

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50206243 (CHEMBL3918431) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from human alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma countin... | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50173944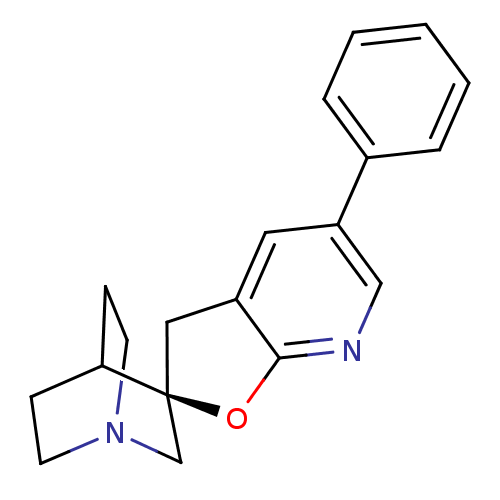 (5'-phenyl-(2'R)-spiro[4-azabicyclo[2.2.2]octane-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to human alpha7 nAChR | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50173944 (5'-phenyl-(2'R)-spiro[4-azabicyclo[2.2.2]octane-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to 5-HT3A receptor (unknown origin) | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50393243 (CHEMBL2151437) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from human alpha7 nAChR expressed in HEK293 cell membranes after 2 hrs by topcount scintillation counting an... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50235306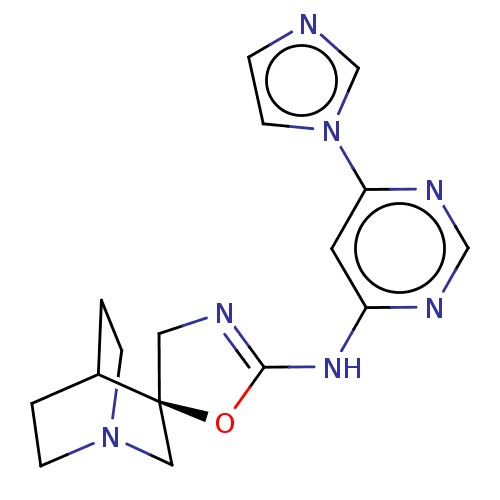 (CHEMBL4084621) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma counting ... | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50206243 (CHEMBL3918431) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]Tyr54-alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes co-expressing human RIC3 measured after 2 hrs... