Chem 61: 8639-8657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00611 BindingDB Entry DOI: 10.7270/Q2HQ42HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388986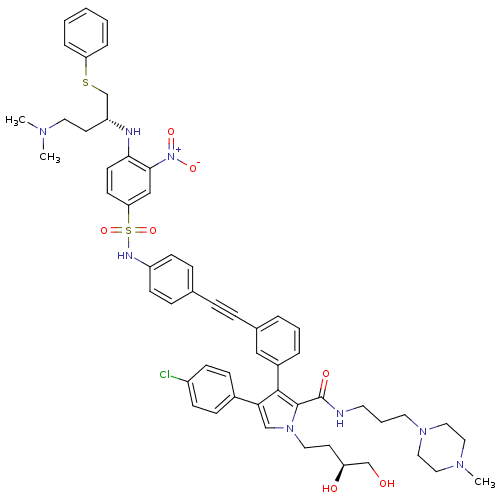 (CHEMBL2063886) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50463777 (CHEMBL4238224) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant mouse Cb2 receptor expressed in HEK293 cells | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50557512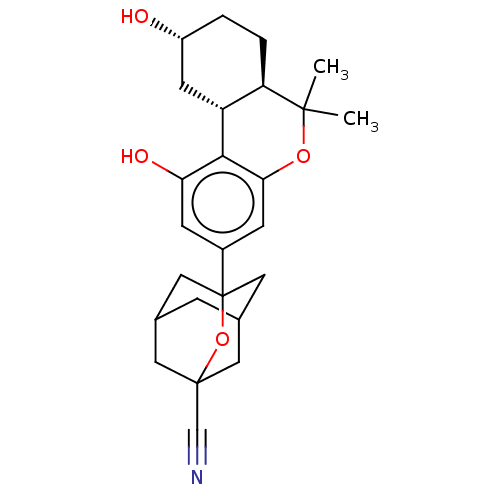 (CHEMBL4779775) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127882 BindingDB Entry DOI: 10.7270/Q2NP283T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50463778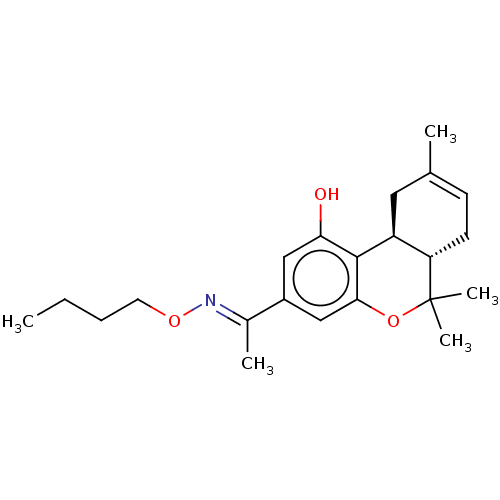 (CHEMBL4240996) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant mouse Cb2 receptor expressed in HEK293 cells | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50397462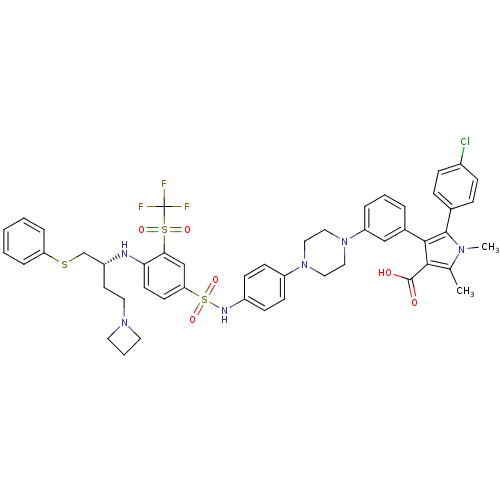 (CHEMBL2170848) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminal 6xHis-tagged human Bcl-2 expressed in Escherichia coli BL21 (DE3) after 2 hrs by fluorescence polarization assay | J Med Chem 55: 8502-14 (2012) Article DOI: 10.1021/jm3010306 BindingDB Entry DOI: 10.7270/Q2V69KQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50388986 (CHEMBL2063886) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 8X His-tagged human Bcl-xL expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization ass... | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 10675 total ) | Next | Last >> |