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50206243 (CHEMBL3918431) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma counting ... | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50393243 (CHEMBL2151437) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes after 2 hrs by topcount scintillation counting anal... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50235306 (CHEMBL4084621) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from human alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma countin... | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50211195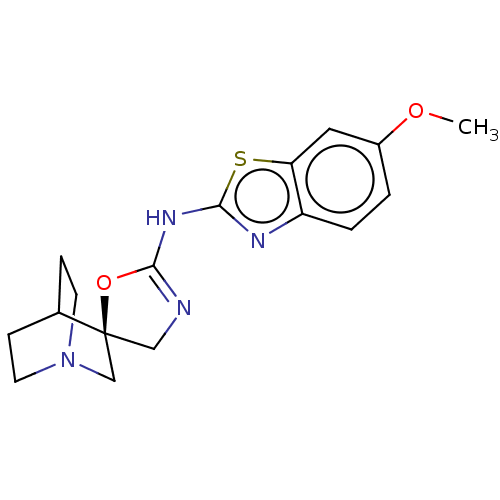 (CHEMBL3944506) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]Tyr54-alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes co-expressing human RIC3 measured after 2 hrs... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164613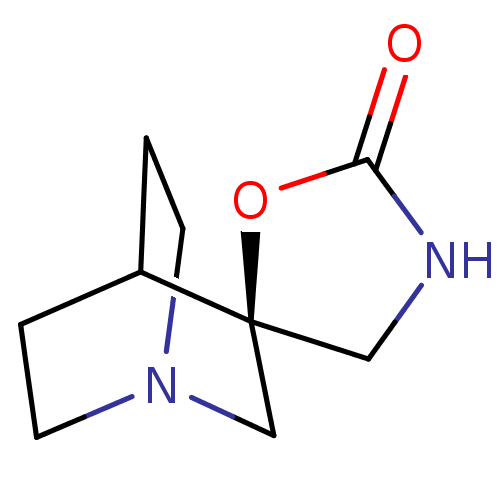 ((-)-Spiro[1-azabicyclo(2.2.2)octane-3,5'-oxazolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from rat hippocampal alpha7 nAChR measured after 2 hrs by TopCount scintillation counting method | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25960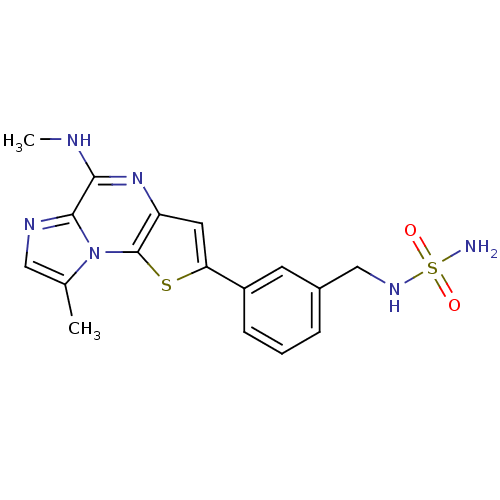 (amino-N-({3-[12-methyl-8-(methylamino)-3-thia-1,7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50232609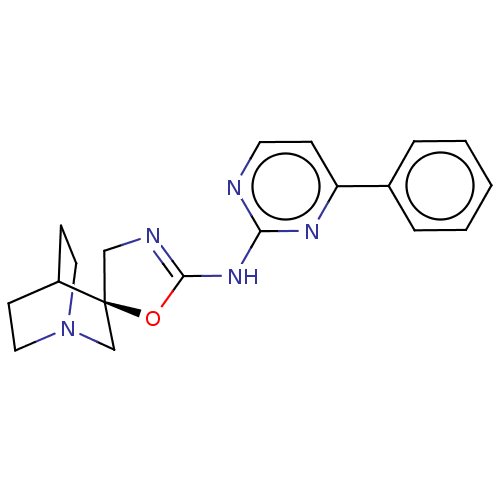 (CHEMBL4098776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT3A receptor assessed as inhibition of 5-HT-induced calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 1261-1266 (2017) Article DOI: 10.1016/j.bmcl.2017.01.058 BindingDB Entry DOI: 10.7270/Q2FJ2K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451609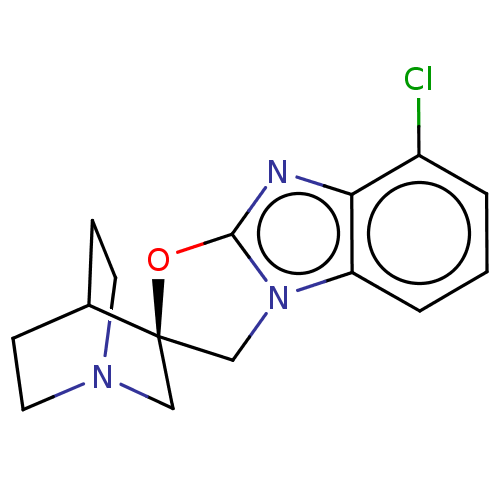 (CHEMBL4204902) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50206258 (CHEMBL3963469) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of Ca2+ flux by Fluo-4-AM dye based FLIPR as... | ACS Med Chem Lett 8: 133-137 (2017) Article DOI: 10.1021/acsmedchemlett.6b00471 BindingDB Entry DOI: 10.7270/Q2765HBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451617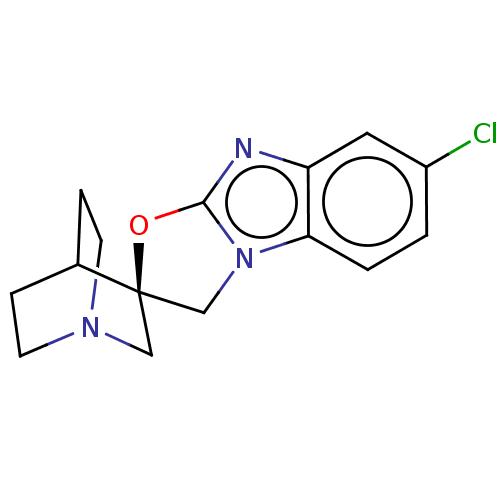 (CHEMBL4206339) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451616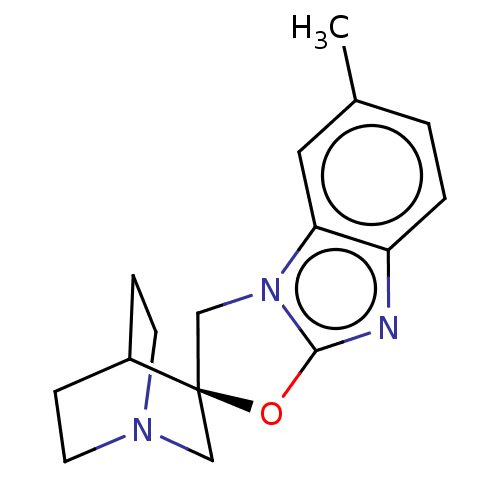 (CHEMBL4209782) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451607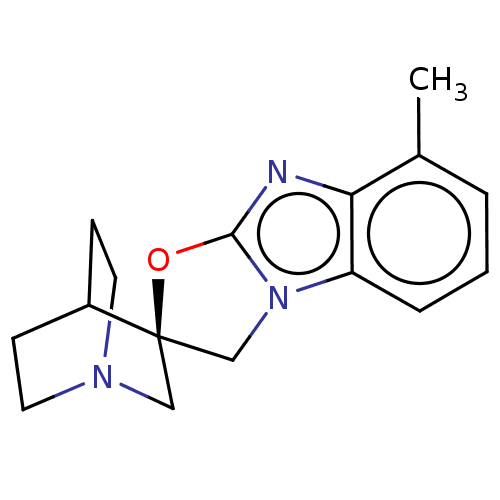 (CHEMBL4202588) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50206256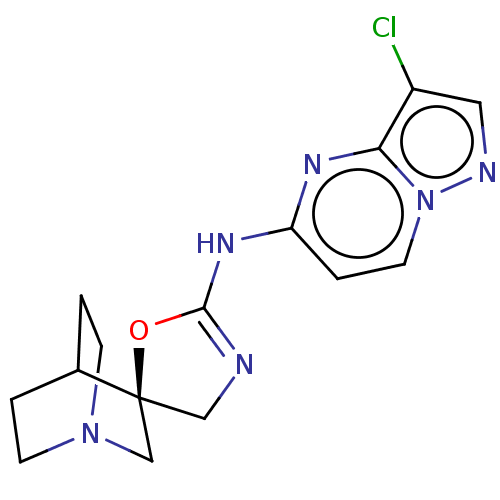 (CHEMBL3917985) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of Ca2+ flux by Fluo-4-AM dye based FLIPR as... | ACS Med Chem Lett 8: 133-137 (2017) Article DOI: 10.1021/acsmedchemlett.6b00471 BindingDB Entry DOI: 10.7270/Q2765HBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50206241 (CHEMBL3901366) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 5-HT3A receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of Ca2+ flux by Fluo-4-AM dye based FLIPR as... | ACS Med Chem Lett 8: 133-137 (2017) Article DOI: 10.1021/acsmedchemlett.6b00471 BindingDB Entry DOI: 10.7270/Q2765HBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211207 (CHEMBL3950038) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25965 (amino-N-(2-{3-[12-methyl-8-(methylamino)-3-thia-1,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211210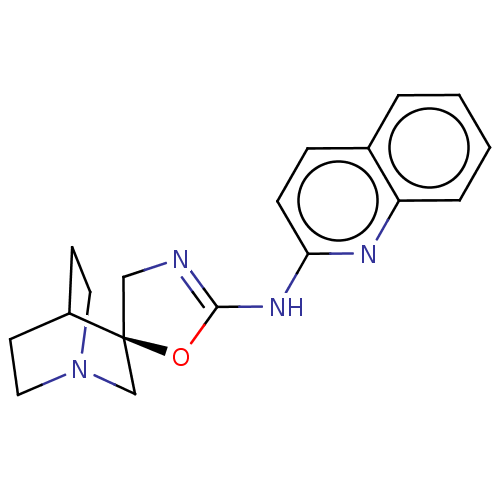 (CHEMBL3984925) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25970 (2-{3-[12-methyl-8-(methylamino)-3-thia-1,7,10-tria...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25942 ((3-{8-[(2-aminoethyl)amino]-12-methyl-3-thia-1,7,1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25957 (4-[3-(aminomethyl)phenyl]-N,12-dimethyl-3-thia-1,7...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50232599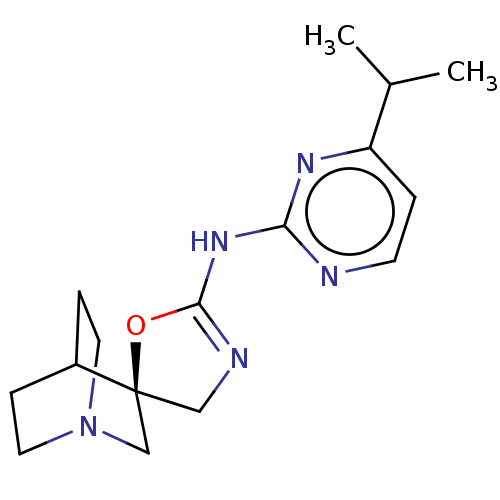 (CHEMBL4095046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT3A receptor assessed as inhibition of 5-HT-induced calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 1261-1266 (2017) Article DOI: 10.1016/j.bmcl.2017.01.058 BindingDB Entry DOI: 10.7270/Q2FJ2K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451618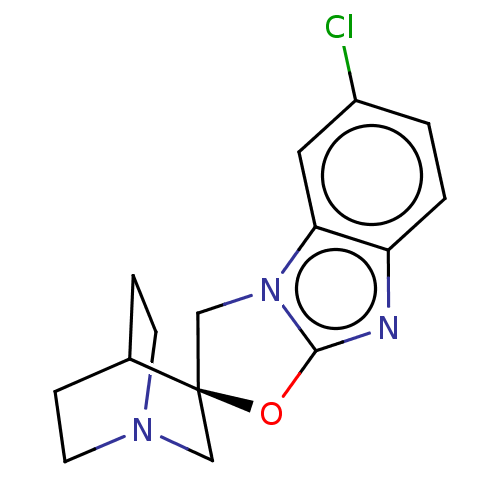 (CHEMBL4218050) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25971 (3-{3-[12-methyl-8-(methylamino)-3-thia-1,7,10-tria...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25934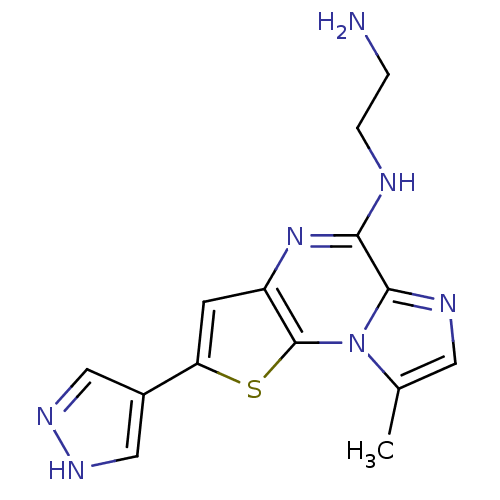 (N-(2-aminoethyl)-12-methyl-4-(1H-pyrazol-4-yl)-3-t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451605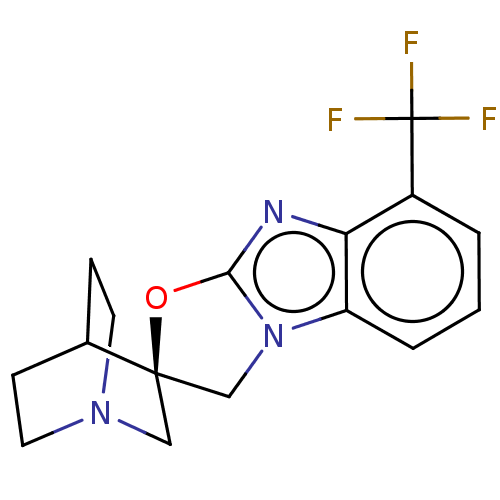 (CHEMBL4216282) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451610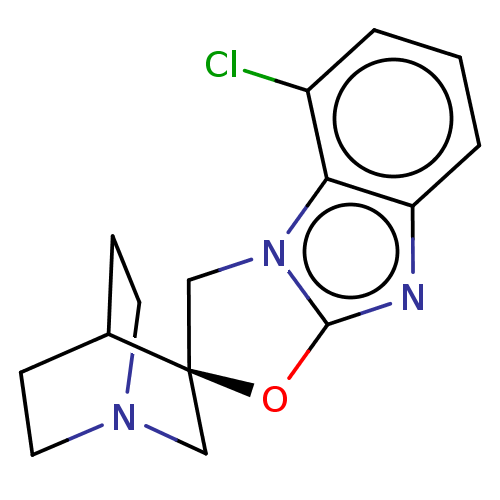 (CHEMBL4218598) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451614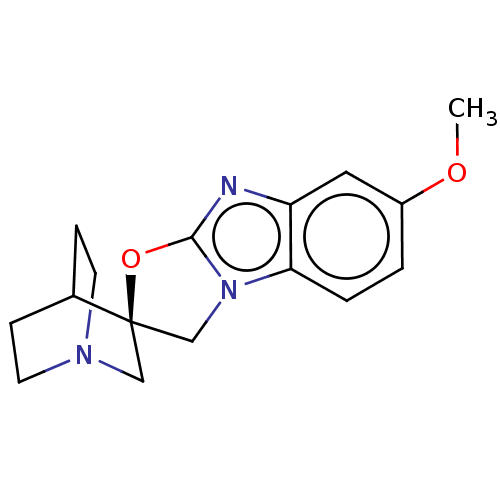 (CHEMBL4211315) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211200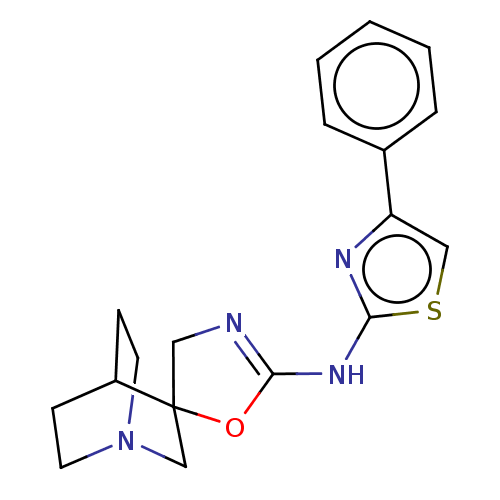 (CHEMBL3974854) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25924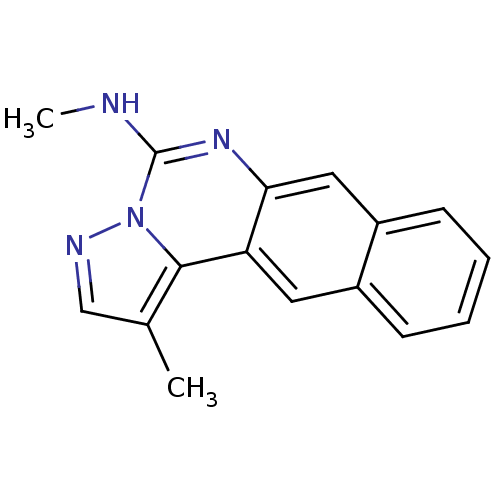 (N,12-dimethyl-14,15,17-triazatetracyclo[8.7.0.0^{3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 1233-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.017 BindingDB Entry DOI: 10.7270/Q2V40SJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451608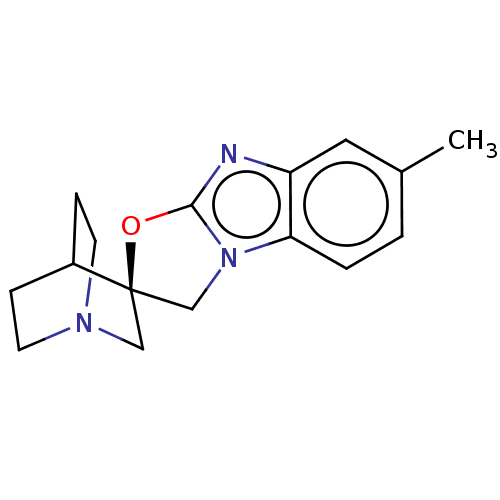 (CHEMBL4207475) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211198 (CHEMBL3961219) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25966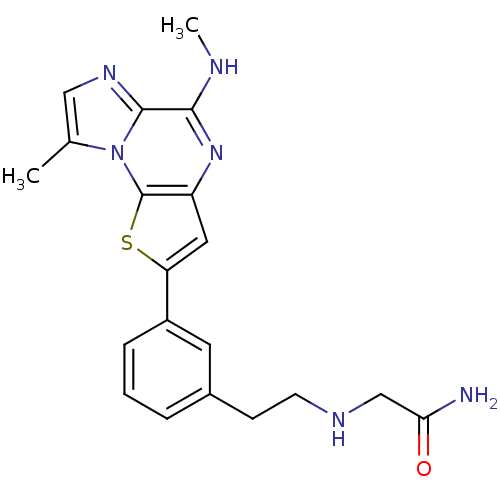 (2-[(2-{3-[12-methyl-8-(methylamino)-3-thia-1,7,10-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25973 (2-amino-3-{3-[12-methyl-8-(methylamino)-3-thia-1,7...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25975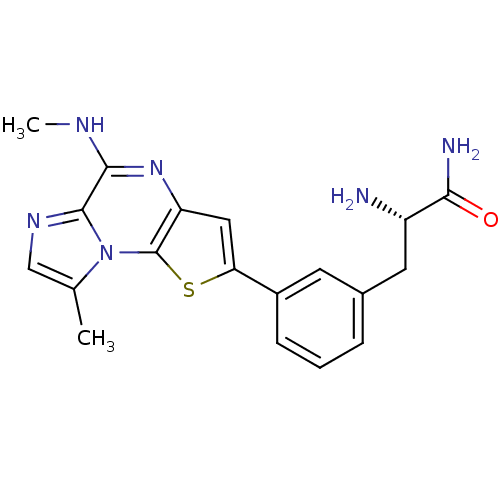 ((2S)-2-amino-3-{3-[12-methyl-8-(methylamino)-3-thi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211215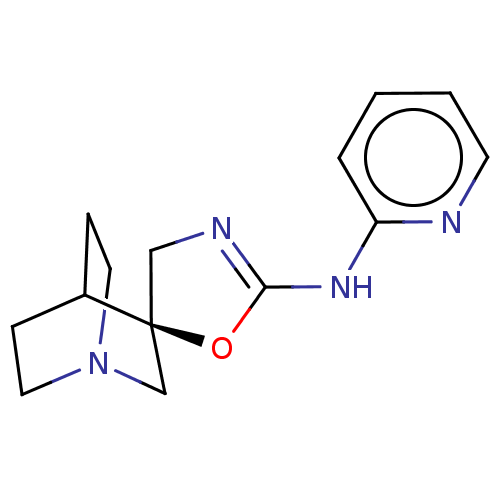 (CHEMBL3898922) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT3A receptor assessed as inhibition of 5-HT-induced calcium influx by Fluo-4-AM dye based FLIPR assay | Bioorg Med Chem Lett 27: 1261-1266 (2017) Article DOI: 10.1016/j.bmcl.2017.01.058 BindingDB Entry DOI: 10.7270/Q2FJ2K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451612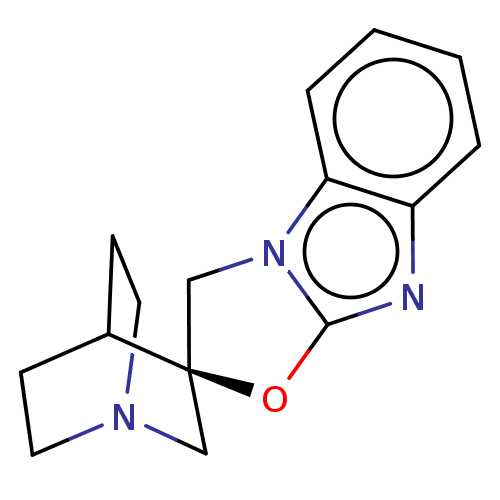 (CHEMBL4215131) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211215 (CHEMBL3898922) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211212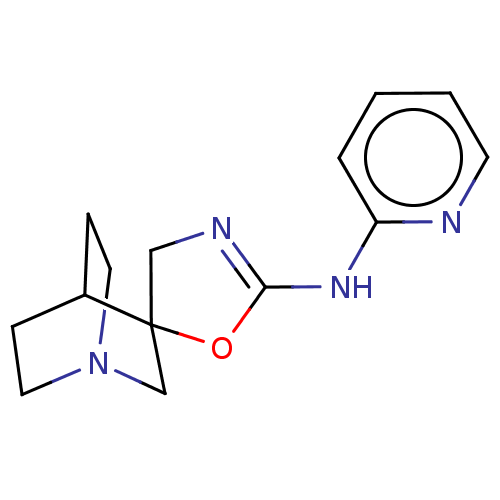 (CHEMBL3927589) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50451615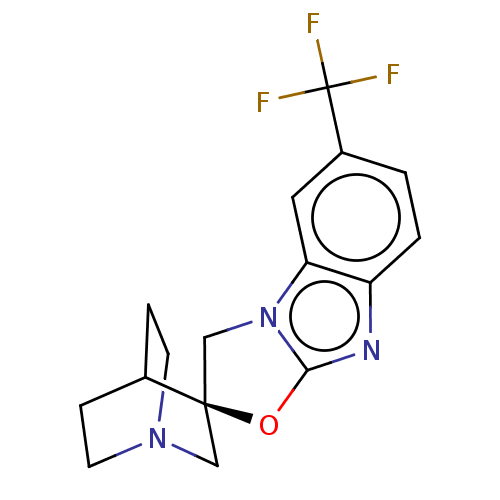 (CHEMBL4203125) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A receptor expressed in HEK293 cells assessed as inhibition of 5-HT-induced calcium flux preincubated for 30 mins p... | Bioorg Med Chem Lett 27: 5002-5005 (2017) Article DOI: 10.1016/j.bmcl.2017.10.009 BindingDB Entry DOI: 10.7270/Q24Q7XJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25964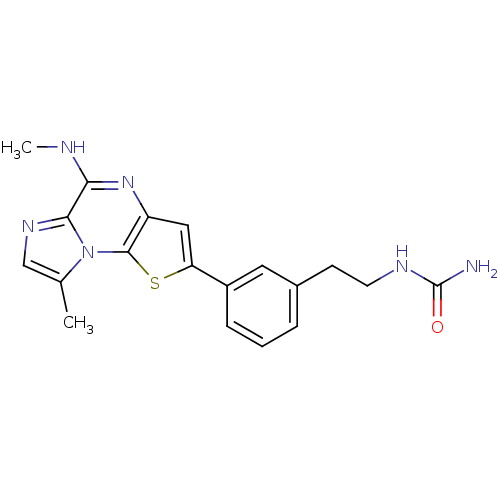 ((2-{3-[12-methyl-8-(methylamino)-3-thia-1,7,10-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25920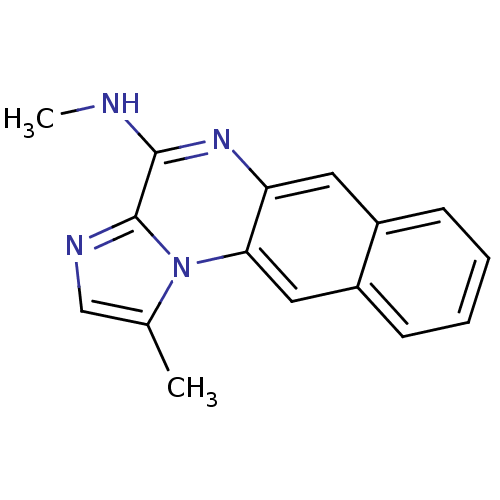 (N,12-dimethyl-11,14,17-triazatetracyclo[8.7.0.0^{3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 1233-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.017 BindingDB Entry DOI: 10.7270/Q2V40SJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25922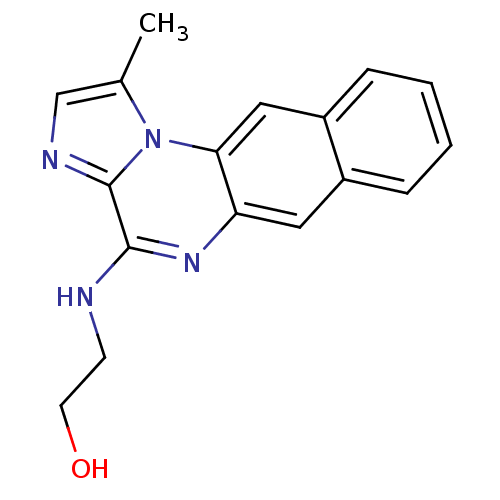 (2-({12-methyl-11,14,17-triazatetracyclo[8.7.0.0^{3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 1233-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.017 BindingDB Entry DOI: 10.7270/Q2V40SJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211202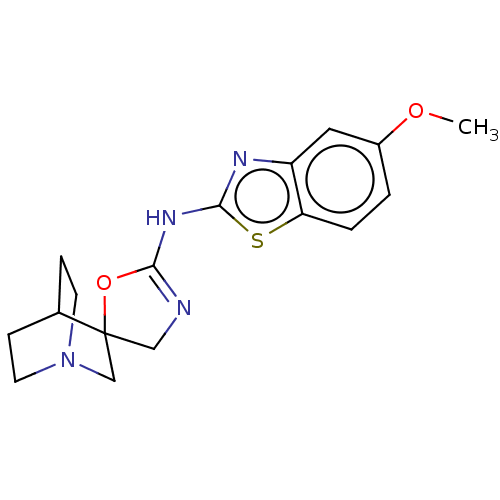 (CHEMBL3981300) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM25972 (3-{3-[12-methyl-8-(methylamino)-3-thia-1,7,10-tria...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... | Bioorg Med Chem Lett 17: 4284-9 (2007) Article DOI: 10.1016/j.bmcl.2007.05.031 BindingDB Entry DOI: 10.7270/Q2QF8R50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 511 total ) | Next | Last >> |